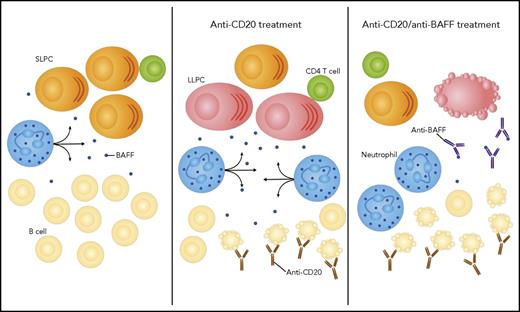
In this issue of Blood, Thai et al demonstrated that after B-cell depletion, elevated levels of unconsumed B-cell activating factor (BAFF) induced the emergence of long-lived plasma cells in the spleen.1 Supported by neutrophils and CD4 T cells, this niche could be targeted by the administration of neutralizing antibodies against BAFF.
In the spleen, B cells consume most of the BAFF secreted by neutrophils. The antibody-secreted cell population is dominated by short-lived plasma cells (SLPCs). After depletion of B cells by an anti-CD20 antibody, the availability of BAFF increases, sustaining the emergence of LLPCs. The combination of the anti-CD20 antibody with an anti-BAFF antibody results in amplified B-cell apoptosis and exhaustion of the LLPC niche.
In the spleen, B cells consume most of the BAFF secreted by neutrophils. The antibody-secreted cell population is dominated by short-lived plasma cells (SLPCs). After depletion of B cells by an anti-CD20 antibody, the availability of BAFF increases, sustaining the emergence of LLPCs. The combination of the anti-CD20 antibody with an anti-BAFF antibody results in amplified B-cell apoptosis and exhaustion of the LLPC niche.
The terminal output of B-cell differentiation can either be a plasmablast or a plasma cell (PC). Plasmablasts are cycling, short-lived, and generated from the early phase of the immune response, providing the first wave of relatively low-affinity antibodies. Postmitotic PCs arise next from the germinal centers, with the ability to secrete high-affinity immunoglobulins of a range of isotypes. Both antibody-secreting cell populations are generated in the secondary lymphoid organs, and a small number of the PCs will settle in the bone marrow (BM). The current dogma is that the longevity of PCs depends on the availability of external survival factors rather than the implementation of an intrinsic program.2 The BM provides a unique microenvironment, able to extend almost indefinitely the lifespan of long-lived plasma cells (LLPCs). The composition of this niche is not yet fully elucidated, but several actors, including neutrophils, megakaryocytes, and CXCL12+ stromal cells, have been proposed. Albeit in a lesser extent, it has also been reported that the spleen, lymph nodes, or mucosa-associated lymphatic tissues could also shelter LLPCs. Furthermore, in chronic inflammations, in particular autoimmune conditions like systemic lupus erythematosus (SLE), inflamed tissues can provide new niches for pathologic LLPCs.3 The mechanisms underlying the formation of these ectopic niches remain to be determined.
In 2013, Mahévas et al identified LLPCs in the spleen of primary immune thrombocytopenia purpura (ITP) patients who had been treated with the B-cell–depleting antibody against CD20, rituximab.4 Interestingly, they suggested that the emergence of the LLPCs was not there due to the inflammation associated with the disease, as they were absent from the spleens of untreated ITP patients, but a consequence of the B-cell depletion. They hypothesized that an increase availability of the prosurvival cytokine BAFF was the driving factor of the new niche. BAFF is a member of the TNF family, which is partially redundant with its relative APRIL.5 One of their 3 receptors, BCMA, plays an important role in LLPC survival in the BM.
Thai et al used mouse models to validate this hypothesis and identify the cells supporting the survival of the splenic LLPCs. They established that in steady-state conditions, as well as in lupus-prone mice, the depletion of B cells led to elevated levels of BAFF and the appearance of splenic LLPCs.
They determined that after B-cell depletion, splenic PCs exhibited a transcriptional signature similar to BM PCs (see figure). In contrast, the normal splenic PC analysis revealed a very low expression of the LLPC signature genes defined by the authors. The low frequency of LLPCs in normal spleen could indicate an early apoptosis of these cells in the absence of BAFF to sustain them and/or an increased differentiation state triggered by the microenvironment resulting from B-cell depletion. Sze et al have previously suggested that the spleen had the capacity to support a finite and low number of antibody-secreting cells and that the excess would die within 2 or 3 days.6 The data from Thai et al implied that BAFF could be one of the major limiting factors of the splenic niche and that the LLPCs found in rituximab-treated ITP patients were not occupying an ectopic niche created by inflammation, but an hypertrophied natural one.
Thai et al proposed that the neutrophils were the main source of BAFF, as they expressed high levels of this cytokine and colocalized with antibody-secreting cells. Because their involvement could not be directly confirmed, and because Mahévas et al previously reported that basophils were recruited in ITP patient spleens,4 this point deserves to be further investigated.
To validate the central role of BAFF in the splenic LLPC niche, Thai et al combined rituximab with a BAFF-neutralizing antibody, which resulted in a stronger depletion of B cells and a decrease in the splenic antibody-secreting cell population. As previously reported,7 BM PCs were not affected, suggesting that either these cells are different from those residing in the spleen or that the medullary niche is able to provide other signals, like APRIL, to compensate for the absence of BAFF.
From a clinical perspective, a long-lasting response to rituximab treatment is currently achieved only in a minority of ITP patients,8 and a splenectomy needs to be performed. The authors proposed that the combination of rituximab with a BAFF-neutralizing antibody, like belimumab, would have the double advantage of potentiating the B-cell–depleting effect and eliminating splenic LLPCs resulting from unconsumed BAFF. This combination was approved to treat SLE patients in 2011, and clinical trials are underway for ITP and Sjogren syndrome. This strategy reflects the growing interest in targeting the pathologic LLPC niches to eliminate cells that have proven refractory to immunosuppressants and difficult to deplete by immunotherapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal