In this issue of Blood, Kastritis et al report growth differentiation factor-15 (GDF-15) as a novel biomarker for predicting early mortality, risk of progression to dialysis, and reduction in levels following treatment to predict improvement in survival in light chain (AL) amyloidosis.1
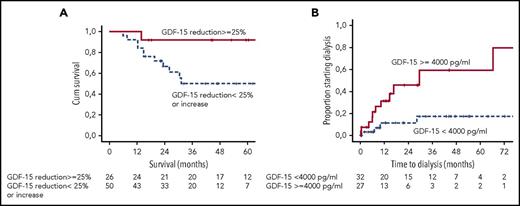
(A) Landmark analysis of survival of patients achieving a reduction in GDF-15 by ≥25% at 6 months. (B) Risk of dialysis for patients presenting with GDF-15 >4000 pg/mL. See Figures 1C and 2A in the article by Kastritis et al that begins on page 1568.
(A) Landmark analysis of survival of patients achieving a reduction in GDF-15 by ≥25% at 6 months. (B) Risk of dialysis for patients presenting with GDF-15 >4000 pg/mL. See Figures 1C and 2A in the article by Kastritis et al that begins on page 1568.
Systemic AL amyloidosis remains a challenge to diagnose and to treat.2 The amyloid fibril deposition and the amyloidogenic light chains both contribute to organ dysfunction; however, exact contribution of each component remains unclear. Because of the insidious nature of the presenting symptoms, two-thirds of all patients have advanced organ failure at diagnosis resulting from a delay in recognition of the etiology. Cardiac involvement, seen in ∼80% patients, continues to cause morbidity and mortality; even with the best available treatments, ∼30% of all patients succumb to the disease in the first few months. Renal involvement, also seen in ∼60% to 70% patients, although not a direct cause of mortality, is a major cause of morbidity because of the high proportion of patients that progress to dialysis within a few years of diagnosis. Exciting progress with agents affecting amyloid deposition (small molecules such as doxycycline or EGCG) or that accelerate amyloid clearance (NEOD001, CAEL 101, and miridesap/demcizumab) has raised new questions about response assessment and trial end points.3
Biomarkers, already important in determining prognosis and choosing the correct treatment pathway, also form the bedrock for trial design and promise to be surrogate end points for drug licensing. The cardiac staging system proposed by the Mayo Clinic group using a combination of N-terminal pro–B-type natriuretic peptide (NT-proBNP) and troponin-T, with later addition of serum-free light chains, is the current gold standard.4 Additionally, very high presenting NT-proBNP (NT-proBNP >8500 ng/L; so-called Mayo stage 3b) identified a very poor-risk group of patients in which treatment benefit remains to be proven.5 NT-proBNP, a marker directly induced in cardiac myocytes because of activation of p38-MAPKinase pathway by the amyloidogenic light chains, changes rapidly following successful treatment.3 The very sensitivity of NT-proBNP in heart failure (of all etiologies) also becomes a crucial problem. It is susceptible to rapid changes with fluid overload, nutritional status, and, critically, changing renal function. Regulatory authorities, with fingers burned by this biomarker in other cardiovascular areas, remain skeptical about its use as a primary end point for clinical trials. A number of new and interesting biomarkers reported over the past few years also show promise in determining prognosis in AL amyloidosis. These include: osteoprotegerin (mechanism unclear),6 von Willebrand factor (probably a marker of endothelial toxicity),7 soluble ST2 (a marker of myocardial remodelling and fibrosis),8 an abnormal Hevylite ratio, and percentage of normal vs abnormal plasma cells in the bone marrow by multiparameter flow cytometry (probably markers of the aggressiveness of the clone).9 None, however, has made it to the main stream. The specter of death from cardiac involvement relegated studies of renal prognosis to a dark place until recent efforts defined a prognostic stratification for renal patients based on a combination of worsening proteinuria and decreasing renal excretory function. However, to date, there has never been a specific renal biomarker in AL amyloidosis. A biomarker marker unifying these various aspects has remained the holy grail of AL amyloidosis.
GDF-15 is a stress/inflammation response marker produced by cardiac myocytes, macrophages, endothelial, and other tissues.10 Raised levels are a marker of poor outcome in heart failure from other etiologies as well as a marker of increased risk of renal failure in diabetes. The present study reports the utility of GDF-15 in a test cohort of patients with AL amyloidosis and validation in an independent patient cohort. Five salient features emerge (see figure), as follows: (1) >90% of all patients with amyloidosis have significantly abnormal GFD-15 levels at diagnosis. (2) More than two-thirds of patients with very high levels of GDF-15 at presentation (>7575 pg/mL) die within a year of diagnosis, a marker of poor prognosis independent of other cardiac biomarkers. (3) GDF-15 levels >4000 pg/mL are the only independent predictors of progression to dialysis; this trumps the recently described renal staging system in a multivariate analysis and, thus, GDF-15 becomes the first renal biomarker in AL amyloidosis. (4) GDF-15 levels appear to decrease rapidly after chemotherapy. At 3 months, a substantially greater number of patients showed decrease in GDF-15 levels compared with NT-proBNP; the survival was significantly better in these patients. GDF-15 shows the potential to replace NT-proBNP as an early response marker in AL amyloidosis. (5) Last, failure for GDF-15 levels to decrease to <4000 pg/mL (or any increase to above this level) predicts for a significantly higher risk of progressing to dialysis.
These impressive results suggest that GDF-15, for the first time, may be a marker providing “unified” prognostic information in AL amyloidosis: from survival, risk of dialysis, to a treatment response. However, the GDF-15 story in AL also raises fascinating questions about the disease pathophysiology and about the use of this marker in clinical practice. The very rapid reduction in GDF-15, even before NT-proBNP, suggests significant contribution from the toxicity of free light chains or amyloid oligomers that causes an unfolded protein stress response rather than from the physical amyloid deposits. So what happens in the longer term as deposits gradually regress, and how will GDF-15 behave if used to assess response with therapies targeting amyloid removal as opposed the current treatments reducing the toxic precursors? Understanding the long-term longitudinal changes to GDF-15 levels resulting from structural improvement in the tissues would be important. The other interesting questions are the reasons for the renal prognostic significance of GDF-15. Is GDF-15 simply a marker of nephrotoxicity of light chains analogous to NT-proBNP for the heart? How much does the cardiorenal syndrome contribute to or confound the marker (or not)? A study in a cohort of patients with predominant renal AL without heart involvement would be important.
Is the time up for the other cardiac biomarkers? NT-proBNP, as a marker of prognosis and organ response, has been studied and validated in thousands of patients. Its pathophysiology is understood, and long-term changes are well known. Furthermore, NT-proBNP remains significant as an independent factor for mortality along with GDF-15. GDF-15 in AL, although compelling, needs to demonstrate reproducibility in larger series and answer some of questions raised. However, the message is clear. Traditional markers watch out: the new kid looks set to win the gold cup.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal