Key Points
IL-18 distinguishes susceptibility to MAS amongst hyperferritinemic and autoinflammatory diseases.
Excess IL-18 in NLRC4 gain-of-function mice derives from intestinal epithelia, and free IL-18 promotes experimental MAS.
Abstract
Hemophagocytic lymphohistiocytosis (HLH) and macrophage activation syndrome (MAS) are life-threatening hyperferritinemic systemic inflammatory disorders. Although profound cytotoxic impairment causes familial HLH (fHLH), the mechanisms driving non-fHLH and MAS are largely unknown. MAS occurs in patients with suspected rheumatic disease, but the mechanistic basis for its distinction is unclear. Recently, a syndrome of recurrent MAS with infantile enterocolitis caused by NLRC4 inflammasome hyperactivity highlighted the potential importance of interleukin-18 (IL-18). We tested this association in hyperferritinemic and autoinflammatory patients and found a dramatic correlation of MAS risk with chronic (sometimes lifelong) elevation of mature IL-18, particularly with IL-18 unbound by IL-18 binding protein, or free IL-18. In a mouse engineered to carry a disease-causing germ line NLRC4T337S mutation, we observed inflammasome-dependent, chronic IL-18 elevation. Surprisingly, this NLRC4T337S-induced systemic IL-18 elevation derived entirely from intestinal epithelia. NLRC4T337S intestines were histologically normal but showed increased epithelial turnover and upregulation of interferon-γ–induced genes. Assessing cellular and tissue expression, classical inflammasome components such as Il1b, Nlrp3, and Mefv predominated in neutrophils, whereas Nlrc4 and Il18 were distinctly epithelial. Demonstrating the importance of free IL-18, Il18 transgenic mice exhibited free IL-18 elevation and more severe experimental MAS. NLRC4T337S mice, whose free IL-18 levels were normal, did not. Thus, we describe a unique connection between MAS risk and chronic IL-18, identify epithelial inflammasome hyperactivity as a potential source, and demonstrate the pathogenicity of free IL-18. These data suggest an IL-18–driven pathway, complementary to the cytotoxic impairment of fHLH, with potential as a distinguishing biomarker and therapeutic target in MAS.
Introduction
Hyperferritinemic inflammation is increasingly recognized as a potentially lethal systemic inflammatory state characterized by cytopenias, hepatobiliary dysfunction, hepatosplenomegaly, coagulopathy, hypertriglyceridemia, and often hemophagocytosis.1 Familial hemophagocytic lymphohistiocytosis (fHLH) describes this syndrome occurring from profound genetic cytotoxic impairment (eg, perforin deficiency). fHLH is quite rare, but secondary forms of HLH occur more commonly in the context of infections (typically Epstein-Barr virus [EBV]), malignancies, sepsis,2,3 chimeric antigen receptor T-cell therapy,4 and in rheumatic diseases, where it is called macrophage activation syndrome (MAS).
In fHLH, cytotoxic impairment alters “pruning” of infected antigen-presenting cells and permits unrestrained T-cell activation.5,6 Human and murine fHLH are associated with excessive CD8 T-cell expansion and cytokine overproduction, most notably interferon-γ (IFN-γ).7-10 The mechanisms driving secondary HLH and MAS are unclear. As in fHLH, IFN-γ and CXCL9 are disease activity markers and potential therapeutic targets in HLH and MAS.11 Also, transient natural killer (NK) dysfunction and heterozygous cytotoxicity gene defects have been observed in up to 40% of secondary HLH and MAS patients.12-14 However, functional NK defects occur in many inflammatory contexts15 and heterozygous cytotoxicity mutations have a high carrier rate, obfuscating the clinical utility of these findings.16 Infection can cause HLH but also trigger fHLH or MAS flares, further complicating the mechanistic understanding of these disorders. HLH treatment guidelines endorse corticosteroids, etoposide, and potentially bone marrow transplantation.17 Despite this regimen’s efficacy,18 investigators often empirically choose from a variety of less toxic/immunosuppressive therapies as substitutes for etoposide in secondary HLH and MAS.19,20
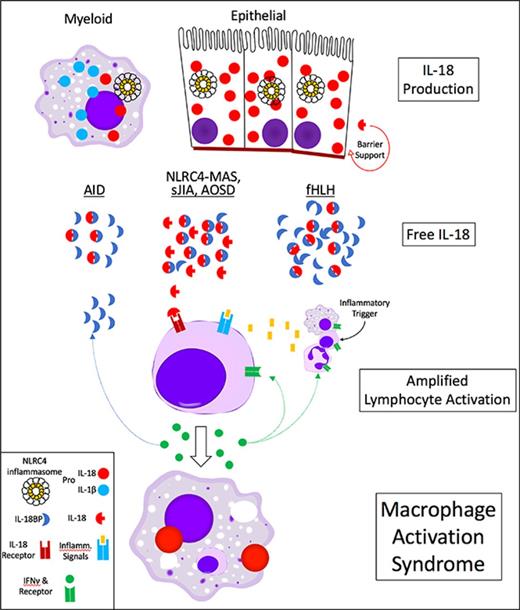
MAS occurs most often in the autoinflammatory diseases systemic juvenile idiopathic arthritis (sJIA) and adult-onset Still disease (AOSD)1,21 and suggests an innate immune mechanism may be at work in MAS.22-25 Inflammasomopathies are monogenic autoinflammatory diseases (AIDs) caused by activating mutations in MEFV/PYRIN (causing familial Mediterranean fever) or NLRP3 (causing cryopyrin-associated periodic syndromes [CAPS]). Inflammasomes are massive pathogen/danger-sensing complexes culminating in maturation of the inflammatory cytokines interleukin-1β (IL-1β) and IL-18, and an inflammatory cell death called pyroptosis.26 The NLRP3 and PYRIN inflammasomopathies are not associated with MAS.26 However, a syndrome of severe/recurrent MAS, infantile enterocolitis, and elevated IL-18 was recently associated with gain-of-function mutations in the NLRC4 inflammasome (NLRC4-MAS)27-29 ; and 1 refractory NLRC4-MAS showed a remarkable response to IL-18 blockade.30
IL-18 is expressed in pro-form by myeloid and epithelial cells. Its receptor is expressed most robustly in cytotoxic lymphocytes, and it is best known for amplifying lymphocyte production of IFN-γ. IL-18 has an endogenous antagonist, IL-18 binding protein (IL-18BP) that is induced by IFN-γ.31 Compared with IL-1β, little is known about IL-18 in human disease. Although IL-18 has been previously associated with MAS in sJIA/AOSD,32,33 the discovery of NLRC4-MAS provided an avenue to explore its origins and effects. Animal models of MAS involve innate immune stimuli and largely support a pathogenic role for IFN-γ, but have not investigated IL-18.34,35 We sought to characterize the association of IL-18 and related cytokines in complex cohorts of hyperferritinemic and AID syndromes and to better understand the sources and effects of IL-18 in experimental MAS.
Methods
Human subjects
Research was conducted under institutional review board–approved protocols at the University of Freiburg, University of Muenster, Cincinnati Children’s Hospital, and the Intramural National Institute of Diabetes and Digestive and Kidney Diseases. Clinical details are provided in Data supplements A and B, available on the Blood Web site.
Murine models
Experiments in wild-type (WT) and NLRP3-deficient/L351P-inducible mice (Jackson), Pycard−/− mice (Genentech), and MEFVV726A/V726A mice36 were performed under protocols approved by National Institute for Arthritis and Musculoskeletal and Skin Diseases or the University of Pittsburgh. The Nlrc4T337S allele was engineered as in Data supplement C. Sublethal endotoxemia was induced with 2.5 mg/kg of lipopolysaccharide (LPS; Sigma) intraperitoneally; TLR9-MAS as in Behrens et al34 ; dextran sodium sulfate colitis as in Nowarski et al37 ; LCMV Armstrong as in Jordan et al7 ; and lymphocytic choriomeningitis virus (LCMV) clone 13 infection was induced with 106 plaque-forming unit IV. Broad-spectrum antibiotics were administered as in Ayres et al.38 Bone marrow chimeras were performed as in Hashimoto et al.39 Murine bone marrow–derived macrophages (BMDMs) were generated and stimulated as in Chae et al36 with LPS (Enzo).
Cytokine measurement
For human IL-18, IL-18BP, and CXCL9, serum was diluted with sample buffer as noted in the figure legends and assayed on a Magpix multiplex instrument per the manufacturer’s instructions (Luminex). BioPlex Pro group II cytokine standard was used for IL-18 and CXCL9; and human IL-18BPa-Fc (R&D Systems) was used for IL-18BP. Mouse and human IL-18 and human IL-18BPa beads were generated by conjugating capture antibody to magnetic beads per the manufacturer’s instructions (Bio-Rad; Data supplement D). For correlation analysis, human IL-18 and IL-18BPa were measured as described previously. IL-6, CXCL9, CXCL10, IFN-α2, IL-2Rα, IL-10, tumor necrosis factor-α, IFN-γ, and IL-12p70 were measured at fourfold dilution using BioPlex Xpress system per the manufacturer’s instructions. IL-37 (which may promote IL-18BP function40 ) was measured by enzyme-linked immunosorbent assay (R&D Systems). C-reactive protein (CRP) and ferritin were derived from clinical measurements.
In preliminary measurements, we found standard dilutions of MAS samples saturated the IL-18 assay, requiring significant dilution and consistent with a hook effect/prozone phenomenon (supplemental Figure 1A); thereafter, serum was diluted 25-fold. Consistent with previous reports,41 we found minimal differences between serum or plasma in paired samples (supplemental Figure 1B). Importantly, we found the measurement of IL-18BPa was inhibited by IL-18 in a dose-dependent manner (supplemental Figure 1C), although total IL-18 detection was unaffected by IL-18BP.42 Thus, reported concentrations of IL-18BPa may represent IL-18–dependent underestimates.
For Figures 6 and 7, supplemental Figure 5B, and supplemental Figure 6, murine cytokines were measured using Cytometric Bead Array per the manufacturer’s instructions (BD). To minimize floor effects, an offset of 1/4 the lowest concentration of each analyte’s standard curve was added to each experimental value.
Human free IL-18 was measured as in Girard et al.42 Murine free IL-18 was measured similarly, as described in Data supplement D.
Immunoprecipitation
IL-18 from THP1 (ATCC TIB-202) lysates, 1 mL of control serum ± recombinant human IL-18 (MBL), or 1 mL patient serum was immunoprecipitated with Protein G Dynabeads per the manufacturer’s instructions (Thermo Fisher).
Flow cytometry
Murine tissues were prepared as in Data supplement E with reagents as in Data supplement D. Events were acquired on a BD FACSCanto II or Fortessa, and analyzed using FlowJo v9.9.4 (Treestar).
Transcriptional analyses
Microarray data were represented as either GC robust multiarray average (Figure 5B), normalized expression value (supplemental Figure 7A; data obtained from http://www.immgen.org), or z score (Figure 5D-E), as curated by and downloaded from https://www.biogps.org or http://www.immgen.org. For RNA-sequencing (RNA-seq), read per kilobase million were obtained from the Gene Expression Omnibus as indicated in the figure legends, converted to transcripts per kilobase million (TPM), and a 0.5 TPM offset added to all values. Intestinal epithelium was isolated by scraping phosphate-buffered saline–washed duodenal lumens with RNase-treated slides and immediately storing in Trizol LS (Thermo Fisher). RNA integrity was analyzed with the Agilent 2200 Tapestation. Messenger RNA purification and fragmentation, complementary DNA synthesis and target amplification were performed with Illumina TruSeq RNA Sample Preparation kit. Pooled complementary DNA libraries were sequenced on Illumina HiSequation 2000 platform, mapped to mm9 using Tophat v2.1, and quantified using Partek GSv6.6 software. Reads were converted to TPM and a 0.5 TPM offset added to all transcripts. Noncoding genes and genes with no sample >1 TPM were removed. Differentially expressed genes are defined as in Data supplement E. Core analysis was then performed on differentially expressed genes using Ingenuity Pathway Analysis v01-08 (Data supplement G). Taqman (Life) quantitative polymerase chain reaction was performed on RNA isolated from Trizol (Thermo Fisher).
Histology
Hematoxylin and eosin, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling, and periodic acid–Schiff staining was performed by Histoserv. Click-It/EdU staining was performed per the manufacturer’s instructions (Life Technologies).
Statistical analysis
Analyses were carried out as indicated in the figure legends using GraphPad Prism v7.
Results
IL-18 distinguishes human MAS from fHLH
We first examined active disease samples from sJIA, MAS, infection-associated HLH (IA-HLH), and fHLH for total IL-18, IL-18BP, free IL-18,42 and CXCL9 (eg, “monokine induced by IFN-γ,” a surrogate for IFN-γ activity11 ). Patients met appropriate criteria for sJIA,43 sJIA-MAS (save 1 lupus patient, open circle),44 and HLH.17 EBV-HLH accounted for 57% of IA-HLH patients, and STXBP2 mutations for 47% of fHLH (Data supplement A).
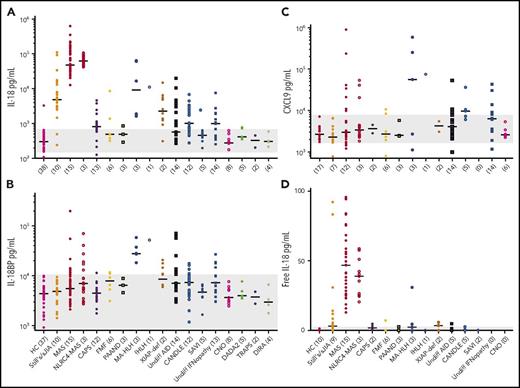
Total IL-18 was extremely high in MAS (∼500-fold above controls; Figure 1A), whereas IL-18BP and CXCL9 were elevated but varied minimally among MAS, IA-HLH, and fHLH (Figure 1B-D). Accordingly, “free IL-18”42 was most consistently elevated in MAS (Figure 1C). A total IL-18 level >24 000 pg/mL distinguished MAS from fHLH with 83% sensitivity and 94% specificity (Figure 1E). Normalizing IL-18 by CXCL9 slightly improved the distinction between MAS and fHLH (Figure 1F-G), although it did not distinguish MAS from active sJIA, a role largely filled by several biomarkers including ferritin44 (supplemental Figure 2).
IL-18 distinguishes MAS from fHLH during active hyperferritinemia. Serum samples from a cohort of healthy controls (HCs) and patients with active sJIA, MAS, IA-HLH, and fHLH were probed for (A) total IL-18, (B) IL-18 binding protein, (C) free IL-18, and (D) CXCL9. See Data supplement A for clinical details. (E) A receiver operating characteristic (ROC) for total IL-18 distinguishing MAS from fHLH was calculated. (F) Total IL-18/CXCL9 ratios were computed and (G) a ROC for this ratio distinguishing MAS from fHLH was calculated. ★Optimal cutoff of 2.3. All concentrations are in picogram/milliliter. *P < .05, **P < .01, ***P < .001, ****P < .0001 by Kruskal-Wallis test with Dunn multiple comparisons posttest, except for ROC analysis. All differences meeting corrected threshold of P < .05 between individual groups are shown except comparisons made to HC for total IL-18 and CXCL9. The open circle in MAS represents systemic lupus erythematosus–associated MAS; all others are sJIA-MAS. Open circles in fHLH represent plasma samples. AUC, area under curve.
IL-18 distinguishes MAS from fHLH during active hyperferritinemia. Serum samples from a cohort of healthy controls (HCs) and patients with active sJIA, MAS, IA-HLH, and fHLH were probed for (A) total IL-18, (B) IL-18 binding protein, (C) free IL-18, and (D) CXCL9. See Data supplement A for clinical details. (E) A receiver operating characteristic (ROC) for total IL-18 distinguishing MAS from fHLH was calculated. (F) Total IL-18/CXCL9 ratios were computed and (G) a ROC for this ratio distinguishing MAS from fHLH was calculated. ★Optimal cutoff of 2.3. All concentrations are in picogram/milliliter. *P < .05, **P < .01, ***P < .001, ****P < .0001 by Kruskal-Wallis test with Dunn multiple comparisons posttest, except for ROC analysis. All differences meeting corrected threshold of P < .05 between individual groups are shown except comparisons made to HC for total IL-18 and CXCL9. The open circle in MAS represents systemic lupus erythematosus–associated MAS; all others are sJIA-MAS. Open circles in fHLH represent plasma samples. AUC, area under curve.
Importantly, clinical data suggested a cohort with comparable disease activity. CRP elevation was comparable in all diseased samples, and hyperferritinemia was comparable in MAS, IA-HLH, and fHLH (supplemental Figure 2). Thrombocytopenia was slightly worse in fHLH than in MAS.
IL-18 distinguishes MAS from other autoinflammatory diseases
We next assessed samples, often longitudinally, from genetically and clinically defined AID (Figure 2; supplemental Figure 3; Data supplement B; supplemental Table 1). We found most patients referred for active or historical MAS (as defined by the referring investigator) had chronically elevated total IL-18 (Figure 2A; supplemental Figure 3, red symbols), typically >40 times normal. By contrast, in NLRP3 and MEFV inflammasomopathies, IL-1, IFN, and nuclear factor-κB–mediated AID, and inflammatory bone disorders, total IL-18 was more modestly and less consistently elevated. Three malignancy-associated HLH patients (2 adult lymphomas, 1 congenital neuroblastoma) demonstrated highly elevated IL-18 and IL-18BP, resulting in minimal free IL-18 (Figure 2; supplemental Figure 3, arrows). Intermediate levels of IL-18 were observed in fHLH (supplemental Figure 3, arrowhead), some patients with sJIA/AOSD (orange), and intermittently in other AID. We also detected high IL-18BP in fHLH and in some MAS patients during active disease. CXCL9 was elevated in a similar group of patients to IL-18BP, consistent with their shared induction by IFN-γ (Figure 2C). Free IL-18 elevation was largely restricted to patients referred for MAS (Figure 2D). In this cohort, a total IL-18 value >11 600 provided 88% sensitivity and 93% specificity in distinguishing samples from patients with sJIA, AOSD, and/or MAS from samples from all other samples tested (supplemental Figure 4A). Again, the total IL-18/CXCL9 ratio improved these characteristics slightly (data not shown).
IL-18 is uniquely elevated in autoinflammatory patients referred for MAS. (A) IL-18, (B) IL-18BP, (C) CXCL9, and (D) free IL-1842 were measured in serum samples from a broad referral cohort of patients with autoinflammatory disease. Acronyms, genetic associations, and statistical testing can be found in supplemental Table 1 and Data supplement B. Patients per group are indicated in parentheses, with multiple samples gathered longitudinally from some patients (eg, Figure 3; supplemental Figure 3). Gray bars, range of values measured in HC samples. CANDLE, chronic atypical neutrophilic dermatosis, lipodystrophy, elevated temperature; CNO, chronic nonbacterial osteomyelitis; DADA2, deficiency of ADA2; DIRA, deficiency of IL-1 receptor antagonist; FMF, familial Mediterranean fever; PAAND, pyrin-associated autoinflammation with neutrophilic dermatosis; SAVI, STING-associated vasculopathy of infancy; TRAPS, TNF receptor–associated periodic syndrome; undiff, undifferentiated; XIAP-def, XIAP-deficiency.
IL-18 is uniquely elevated in autoinflammatory patients referred for MAS. (A) IL-18, (B) IL-18BP, (C) CXCL9, and (D) free IL-1842 were measured in serum samples from a broad referral cohort of patients with autoinflammatory disease. Acronyms, genetic associations, and statistical testing can be found in supplemental Table 1 and Data supplement B. Patients per group are indicated in parentheses, with multiple samples gathered longitudinally from some patients (eg, Figure 3; supplemental Figure 3). Gray bars, range of values measured in HC samples. CANDLE, chronic atypical neutrophilic dermatosis, lipodystrophy, elevated temperature; CNO, chronic nonbacterial osteomyelitis; DADA2, deficiency of ADA2; DIRA, deficiency of IL-1 receptor antagonist; FMF, familial Mediterranean fever; PAAND, pyrin-associated autoinflammation with neutrophilic dermatosis; SAVI, STING-associated vasculopathy of infancy; TRAPS, TNF receptor–associated periodic syndrome; undiff, undifferentiated; XIAP-def, XIAP-deficiency.
To test for cytokines coregulated with IL-18, we compared several candidate cytokines to IL-18, CRP, and ferritin (supplemental Figure 4B-C). IFN-γ, tumor necrosis factor-α, IL-10, and IL-12p70 measurements were excluded from this analysis because of minimal variability. IL-18 correlated only modestly with sCD25 and IL-18BP (supplemental Figure 4C). The strongest correlations remaining were between the IFN-induced chemokines CXCL9 and CXCL10 (r = 0.55) and the disease activity markers ferritin and sCD25 (r = 0.61).
IL-18 immunoprecipitated from MAS patients’ serum (with the same antibody used for cytokine measurement) ran at the same molecular weight as recombinant, mature IL-18 (supplemental Figure 4D), confirming that measurements in MAS serum represent proteolytically mature IL-18.
Patterns of IL-18 in MAS
Given the chronicity of IL-18 elevation in MAS patients in our AID cohort, we assessed cytokine dynamics in relation to the disease activity markers ferritin and CRP in 3 patients followed longitudinally (Figure 3; patients labeled in supplemental Figure 3). We observed a total IL-18 “set-point” of ∼60 000 pg/mL, with proportionally small increases during brief influenza vaccine-triggered flares, over 4 years of observation of an NLRC4-MAS patient (Figure 3A).27 We observed a similar IL-18 set-point (∼40 000 pg/mL) in a female with urticaria and bony overgrowth reminiscent of CAPS,45 but with a history of recurrent MAS and no germ line or somatic inflammasome mutations (Figure 3B). We have not detected serum IL-18 values approximating MAS in any samples from patients bearing NLRP3 or MEFV mutations (Figure 2A). By contrast, an adult with AOSD presented with MAS and extraordinary IL-18 at the onset of disease (Figure 3C). He responded quickly to steroids and IL-1 inhibition, but his total IL-18 did not improve (from its 110 000 pg/mL peak) until months after normalization of CRP, ferritin, free IL-18, and reduction of steroids. This pattern is consistent with reports in sJIA of partial IL-18 improvement months after normalization of other disease activity markers.46
Different patterns of chronic IL-18 in MAS-prone patients. Time course of cytokine and disease activity measurements in a patient with (A) NLRC4-associated MAS, (B) idiopathic MAS, and (C) adult-onset Still disease presenting with MAS. Dashed lines crossing the y-axes indicate upper limits of normal . In panel A, influenza vaccination is indicated by asterisks along the x-axis. CRP in milligram/liter, ferritin in nanogram/milliliter, all others in picogram/milliliter.
Different patterns of chronic IL-18 in MAS-prone patients. Time course of cytokine and disease activity measurements in a patient with (A) NLRC4-associated MAS, (B) idiopathic MAS, and (C) adult-onset Still disease presenting with MAS. Dashed lines crossing the y-axes indicate upper limits of normal . In panel A, influenza vaccination is indicated by asterisks along the x-axis. CRP in milligram/liter, ferritin in nanogram/milliliter, all others in picogram/milliliter.
IL-18 in murine Nlrc4 hyperactivity
To investigate the sources and effects of IL-18 in MAS, we engineered the NLRC4-MAS-inspired Thr337Ser mutation in murine Nlrc4 (Data supplement C), which is highly homologous to human NLRC4.27 NLRC4T337S mice, unlike those bearing inflammasome-activating mutations in NLRP3,47 MEFV,36 or with transgenic expression of mutant Nlrc4,48 showed no spontaneous signs of inflammation. Analyzing the earliest (likely mosaic) pups, we found elevated serum IL-18 in mice bearing the T337S mutation, but not in mice bearing only indel or silent mutations (supplemental Figure 5A). After breeding to ensure germ line transmission, we consistently observed allele-dependent serum IL-18 elevation in NLRC4T337S mice (Figure 4A).
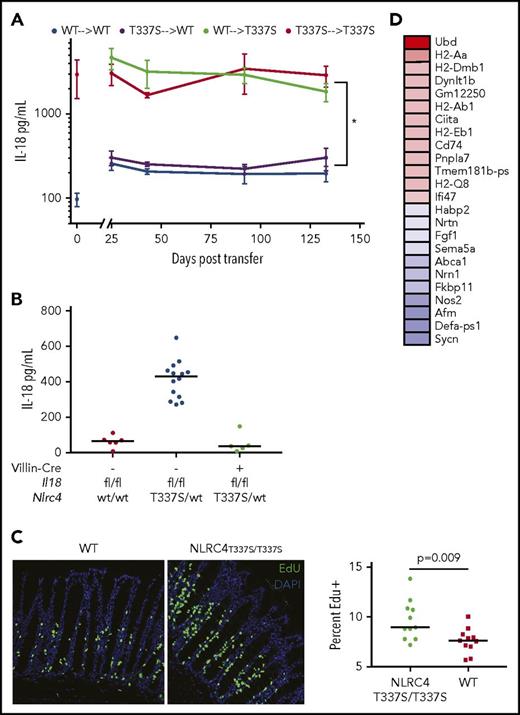
Mice bearing Nlrc4T337Smutation have chronic, inflammasome-dependent IL-18 elevation. (A) Serum IL-18 from age- and sex-matched NLRC4T337S/T337S, NLRC4T337S/WT, and WT mice. (B) NLRC4T337S/WT mice or littermate controls were assessed for serum IL-18 upon weaning at 21 days of age. (C) Serum was obtained from NLRC4T337S/T337S and WT mice housed separately until 7 weeks of age, then cohoused for 4 weeks, and then cohoused and administered broad-spectrum antibiotics for an additional 4 weeks. (D) Serum IL-18 was assessed in mice of the indicated genotypes. Each graph is representative of at least 2 separate experiments. ***P < .001, ***P < .0001, 1-way analysis of variance (ANOVA) with Tukey posttest.
Mice bearing Nlrc4T337Smutation have chronic, inflammasome-dependent IL-18 elevation. (A) Serum IL-18 from age- and sex-matched NLRC4T337S/T337S, NLRC4T337S/WT, and WT mice. (B) NLRC4T337S/WT mice or littermate controls were assessed for serum IL-18 upon weaning at 21 days of age. (C) Serum was obtained from NLRC4T337S/T337S and WT mice housed separately until 7 weeks of age, then cohoused for 4 weeks, and then cohoused and administered broad-spectrum antibiotics for an additional 4 weeks. (D) Serum IL-18 was assessed in mice of the indicated genotypes. Each graph is representative of at least 2 separate experiments. ***P < .001, ***P < .0001, 1-way analysis of variance (ANOVA) with Tukey posttest.
Considering the pathogenic and homeostatic interactions reported between inflammasomes, IL-18, and microbiota reported,37,49,50 we assessed the effects of age and environment on IL-18 overproduction. We found NLRC4T337S mice displayed IL-18 elevation as early as 3 days of age, with no effects of cohousing or broad-spectrum antibiotics (Figure 4B-C; supplemental Figure 5B).
Unlike NLRP3 or MEFV, the NLRC4 inflammasome can function without the adaptor ASC (Pycard), but typically requires ASC for infection-induced cytokine production. We found NLRC4T337S/wt mice deficient in ASC (Pycard) had WT levels of serum IL-18, demonstrating that NLRC4T337S-induced IL-18 overproduction occurred through a canonical inflammasome (Figure 4D). Likewise, Salmonella induces an inflammasome containing both NLRC4 and NLRP3, and both are required for full function.51,52 However, we saw no IL-18 diminution in NLRP3-deficient NLRC4T337S/wt mice (supplemental Figure 5C).
Sublethal endotoxemia, acute dextran sodium sulfate colitis, and infection with abortive or chronic lymphocytic choriomeningitis virus were not more severe in NLRC4T337S/T337S mice (supplemental Figure 6A-C). Furthermore, when we stimulated BMDMs) with LPS, we saw comparable IL-1β secretion from both WT and NLRC4T337S BMDMs, whereas MEFVV726A/V726A BMDMs36 showed specific increases in IL-1β secretion (supplemental Figure 6D). Importantly, Nlrc4 messenger RNA was not reduced in NLRC4T337S/T337S macrophages compared with WT (supplemental Figure 6E).
Il1b, Il18, and inflammasomes in immune vs epithelial cells
Given the phenotypic differences between NLRP3, MEFV, and NLRC4 inflammasomopathies in humans and mice, and noting the lower expression of Nlrc4 compared with Nlpr3 in BMDMs (supplemental Figure 6E), we evaluated expression of various inflammasome components in publicly available transcriptional datasets. Transcriptional microarray of murine immune cells showed that certain tissue-resident macrophages and Langerhans cells had the highest Il18 expression. Even in these high Il18 immune cells, Il1b transcription was generally comparable to Il18. By contrast, Il1b transcription in high Il1b cells (neutrophils and intestinal monocytes) was much greater than Il18 (supplemental Figure 7A). In an RNA-seq dataset, we similarly observed that transcription of Il1b in neutrophils dwarfed expression of either Il1b or Il18 in any other cell type (Figure 5A, left). Examining inflammasome expression, we found Nlrc4 was expressed far less than Nlrp3 and Mefv in murine neutrophils (Figure 5A; supplemental Figure 7A). Expression in whole tissues showed a dramatically different pattern: Il18 expression overshadowed Il1b, particularly in barrier epithelium such as the trachea, gastrointestinal tract, and skin (Figure 5B).
Expression of Il18 and Nlrc4 converge in barrier epithelial tissues. (A) RNA-seq expression values of Il1b and Il18 (left) and inflammasomopathy-associated genes (right) in murine immune cells derived from analysis of data from ImmGen project.63 (B) The gcrma microarray expression values of Il18 and Il1b in diverse murine tissues from BioGPS dataset GNF1M.64 Expression values of ex vivo LPS-stimulated macrophages are included as an internal high Il1b control. Microarray expression of (C) human immune cells or (D) tissues with consistent expression (Z > 5 in both duplicates) of IL1B or IL18. Data were derived from BioGPS dataset “Barcode on Normal Tissues.”65 An Nlrc4 probeset was not present in multiple large murine tissue datasets, precluding a comparison of inflammasomes in human tissues. All graphs depict mean and standard error of the mean of all available replicates. Data for panels A-B were obtained from http://www.immgen.org and BioProject PRJNA2B1360. Data for panels B-D were obtained via http://www.biogps.org. DC, dendritic cell; GI, gastrointestinal; NKT, NK T cell; resp, respiratory; Treg, T regulatory cell.
Expression of Il18 and Nlrc4 converge in barrier epithelial tissues. (A) RNA-seq expression values of Il1b and Il18 (left) and inflammasomopathy-associated genes (right) in murine immune cells derived from analysis of data from ImmGen project.63 (B) The gcrma microarray expression values of Il18 and Il1b in diverse murine tissues from BioGPS dataset GNF1M.64 Expression values of ex vivo LPS-stimulated macrophages are included as an internal high Il1b control. Microarray expression of (C) human immune cells or (D) tissues with consistent expression (Z > 5 in both duplicates) of IL1B or IL18. Data were derived from BioGPS dataset “Barcode on Normal Tissues.”65 An Nlrc4 probeset was not present in multiple large murine tissue datasets, precluding a comparison of inflammasomes in human tissues. All graphs depict mean and standard error of the mean of all available replicates. Data for panels A-B were obtained from http://www.immgen.org and BioProject PRJNA2B1360. Data for panels B-D were obtained via http://www.biogps.org. DC, dendritic cell; GI, gastrointestinal; NKT, NK T cell; resp, respiratory; Treg, T regulatory cell.
Making similar comparisons in human immune cells and tissues, a similar pattern emerged. IL1B expression was much greater than IL18 in neutrophils and monocytes (Figures 5C; supplemental Figure 7B). Human IL18 was transcribed at much higher levels than IL1B in barrier tissues such as gut or skin (Figure 5D). Assessment of NLRC4, NLRP3, and MEFV expression in human immune cells and tissues was hampered by low and inconsistent expression of probesets. In human peripheral leukocytes,53 expression of NLRC4 was comparable to or less than NLRP3 and MEFV (supplemental Figure 7B).
Because IL18 expression appeared higher in tissues than immune cells, we evaluated RNA-Seq datasets of isolated murine intestinal epithelial cells (IECs) and human skin explants (supplemental Figure 7C-E). As with the tissue datasets, we found much higher expression of Il18 than Il1b in these epithelia. Likewise, we noted higher IEC expression of Nlrc4 than Nlrp3 and Mefv. Strikingly, IEC expression of Nlrc4 and Il18 persisted even under germ-free conditions, suggesting constitutive expression (supplemental Figure 7E). Overall, these transcriptional data suggest neutrophils express Il1b highly, whereas epithelia may be programmed for constitutive Nlrc4 and Il18 expression.
Systemic IL-18 in NLRC4T337S mice originates from intestinal epithelia
The chronic nature of IL-18 elevation in NLRC4T337S mice, coupled with the previous functional and transcriptional observations, suggested the possibility that NLRC4-driven IL-18 elevation could be nonhematopoietic. To directly test this, we performed bone marrow transplants, conditioning recipient animals with high-dose irradiation directed at replacing the tissue-resident immune cells.39 Syngeneic transplants (WT into WT or NLRC4T337S/T337S into NLRC4T337S/T337S) did not alter the differences in systemic IL-18. However, we found that WT mice transplanted with NLRC4T337S/T337S marrow had WT levels of IL-18, whereas NLRC4T337S/T337S mice transplanted with WT marrow showed IL-18 elevation identical to NLRC4T337S/T337S controls. This confirmed that the Nlrc4 mutation in nonhematopoietic cells was both necessary and sufficient for serum IL-18 elevation (Figure 6A). Importantly, these mice showed near-complete chimerism: all neutrophils, monocytes, B and NK cells, and the majority of tissue macrophages, were of donor origin (supplemental Figure 8). Furthermore, we found that the Nlrc4T337S/wt mutation was unable to elevate serum IL-18 when the Il18 gene was deleted specifically in intestinal epithelia (Nlrc4T337S/WT, Villin-Cre, Il18flox/flox; Figure 6B), genetically proving that intestinal epithelia were the source of elevated IL-18 in NLRC4T337S mice.
IL-18 overproduction in NLRC4T337S/T337Smice derives from IECs and induces proliferation and major histocompatibility complex II upregulation. (A) Serum IL-18 concentration in bone marrow chimeras as depicted. Values at 0 time point represent serum concentration in donor mice. *P < .05 for all comparisons of top lines to bottom lines; repeated measures ANOVA with Tukey test for multiple comparisons. Representative of 2 experiments with at least 4 mice per group. (B) Serum IL-18 in Il18flox/flox mice of the indicated genotypes. (C) Mice were injected with EdU and histologically evaluated 4 hours later for the proportion of EdU positive (green) cells among all villous cells (4′,6-diamidino-2-phenylindole). Original magnification ×200. Analysis by Student t test of pooled data from 3 separate experiments. Each point represents a distinct organ. (D) Genes most differentially upregulated (red) or downregulated (blue) in NLRC4T337S/T337S duodenal epithelium by RNA-seq; see also Data supplements F and G.
IL-18 overproduction in NLRC4T337S/T337Smice derives from IECs and induces proliferation and major histocompatibility complex II upregulation. (A) Serum IL-18 concentration in bone marrow chimeras as depicted. Values at 0 time point represent serum concentration in donor mice. *P < .05 for all comparisons of top lines to bottom lines; repeated measures ANOVA with Tukey test for multiple comparisons. Representative of 2 experiments with at least 4 mice per group. (B) Serum IL-18 in Il18flox/flox mice of the indicated genotypes. (C) Mice were injected with EdU and histologically evaluated 4 hours later for the proportion of EdU positive (green) cells among all villous cells (4′,6-diamidino-2-phenylindole). Original magnification ×200. Analysis by Student t test of pooled data from 3 separate experiments. Each point represents a distinct organ. (D) Genes most differentially upregulated (red) or downregulated (blue) in NLRC4T337S/T337S duodenal epithelium by RNA-seq; see also Data supplements F and G.
Given recent reports linking NLRC4 and IL-18 to intestinal epithelial inflammation,37,50 we examined the intestines of NLRC4T337S/T337S mice. We did not observe pathology, increased apoptosis, or goblet cell abnormalities in NLRC4T337S/T337S intestines (supplemental Figure 9A). However, we did observe increased IEC turnover (as assessed by EdU incorporation; Figure 6C). This is consistent with the recently described Nlrc4-dependent IEC expulsion that occurs with Salmonella infection.50 Likewise, the most upregulated gene in NLRC4T337S/T337S intestinal epithelium, Ubd, is associated with cell-cycle promotion and IEC proliferation in colorectal cancer54 (Figure 6D; supplemental Figure 9B). Interestingly, although Il18 transcription was modestly downregulated, Il18bp was upregulated along with multiple genes associated with antigen presentation (enrichment P = 9.5 × 10−10; Data supplement F). There was neither histologic nor transcriptional evidence for increased hematopoietic infiltration in NLRC4T337S/T337S intestinal epithelium, suggesting these signatures derived from the IECs themselves (supplemental Figure 9A-C; Data supplement G). Supporting the intestinal specificity of Nlrc4, when we expressed an Nlrp3 mutation in IECs that is lethal when expressed in myeloid cells,55 it caused neither intestinal pathology nor elevated IL-18 (supplemental Figure 9D). Thus, the NLRC4T337S mutation caused chronic, inflammasome-dependent, systemic IL-18 elevation derived entirely from intestinal epithelia.
Free IL-18 is required for enhanced MAS
As opposed to the free IL-18 observed in human MAS serum, we suspected that NLRC4T337S/T337S IL-18 overproduction did not surpass endogenous inhibition by IL-18BP. We confirmed that neither WT nor NLRC4T337S/T337S mice had detectable free IL-18, even upon stimulation (Figure 7A). By contrast, mice carrying a transgene for expression of mature, exportable Il18 (Il18tg)34 had detectable free IL-18 both at rest and with stimulation. These mice have extremely high total IL-18, but no overt inflammatory phenotype when unstimulated, much like many MAS patients during quiescence (Figure 3). We hypothesized that chronically elevated free IL-18 was required for more severe MAS, and performed the TLR9-driven model of MAS in WT, NLRC4T337S/T337S, and Il18tg mice. Upon repeated TLR9 stimulation, only Il18tg mice developed more severe MAS than WT, including enhanced splenomegaly, hepatitis, thrombocytopenia, and serum IL-6, MCP-1, IL-10, CXCL9, and IFN-γ (Figure 7B-C; data not shown). Thus, only mice with chronic elevation of free IL-18 developed more severe TLR9-induced MAS.
Free IL-18 determines susceptibility to more severe TLR9-induced MAS. Mice of the indicated genotypes were repeatedly injected with phosphate-buffered saline or the TLR9-stimulus CpG and were assessed for (A) serum total and free IL-18; (B) relative spleen size, aspartate aminotransferase, and platelet count; and (C) serum IL-10, CXCL9, and IFN-γ. *Adjusted P < .01, **adjusted P < .001, 2-way ANOVA with Tukey posttest. Significance is only shown for comparisons where adjusted P < .05. Results are representative of at least 2 independent experiments.
Free IL-18 determines susceptibility to more severe TLR9-induced MAS. Mice of the indicated genotypes were repeatedly injected with phosphate-buffered saline or the TLR9-stimulus CpG and were assessed for (A) serum total and free IL-18; (B) relative spleen size, aspartate aminotransferase, and platelet count; and (C) serum IL-10, CXCL9, and IFN-γ. *Adjusted P < .01, **adjusted P < .001, 2-way ANOVA with Tukey posttest. Significance is only shown for comparisons where adjusted P < .05. Results are representative of at least 2 independent experiments.
Discussion
Our data both clinically and mechanistically support unopposed IL-18 as an independent host susceptibility factor for MAS and provide a rationale for the distinction of MAS from other forms of hyperferritinemic inflammation. They also illustrate the potential for IEC inflammasome hyperactivity to mobilize the large reservoir of epithelial proIL-18. Finally, they reinforce that IL-18 must rise above endogenous inhibition by IL-18BP to promote more severe MAS.
Despite the association of IL-18 with sJIA and MAS made many years ago, questions about specificity, correlation with disease activity, and utility have remained. Preparing to address these, we first uncovered a technical detail that may explain why free IL-18 imputed mathematically is higher than that measured directly:42 IL-18 interfered with detection of IL-18BP. Therefore, IL-18BP measurements may be underestimates,30,33,56 and mathematical imputations of free IL-18 are likely overestimates. We then assessed a unique cohort of patients with active hyperferritinemic disease and relevant sJIA and healthy controls. Unlike previous reports,57 typical clinical markers were inadequate to distinguish MAS from IA-HLH or fHLH (supplemental Figure 2B). However, IL-18 clearly stood out in MAS. Free IL-18 was also most highly elevated in MAS. IL-18BP was similarly elevated in all hyperferritinemic groups, and CXCL9 trended higher in fHLH.
Patients classified as IA-HLH displayed a bimodal pattern of IL-18 elevation that did not correlate with EBV as the infectious trigger (data not shown), suggesting IL-18 activity may also be an important host susceptibility factor in some IA-HLH patients. Partial cytotoxic impairment may also promote IA-HLH,13 and the 2 mechanisms may be complementary. Likewise, a sizeable minority of our sJIA-MAS patients are predicted to carry partial cytotoxic-gene mutations (not tested in our cohort, but up to 40%12 ), but our data suggest these patients would still have highly elevated IL-18. Because cytotoxic lymphocytes highly express the IL-18 receptor, our data support the potentially central role of these cells in both MAS and fHLH.58
We observed a similar correlation of extraordinary serum IL-18, free IL-18, and MAS susceptibility in an unprecedented cohort of rare autoinflammatory diseases. IL-18 remained elevated long after normalization of disease activity markers, and many patients displayed a set-point regardless of flares or years of quiescence. Importantly, MAS-associated peripheral IL-18 was proteolytically mature, implying inflammasome activity even during quiescence. Free IL-18 also correlated with MAS, particularly in the autoinflammatory cohort, but did not perform as well as total IL-18 (± normalization by CXCL9) in either cohort, possibly because of stability or dynamic range limitations. Overall, these observations may provide a method for determining MAS susceptibility regardless of context or disease activity, but clearly require prospective validation.
Building on the insights provided by murine models of fHLH and NLRP3 and PYRIN inflammasomopathies,7,47,55 we generated a murine model of NLRC4 hyperactivity. As with NLRC4-MAS patients, NLRC4T337S mice showed chronically elevated serum IL-18 soon after birth. The origin of this IL-18 from villin-expressing IECs provides a reasonable explanation for its chronicity and stability. In light of NLRC4-associated enterocolitis28-30 and the murine literature on intestinal inflammasomes,37,50 we looked more deeply at the NLRC4T337S intestinal epithelium. NLRC4T337S intestines showed no obvious histologic abnormalities, but did show increased Ubd expression and IEC turnover. Rauch et al50 recently described NLRC4-dependent IEC expulsion, providing a possible mechanism of IL-18 release in NLRC4T337S mice. We also observed upregulation of Il18bp and major histocompatibility complex II antigen presentation transcripts in NLRC4T337S intestinal epithelium: these may indicate IEC-specific responses to IFN-γ.59,60 Thus, our data support a paradigm in which IEC IL-18 serves a barrier-supportive role under homeostatic conditions, but with infection or intrinsic inflammasome hyperactivity, this reservoir of IL-18 reaches levels and cells that promote MAS.26,28-30,37,50
NLRC4T337S mice also reinforced that IL-18 must overcome endogenous inhibition to promote MAS. To positively demonstrate this, we examined responses to TLR9 stimulation in Il18tg mice and found increased MAS susceptibility both “clinically” and in their cytokine production. Thus, stimulation of mice with chronic free IL-18 may better model human MAS than currently available systems.34,35
The differences between NLRC4T337S mice and human NLRC4-MAS are also instructive. Human NLRP3 and PYRIN inflammasomopathies are associated with neutrophil infiltration and IL-1, consistent with our transcriptional survey (Figure 5; supplemental Figure 7). NLRC4-MAS, by contrast, is characterized by histiocytosis, IL-18, and MAS.27-29,61 However, the macrophage-intrinsic inflammasome hyperactivity observed in all human inflammasomopathies was not observed in NLRC4T337S BMDMs (supplemental Figure 6D), potentially because murine monocytes express less Nlrc4 than human. Myeloid expression may also explain the spontaneous dermatitis, arthritis, and cachexia of NLRP3-mutant,47,55 MEFV-mutant,36 and Nlrc4-transgenic mice,48 but not NLRC4T337S mice. Interestingly, some NLRC4-MAS patients have had periods of inactive disease off medication lasting several years,27,28 whereas the periodicity of CAPS and familial Mediterranean fever flares rarely exceeds a few weeks. Murine housing conditions and/or the source of IL-18 (intestinal vs transgenic) may also affect the observed levels of free IL-18 and severity of MAS in NLRC4T337S mice.
We cannot distinguish epithelial from myeloid IL-18 in human NLRC4-MAS, but our data raise the interesting hypothesis that it derives from mobilization of epithelial stores. If instead it derives from a special (ostensibly macrophage) population chronically producing and cleaving IL-18, then this population would be quite unique because both the NLRP3 and MEFV inflammasomopathies have profound inflammasome activation, but minimal peripheral IL-18 elevation and no MAS (Figure 2A). IL-18’s chronicity and specificity for NLRC4 (over NLRP3 or PYRIN) suggest there may be an unappreciated role for epithelial inflammasome activation in MAS more generally.
Eighteen years ago, the association of perforin deficiency with HLH propelled advances in understanding and eventually treating deadly hyperferritinemic inflammation in fHLH.62 Spurred by the discovery of NLRC4-MAS, we believe IL-18 can be used similarly to guide more rational and effective diagnosis and treatment of MAS.
The murine intestinal epithelium RNA-seq data are available at the Gene Expression Omnibus database (accession number GSE109032).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ivona Aksentijevich, Amanda Ombrello, Shanmuganathan Chandrakasan, James Katz, Juan Arostegui, Seth Masters, and Dorian McGavern for referring patients and/or sharing reagents or samples; Jae-Jin Chae, Daniel Kastner, and Richard Flavell for sharing transgenic mice; and John O’Shea, Behdad Afzali, Hal Hoffman, Edward Behrens, Amanda Poholek, and Timothy Hand for advice and critical review.
This work was supported by grants from the Intramural Research Program (IRP) of the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the RK Mellon Foundation Institute for Pediatric Research (RKMFI) (E.S.W.); NIAMS and National Institute of Allergy and Infectious Diseases (NIAID) IRPs (A.A.d.J., R.G.-M.); NIAMS IRP (Z.T.); Bundesministerium für Bildung und Forshung (01E01303) (S.A.); German Research Foundation (SFB1160 TP1) (S.E.); the Swiss National Science Foundation (310030-172674/1), CTI project (18772.1PFLS-LS), Rheumasearch Foundation, and Institute of Arthritis Research (C.G.); NIAMS IRP, RKMFI, and NIAID (K22 AI123366) (S.W.C.); and an unrestricted educational grant from AB2Bio, Ltd. (C.G.-G.).
Authorship
Contribution: E.S.W., C.G.-G., A.A.d.J., Z.T., and S.W.C. planned and performed experiments and analyzed and reviewed data; D.H., D.F., A.A.G., S.A., S.E., T.H., J.P., E.J.S., and S.W.C. generated critical specimens or reagents and reviewed data; R.G.-M., C.G., and S.W.C. conceptualized the study and reviewed data; S.W.C. wrote the manuscript; and all authors reviewed and approved of the submitted manuscript.
Conflict-of-interest disclosure: AB2Bio, Ltd., holds exclusive rights to the human free IL-18 assay. E.J.S. is an employee of AB2Bio, Ltd. S.W.C. and C.G. are consultants for AB2Bio, Ltd. The remaining authors declare no competing financial interests.
Correspondence: Scott W. Canna, RK Mellon Institute, Children’s Hospital of Pittsburgh of UPMC, 8124 Rangos Research Building, 4401 Penn Ave, Pittsburgh, PA 15224; e-mail: scott.canna@chp.edu.
REFERENCES
Author notes
E.S.W. and C.G.-G. contributed equally to this study.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal