In this issue of Blood, Pellicano et al1 identify regulation of the transcription factor E2F1 by hsa-mir183/EGR1 as a novel pathway important for leukemic stem cell survival in chronic myeloid leukemia (CML).
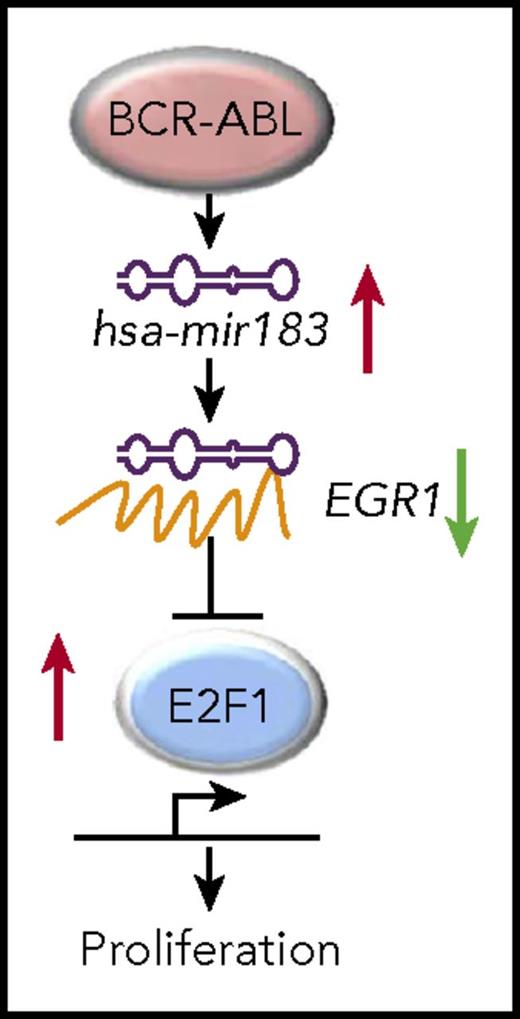
Schematic of the hsa-mir183/EGR1–mediated regulation of E2F1. See Figure 3 in its entirety in the article by Pellicano et al that begins on page 1532.
Schematic of the hsa-mir183/EGR1–mediated regulation of E2F1. See Figure 3 in its entirety in the article by Pellicano et al that begins on page 1532.
In ancient Rome, it was not just Brutus who gave Julius Caesar the final stab with his sword on the Ides of March in 44 BC. More than 40 conspirators had gathered to end the dictator’s life to proclaim “Liberty! Freedom! Tyranny is dead!” (at least in Shakespeare’s play).
Similarly, in hematology, multiple different signaling pathways contribute to leukemic stem cell survival in the face of treatment with tyrosine kinase inhibitors (TKIs) or chemotherapy. Treatments attacking several of these pathways in unison are needed to eradicate leukemic stem cells and lead the patient to freedom from the tyranny of disease and, finally, cure.
The current conundrum in CML therapy lies in the fact that TKIs are highly effective at inducing remissions, reducing disease progression, and prolonging survival of patients with chronic-phase CML but that this, literally, comes at a high price, and adverse events, noncompliance, teratogenicity, and treatment failure are not uncommon events.2 In addition, because of the noneradication of leukemic stem cells, relapse after discontinuation of tyrosine kinase inhibitors occurs in approximately 61% of patients,3 and the treatment-free remission rate remains low.4 Therefore, the rational design of strategies to increase treatment-free remissions is the unwavering goal in CML research and therapy.
Prior to the current study, it was known that quiescent leukemic stem cells (LSC) are resistant to TKI-induced apoptosis,5 although their kinase activity is inhibited.6
Several pathways such as the JAK/STAT, nuclear factor κB, and β-catenin pathways2 and networks involving p53,7 c-MYC, and SIRT1 have been identified as mediators of LSC maintenance and resistance to TKIs. Several of these pathways, including the inhibition of histone deacetylase, the methyltransferase EZH2,2 and BCL2, are now being considered as targetable entities, and therapeutic strategies targeting some of these proteins have entered clinical trials.2
In this article by Pellicano et al, the authors demonstrate a novel BCR-ABL1–dependent pathway, which involves the upregulation of hsa-mir183 and in turn the downregulation and inhibition of its target, the transcription factor early growth response 1 (EGR1), which consequently, leads to the upregulation of the transcription factor E2F1 (see figure), previously shown to be involved in cell cycle progression.8 The authors first confirm the quiescence of CML progenitors and by performing microRNA transcriptomic analyses, the authors show the cancer-related microRNA hsa-mir183 to be highly expressed in CML progenitors in a BCR-ABL1–dependent fashion. Following up on published reports and on computational target prediction performed by the authors, it is shown that hsa-mir183 downregulates EGR1 by direct binding and that knockdown of hsa-mir183 leads to impaired formation of colonies by CML progenitors. Comparing transcriptomic data from quiescent normal versus CML cells, the authors reveal a significant upregulation of E2F1 cell cycle–related target genes in quiescent CML versus normal progenitor cells. In confirmation of the link between EGR1 and E2F1, knockdown of EGR1 led to an increase of E2F1 and cell cycle progression genes. Consistently, knockdown of E2F1 in CML progenitor cells led to cell cycle arrest, decreased colony formation, and an increase in the percentage of dead cells in CML but not of normal progenitors. Knockdown of E2F1 did not alter overall levels of p53, but it increased the phosphorylation of serine 15 on p53, which is a site important for induction of cell cycle arrest. This suggested that decreased expression of E2F1 led to activation of p53. Testing whether targeting of E2F1 may be deleterious for normal hematopoietic stem cells, the authors performed transplantation assays revealing that E2F1 loss in hematopoietic stem cells did not significantly decrease their reconstitution ability.
In summary, this study by Pellicano et al has shown impressively the role of the transcription factor E2F1 for the survival of primary human CML progenitor, but not of normal progenitor, cells. Furthermore, the authors show E2F1 in CML progenitor cells to be regulated by the BCR-ABL1/hsa-mir183/EGR1 axis. Although the authors have previously demonstrated p53 to be downregulated in CML stem cells,7 the authors here provide a tentative link between E2F1 and p53, namely that inhibition of E2F1 increases p53 activity, although the exact mechanism will need to be clarified in the future. The authors’ data suggest that despite E2F1 upregulation being a BCR-ABL1–dependent process, inhibition of E2F1 or partners in the BCR-ABL1/hsa-mir183/EGR1 axis may provide an efficient therapeutic strategy, because expression of genes related to proliferation downstream of E2F1 is active in CML cells persisting after TKI therapy. This finding is particularly noteworthy, because normal hematopoietic stem cells were unaffected by E2F1 loss, underlining the specificity of the discovered pathway for BCR-ABL1+ cells. However, the effect of loss of E2F1 in leukemic stem cells will have to be tested in vivo.
Although drug development, as in the case of E2F1, may be difficult or laden with unexpected toxicities or ineffectiveness once moved into clinical trial, the leukemia field is moving toward the design of effective combination therapies. These may include a combination of drugs or the combination of immunological approaches or targeting of the bone marrow microenvironment with conventional therapies, in a unified effort to eradicate the last leukemic stem cell and toward a final “Then fall, leukemic stem cell!”
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal