Key Points
P4HA2, associated with progression and poor overall survival in DLBCL patients, could serve as a novel biomarker and therapeutic target.
P4HA2 counteracts the negative effect of Carabin on lymphoma by hydroxylation of Carabin at Pro306.
Abstract
B-cell lymphomas are heterogeneous blood disorders with limited therapeutic options, largely because of their propensity to relapse and become refractory to treatments. Carabin, a key suppressor of B-cell receptor signaling and proliferation, is inactivated in B-cell lymphoma by unknown mechanisms. Here, we identify prolyl 4-hydroxylase 2 (P4HA2) as a specific proline hydroxylase of Carabin. Carabin hydroxylation leads to its proteasomal degradation, thereby activating the Ras/extracellular signal-regulated kinase pathway and increasing B-cell lymphoma proliferation. P4HA2 is undetectable in normal B cells but upregulated in the diffuse large B-cell lymphoma (DLBCL), driving Carabin inactivation and lymphoma proliferation. Our results indicate that P4HA2 is a potential prognosis marker for DLBCL and a promising pharmacological target for developing treatment of molecularly stratified B-cell lymphomas.
Introduction
B-cell lymphomas are clinically and genetically heterogeneous diseases with diverse morphology, immunophenotype, and molecular features. The diffuse large B-cell lymphoma (DLBCL) is the most prevalent subtype of lymphomas in adulthood, comprising the germinal center B-cell–like (GCB) and non-GCB DLBCL, including activated B-cell–like (ABC) and the rare primary mediastinal B-cell lymphoma (PMBCL) DLBCL according to their different gene expression profiles and putative origins of cells.1 Non-GCB DLBCL is the most aggressive subtype and shows features of constitutive B-cell receptor (BCR)-activated signaling and concomitant activation of the anti-apoptotic NF-κB pathway.2-4 The BCR signal pathway is indispensable for normal B-cell development, selection, survival, proliferation, and differentiation into antibody-secreting plasma cells.5,6 BCR signaling is also a key driver of certain B-cell malignancies including DLBCL.5,7 Targeting active kinases such as spleen tyrosine kinase (SYK) and Bruton tyrosine kinase (BTK) separately with fostamatinib and ibrutinib in this signaling pathway has proven to be effective for treating B lymphoma.8-10 However, the inherent heterogeneity and relapse after chemotherapy in DLBCL underscores the complexity of this disease, calling for identification of new molecular targets for developing novel drugs to treat distinct types of B-cell lymphomas.
Three major signaling pathways emanate from the BCR: the Ras-signaling pathway, the phospholipase C-γ-Ca2+ pathway and the phosphoinositde 3-kinase (PI3K) pathway.11 Carabin, also known as TBC1D10C, is highly expressed in spleen and blood leukocytes and negatively regulates T-cell activation through direct inhibition of the calcineurin and Ras-extracellular signal-regulated kinase (ERK) pathway upon antigen stimulation.12,13 Moreover, it also negatively regulates NF-κB through its Ras GTPase activating protein activity. Recently, it was reported that Carabin is downregulated in B cells from lupus patients.14 Using knockout mice, it was shown that Carabin has a similar function in B cells as in T cells through inhibition of the Ras-ERK–signaling pathway, blocking B-cell activation upon activation of Toll-like receptor 9 and BCR-signaling pathway.14 The finding that BCR signaling was not missing but suppressed in lymphoma-negative prognostic cells together with the observation that Carabin is a negative regulator for Ras-ERK cascade in primary B cells prompted us to hypothesize that Carabin may be responsible for suppression of BCR signaling in B-cell lymphoma.
We sought to identify Carabin-interacting proteins to gain a deeper understanding of its function and regulation. Using tandem affinity purification (TAP),15 we identified the proline 4-hydroxylase P4HA2 as a new Carabin-binding protein. P4H is 1 of the 2 types of proline hydroxylases, the other being prolyl hydroxylase domain-containing proteins (PHDs). PHDs are responsible for the hydroxylation of hypoxia-inducing factor 1α (HIF1α) and its degradation under normoxic conditions.16 Unlike PHDs, P4H is known to play essential roles in collagen biogenesis via catalyzing proline hydroxylation of collagen in the X-Pro-Gly (X-P-G) triplets.17,18 There are 3 isoforms of the α subunit of P4HA, that is, P4HA1, P4HA2, and P4HA3, which form α2β2 tetramers with P4HB.19 In these tetramers, the α subunit contains the catalytic and substrate-binding domains, whereas the β-subunit is a disulfide isomerase. In addition to collagens, other proteins containing collagen-like sequence have also been shown to be substrates of P4H, including apoproteins, Agonaute 2, and surfactant.20-22 It has been reported that P4HA1 and P4HA2 promote breast cancer and oral cavity squamous cell carcinoma invasion and metastasis to lymph nodes.23-25 However, the mechanisms by which P4Hs regulate tumorigenesis including B-cell lymphoma remain largely unknown.
Herein, we report that P4HA2 and Carabin are a pair of positive and negative regulators of B-cell lymphoma cell proliferation both in vitro and in vivo. In contrast to normal B cells, P4HA2 is upregulated whereas Carabin is downregulated in B-cell lymphoma. Through its regulation of the Ras-ERK pathway, Carabin blocks B-lymphoma proliferation. This inhibition is relieved by P4HA2 through hydroxylation of proline 306 in Carabin, which leads to polyubiquitination and proteasome-mediated degradation of Carabin. Importantly, we demonstrate that knockdown of P4HA2 causes significant inhibition of B-cell lymphoma proliferation both in vitro and in vivo, suggesting that inhibition of P4HA2 is a viable new strategy for treating B-cell lymphoma. Moreover, we show that P4HA2 expression is a prognostic marker for poor survival, positively correlated with phosphorylated Erk (p-Erk) expression and response to existing therapy in DLBCL patients. Together, these results revealed that P4HA2 and Carabin play pivotal roles in B-cell malignancy and suggest that P4HA2 can serve as a potential prognosis marker and a novel therapeutic target for DLBCL.
Methods
Additional methods related to TAP, mass spectrum, hydroxylation assay, and statistical analysis et al are described in supplemental Methods (available on the Blood Web site).
Cell culture and proliferation assay
All the cell lines were cultured as per the introduction of the American Type Culture Collection. For the proliferation assay, all of the B-cell lymphoma cells were seeded on 96-well plates at a density of 6000 cells per well. During a 5-day culture period, living cells were detected by Alarma Blue every 24 hours.
Patient selection and IHC
Formalin-fixed and paraffin-embedded (FFPE) samples of 205 DLBCLs and 20 reactive hyperplasias of lymph nodes during an 8-year period (2005-2012) from the Department of Pathology at the Fudan University Shanghai Cancer Center were collected. Diagnoses were histopathologically confirmed and reviewed on the basis of the 2016 World Health Organization (WHO). Clinicopathological information was electronically retrieved from the Hospital Information System (HIS) of the Fudan University Shanghai Cancer Center. P4HA2 or p-Erk expression was detected with P4HA2 (Proteintech, Wuhan, China) or p-Erk (Abcam) antibody on an automated system (Ventana BenchMARK XT) using immunohistochemistry (IHC).
Results
Carabin negatively regulates the proliferation and tumorigenesis of B-cell lymphoma
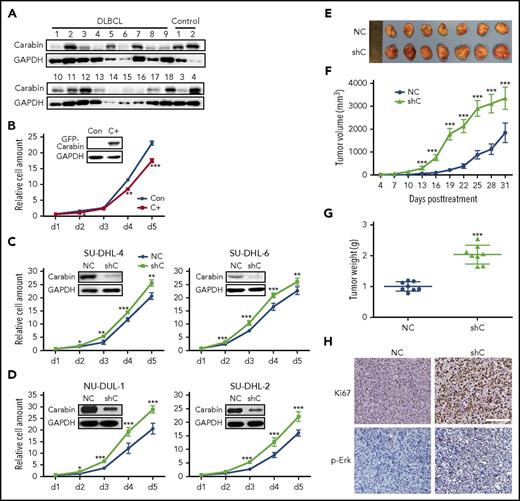
Previously, it was reported that Carabin was predominantly expressed in blood cells.12,13,26 To explore its function in B lymphoma, we analyzed the expression of Carabin in DLBCL tissues (supplemental Table 1). Carabin was downregulated in 12 of 18 samples, but only 1 of 4 benign controls (Figure 1A). We next assessed the effect of Carabin overexpression and depletion in several different subtypes of DLBCL cell lines, which have low (SU-DHL-6) and high (SU-DHL-4, NU-DUL-1, and SU-DHL-2) expression levels of Carabin. Overexpression of Carabin in SU-DHL-6 decreased the cellular growth rates (Figure 1B), whereas knockdown of Carabin in GCB-DLBCL (SU-DHL-6 and SU-DHL-4) or ABC-DLBCL (NU-DUL-1 and SU-DHL-2) accelerated cell proliferation (Figure 1C-D). Given that Carabin plays a negative role in modulating Ras/Erk activity,12,14,27 we then examined the effect of Carabin on the Ras/Erk-signaling pathway in the same DLBCL tissues and cell lines. Phosphorylation of Erk1/2 negatively correlated with Carabin in most tissues examined (supplemental Figure 1A). In addition, p-Erk was reduced upon overexpression of Carabin. By contrast, knockdown of Carabin expression by RNA inference resulted in increased Erk1/2 but not JNK phosphorylation (supplemental Figure 1B).
Carabin inhibits B-lymphoma cell proliferation. (A) Downregulated Carabin expression in DLBCL tissues. Carabin protein was analyzed in 18 DLBCL specimens, 4 benign tissues as control. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was the immunoblot loading marker. (B) Overexpression of Carabin suppresses B-lymphoma cell proliferation. Stable expression of green fluorescent protein (GFP)-Carabin was detected by western blot (WB) in SU-DHL-6 cells. A cell growth assay was carried out over a 5-day culture period and living cells were detected by Alarma blue every 24 hours. Points, mean (n = 6); bar, standard deviation (SD); *P < .05; **P < .01; ***P < .001. Knockdown of Carabin promotes B-lymphoma cell proliferation. Knockdown of Carabin was carried out by lentivirus in (C) GCB-DLBCL cell lines, SU-DHL-4 (left), and SU-DHL-6 (right) cells; (D) ABC-DLBCL cell lines, NU-DUL-1 (left), and SU-DHL-2 (right). Cell growth assays were analyzed as described above. Points, mean (n = 6); bar, SD; *P < .05; **P < .01; ***P < .001. (E) The resected tumors from individual nude mice. Carabin knockdown and control SU-DHL-4 stable cells were injected into 2 nude mice groups, respectively. Seven mice were used in each group. (F) The tumor volume of the xenografts. Points, mean (n = 7); bar, SD; ***P < .001. (G) The tumor weight of the xenografts after dissection. Scatter plot, mean (n = 7); bars, SD; ***P < .001. (H) Immunohistochemical analysis of Ki-67 (top) and p-Erk (bottom) in the xenograft tumor tissues (hematoxylin and eosin stain; scale bar, 50 μM). C+, cells stably expressing GFP-Carabin; Con, control cell; NC, control cell; shC, cells stably expressing Carabin-specific shRNA.
Carabin inhibits B-lymphoma cell proliferation. (A) Downregulated Carabin expression in DLBCL tissues. Carabin protein was analyzed in 18 DLBCL specimens, 4 benign tissues as control. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was the immunoblot loading marker. (B) Overexpression of Carabin suppresses B-lymphoma cell proliferation. Stable expression of green fluorescent protein (GFP)-Carabin was detected by western blot (WB) in SU-DHL-6 cells. A cell growth assay was carried out over a 5-day culture period and living cells were detected by Alarma blue every 24 hours. Points, mean (n = 6); bar, standard deviation (SD); *P < .05; **P < .01; ***P < .001. Knockdown of Carabin promotes B-lymphoma cell proliferation. Knockdown of Carabin was carried out by lentivirus in (C) GCB-DLBCL cell lines, SU-DHL-4 (left), and SU-DHL-6 (right) cells; (D) ABC-DLBCL cell lines, NU-DUL-1 (left), and SU-DHL-2 (right). Cell growth assays were analyzed as described above. Points, mean (n = 6); bar, SD; *P < .05; **P < .01; ***P < .001. (E) The resected tumors from individual nude mice. Carabin knockdown and control SU-DHL-4 stable cells were injected into 2 nude mice groups, respectively. Seven mice were used in each group. (F) The tumor volume of the xenografts. Points, mean (n = 7); bar, SD; ***P < .001. (G) The tumor weight of the xenografts after dissection. Scatter plot, mean (n = 7); bars, SD; ***P < .001. (H) Immunohistochemical analysis of Ki-67 (top) and p-Erk (bottom) in the xenograft tumor tissues (hematoxylin and eosin stain; scale bar, 50 μM). C+, cells stably expressing GFP-Carabin; Con, control cell; NC, control cell; shC, cells stably expressing Carabin-specific shRNA.
To determine whether Carabin affects B-cell lymphoma growth in vivo, we carried out a xenograft assay. B-lymphoma cells transfected with control and Carabin–short hairpin RNA (Carabin-shRNA) were subcutaneously injected into nude mice, respectively. We found that knockdown of Carabin resulted in a significant increase in the volume and weight of tumors (Figure 1E-G). We confirmed knockdown of Carabin in xenograft tissues (supplemental Figure 2A). The levels of cellular proliferation marker, Ki-67, and p-Erk were markedly increased in Carabin-knockdown xenografts (Figure 1H; supplemental Figure 2B). Together, these results suggest that Carabin suppresses the proliferation of B-lymphoma cells in vitro and in vivo.
P4HA2 physically interacts with Carabin
The fact that Carabin expression is downregulated upon BCR signaling and its expression is nearly absent in a significant number of B-cell lymphoma cells suggested that the stability or accumulation of Carabin might be tightly regulated by yet-to-be identified mechanisms. To search for proteins involved in the regulation of Carabin stability, we used TAP to identify the interacting partners of Carabin. The immunoprecipitated complex was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), excised, and analyzed by mass spectrum (MS). We found the hydroxylase P4HA2 and its β subunit, P4HB, were identified in the complex and focused on characterizing P4HA2 and P4HB in Carabin interaction and regulation (supplemental Figure 3A).
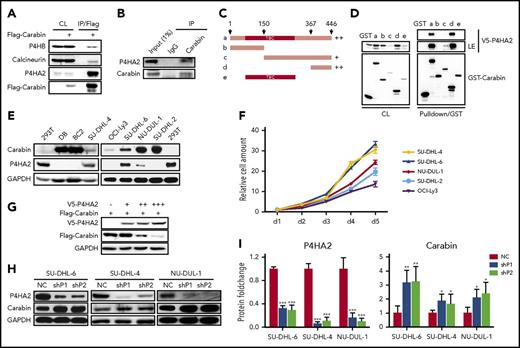
To further confirm that P4HA2 and P4HB are associated with Carabin, we carried out a coimmunoprecipitation (Co-IP) assay in HEK293T cells ectopically overexpressing the FLAG-tagged Carabin. Calcineurin, a known interacting protein of Carabin,12 was used as a positive control. As shown in Figure 2A, endogenous P4HA2, P4HB, and calcineurin were coimmunoprecipitated with FLAG-Carabin. Moreover, we detected the interaction between endogenous Carabin and P4HA2 by Co-IP using specific antibody against Carabin (Figure 2B).
Hydroxylase P4HA2 forms a complex with Carabin and downregulates Carabin. (A) Carabin exotically interacts with P4HA2. 293T cells were transfected with Flag-Carabin. Cell lysates were prepared and subjected to immunoprecipitation with (M2) anti-Flag beads. The immunoprecipitates were analyzed by WB with anti-Flag, P4HA2, P4HB, and calcineurin antibodies. (B) Carabin combines with P4HA2 in vivo. SU-DHL-6 cell lysates were incubated with protein A/G-Sepharose with either anti-Carabin or control IgG antibody. The immunoprecipitates were resolved by SDS-PAGE and followed by WB with anti-Carabin and anti-P4HA2 antibodies. (C) Schematic diagram of truncated GST-Carabin. Various truncated mutation constructs of GST-Carabin are shown schematically. (D) Mapping the interaction domain of Carabin with P4HA2. Purified GST fusion protein of Carabin WT (a) or mutants (b-e) were bound to glutathione-Sepharose beads and incubated with lysates of 293T cells transfected with the V5-P4HA2 expression construct. Cell lysates (CL) and GST pulldown immunoprecipitates were analyzed by WB. (E) Negative relevant expression of Carabin and P4HA2 in B-lymphoma cell lines. Detection protein expression of Carabin and P4HA2 in DB, BC2, SU-DHL-4, SU-DHL-6, OCI-Ly3 NU-DUL-1, and SU-DHL-2 B-lymphoma cell lysates. 293T are the positive controls of P4HA2. (F) Positive correlation between P4HA2 expression and DLBCL cell proliferation. Cell growth assays were analyzed as described above. Points, mean (n = 6); bar, SD. (G) Overexpression of P4HA2 leads to degradation of Carabin in a dose-dependent manner. The same amount of Flag-Carabin was cotransfected with increasing amounts of V5-P4HA2 plasmids into 293T cells. The protein levels of Flag-Carabin and V5-P4HA2 were detected by WB. (H) Carabin protein is upregulated by knockdown of P4HA2 in B-lymphoma cells. SU-DHL-4, SU-DHL-6, and NU-DUL-1 cells of P4HA2 knockdown were analyzed Carabin expression by WB. (I) Quantification of Carabin upon P4HA2 knockdown. Columns, mean (n = 3); bar, SD; *P < .05; **P < .01; ***P < .001. NC, control cell; shP1, cells stably expressing P4HA2-specific shRNA1; shP2, cells stably expressing P4HA2-specific shRNA2.
Hydroxylase P4HA2 forms a complex with Carabin and downregulates Carabin. (A) Carabin exotically interacts with P4HA2. 293T cells were transfected with Flag-Carabin. Cell lysates were prepared and subjected to immunoprecipitation with (M2) anti-Flag beads. The immunoprecipitates were analyzed by WB with anti-Flag, P4HA2, P4HB, and calcineurin antibodies. (B) Carabin combines with P4HA2 in vivo. SU-DHL-6 cell lysates were incubated with protein A/G-Sepharose with either anti-Carabin or control IgG antibody. The immunoprecipitates were resolved by SDS-PAGE and followed by WB with anti-Carabin and anti-P4HA2 antibodies. (C) Schematic diagram of truncated GST-Carabin. Various truncated mutation constructs of GST-Carabin are shown schematically. (D) Mapping the interaction domain of Carabin with P4HA2. Purified GST fusion protein of Carabin WT (a) or mutants (b-e) were bound to glutathione-Sepharose beads and incubated with lysates of 293T cells transfected with the V5-P4HA2 expression construct. Cell lysates (CL) and GST pulldown immunoprecipitates were analyzed by WB. (E) Negative relevant expression of Carabin and P4HA2 in B-lymphoma cell lines. Detection protein expression of Carabin and P4HA2 in DB, BC2, SU-DHL-4, SU-DHL-6, OCI-Ly3 NU-DUL-1, and SU-DHL-2 B-lymphoma cell lysates. 293T are the positive controls of P4HA2. (F) Positive correlation between P4HA2 expression and DLBCL cell proliferation. Cell growth assays were analyzed as described above. Points, mean (n = 6); bar, SD. (G) Overexpression of P4HA2 leads to degradation of Carabin in a dose-dependent manner. The same amount of Flag-Carabin was cotransfected with increasing amounts of V5-P4HA2 plasmids into 293T cells. The protein levels of Flag-Carabin and V5-P4HA2 were detected by WB. (H) Carabin protein is upregulated by knockdown of P4HA2 in B-lymphoma cells. SU-DHL-4, SU-DHL-6, and NU-DUL-1 cells of P4HA2 knockdown were analyzed Carabin expression by WB. (I) Quantification of Carabin upon P4HA2 knockdown. Columns, mean (n = 3); bar, SD; *P < .05; **P < .01; ***P < .001. NC, control cell; shP1, cells stably expressing P4HA2-specific shRNA1; shP2, cells stably expressing P4HA2-specific shRNA2.
To determine which domain of Carabin mediated the interaction with P4HA2, we generated a series of truncated mutants of Carabin, including the N terminus (1-150 aa), the C terminus (150-446 aa and 367-446 aa), and the Tre-2/Bub2/Cdc 16 domain, respectively (Figure 2C). Using glutathione S-transferase (GST) pulldown assays, we found that P4HA2 could be pulled down by the full length as well as two C-terminal fragments of Carabin (Figure 2D). Subsequently, we mapped the minimal interacting motif of Carabin to its C-terminal (407-437 aa) fragment (supplemental Figure 3B-D).
Carabin is a substrate of P4HA2 and undergoes hydroxylation-dependent degradation
The identification of P4HA2 as a Carabin-binding protein prompted us to investigate whether Carabin is a substrate of P4HA2 and thus meditates the function of P4HA2 in tumorigenesis. To test this, we examined the levels of Carabin and P4HA2 in different cell lines and found that their levels showed an inverse correlation (Figure 2E). Furthermore, we found the proliferation rates of 5 DLBCL cell lines correlated with P4HA2 levels (Figure 2F). By coexpression of V5-P4HA2 and Flag-Carabin, we found that the expression of P4HA2 caused a decrease in Carabin in a dose-dependent manner (Figure 2G). Conversely, knockdown of P4HA2 by 2 specific shRNAs in SU-DHL-6, SU-DHL-4, or NU-DUL-1 cells resulted in enhanced accumulation of Carabin (Figure 2H-I) without affecting its messenger RNA (mRNA) level (supplemental Figure 4). These results indicate that P4HA2 is a negative regulator of Carabin and raises the possibility that P4HA2 may be responsible for regulating Carabin stability/accumulation through proline hydroxylation.
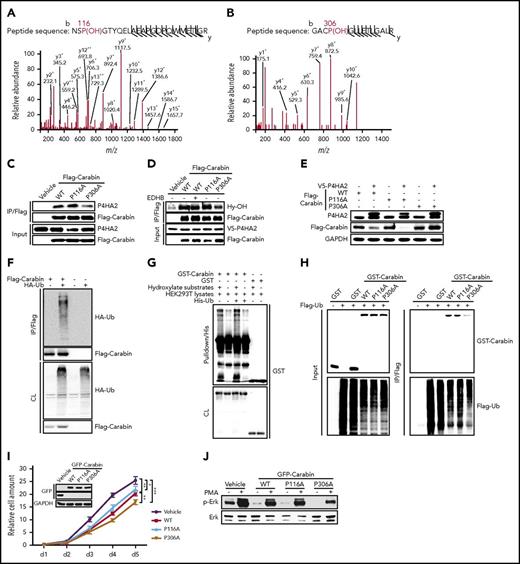
To test this hypothesis, we determined whether Carabin underwent hydroxylation using MS analysis. HEK293T cells expressing FLAG-Carabin protein were cultured in the presence or absence of the general hydroxylase inhibitor ethyl-3,4-dihydroxybenzoate (EDHB) before the cell lysates were prepared and applied to an affinity purification. The Carabin-enriched complex was subjected to MS analysis. Interestingly, both Pro116 and Pro306 residues of Carabin were found to be hydroxylated (Figure 3A-B), which matches the “X-P-G” sequence motif characteristic of substrates of collagen hydroxylases.28,29 To determine whether both P116 and P306 of Carabin are critical for its interaction and function, we constructed 2 hydroxylation-defective mutants of Carabin by substituting P116 and P306 residues with alanine, respectively. The Co-IP assays revealed that the P306A mutant, but not P116A, suffered from a significant loss in the ability to interact with P4HA2 (Figure 3C), suggesting that P306 may serve as the authentic hydroxylation site mediating the interaction between Carabin and P4HA2. To assess the importance of P306 in the hydroxylation of Carabin by P4HA2, the level of hydroxylation of wild-type Carabin and 2 mutants was determined. As shown in Figure 3D, a significant decrease in hydroxylation was seen only in the P306A mutant as revealed by a specific antibody against hydroxyl-proline.30 Moreover, we found that P306A, but not P116A, was refractory to degradation by coexpressed P4HA2 (Figure 3E), suggesting that hydroxylation of P306 is required for P4HA2-stimulated degradation of Carabin.
P4HA2 hydroxylates Carabin on Pro306. (A-B) MS/MS spectra of 2 tryptic peptides (A, Pro116; B, Pro306) derived from MS/MS spectra of 2 tryptic peptides derived from Carabin. The b ion and y ion are fragment ions of tryptic peptide in tandem mass spectrometry. The x and y axes represent m/z and relative ion intensity, respectively. (C) P306A mutant of Carabin binds weakly to P4HA2. 293T cells were transfected with WT Carabin or mutant Flag Carabin as shown. Endogenous P4HA2 was detected in Flag-immunoprecipitated complexes by anti-P4HA2 antibody. (D) Diminished hydroxylation of P306A Carabin mutant. 293T cells were cotransfected with V5-P4HA2 and the illustrated plasmids. As shown, cell sample was treated with EDHB (200 μM for 12 hours). The Flag-immunoprecipitates were resolved by SDS-PAGE. Specific hydroxylation antibody (Hy-OH) for the substrates of hydroxylases was used to reveal the hydroxylation of Flag-Carabin. (E) P306A mutant of Carabin is stable. 293T cells were cotransfected with plasmids as shown. Cell lysates were determined by WB. (F) Carabin can be polyubiquitinated. 293T cells were transfected as shown. Before harvest, cells were treated with MG132 (5 µM for 8 hours). Cell lysates were incubated with Flag beads. Then, the immunoprecipitates were resolved by SDS-PAGE. The polyubiquitinated forms of GST-Carabin were detected by WB with anti-hemagglutinin (HA) antibody. (G) Ubiquitination of Carabin is dependent on the hydroxylation in vitro. Purified GST-Carabin proteins were combined with hydroxylate substrates and His-Ub as shown. After His pulldown, complexes were analyzed by WB. (H) Declined ubiquitination of the P306A Carabin mutant. 293T cells were cotransfected as shown. After MG132 treatment (5 μM for 8 hours), cell lysates were prepared and subjected to immunoprecipitation with Flag beads or GST beads, respectively. The immunoprecipitates were analyzed by WB with anti-Flag and anti-GST antibodies. (I) Growth of SU-DHL-6 cell expressed P306A mutant of Carabin is slow. Overexpression of WT or mutant Carabin in SU-DHL-6 cells was carried out by lentivirus and detected by WB. Cell growth assay was performed as depicted in Figure 1B. Points, mean (n = 6); bar, SD; *P < .05, ***P < .001. (J) SU-DHL-6 cell-expressed P306A mutant of Carabin has the lowest kinase activity of Erk. Cells depicted as in panel I were stimulated with PMA (40 ng/mL) for 10 min (+) or not (−). Cell lysates were analyzed using an anti-phospho Erk antibody. Erk were used as a loading marker.
P4HA2 hydroxylates Carabin on Pro306. (A-B) MS/MS spectra of 2 tryptic peptides (A, Pro116; B, Pro306) derived from MS/MS spectra of 2 tryptic peptides derived from Carabin. The b ion and y ion are fragment ions of tryptic peptide in tandem mass spectrometry. The x and y axes represent m/z and relative ion intensity, respectively. (C) P306A mutant of Carabin binds weakly to P4HA2. 293T cells were transfected with WT Carabin or mutant Flag Carabin as shown. Endogenous P4HA2 was detected in Flag-immunoprecipitated complexes by anti-P4HA2 antibody. (D) Diminished hydroxylation of P306A Carabin mutant. 293T cells were cotransfected with V5-P4HA2 and the illustrated plasmids. As shown, cell sample was treated with EDHB (200 μM for 12 hours). The Flag-immunoprecipitates were resolved by SDS-PAGE. Specific hydroxylation antibody (Hy-OH) for the substrates of hydroxylases was used to reveal the hydroxylation of Flag-Carabin. (E) P306A mutant of Carabin is stable. 293T cells were cotransfected with plasmids as shown. Cell lysates were determined by WB. (F) Carabin can be polyubiquitinated. 293T cells were transfected as shown. Before harvest, cells were treated with MG132 (5 µM for 8 hours). Cell lysates were incubated with Flag beads. Then, the immunoprecipitates were resolved by SDS-PAGE. The polyubiquitinated forms of GST-Carabin were detected by WB with anti-hemagglutinin (HA) antibody. (G) Ubiquitination of Carabin is dependent on the hydroxylation in vitro. Purified GST-Carabin proteins were combined with hydroxylate substrates and His-Ub as shown. After His pulldown, complexes were analyzed by WB. (H) Declined ubiquitination of the P306A Carabin mutant. 293T cells were cotransfected as shown. After MG132 treatment (5 μM for 8 hours), cell lysates were prepared and subjected to immunoprecipitation with Flag beads or GST beads, respectively. The immunoprecipitates were analyzed by WB with anti-Flag and anti-GST antibodies. (I) Growth of SU-DHL-6 cell expressed P306A mutant of Carabin is slow. Overexpression of WT or mutant Carabin in SU-DHL-6 cells was carried out by lentivirus and detected by WB. Cell growth assay was performed as depicted in Figure 1B. Points, mean (n = 6); bar, SD; *P < .05, ***P < .001. (J) SU-DHL-6 cell-expressed P306A mutant of Carabin has the lowest kinase activity of Erk. Cells depicted as in panel I were stimulated with PMA (40 ng/mL) for 10 min (+) or not (−). Cell lysates were analyzed using an anti-phospho Erk antibody. Erk were used as a loading marker.
Proteasomes mediate the degradation of HIF1α upon its hydroxylation by PHD.31,32 We thus assessed whether the P4HA2-induced degradation of Carabin is also mediated by the proteasome. Treatment with either the hydroxylase inhibitor EDHB or proteasome inhibitor MG132 caused an increase in Carabin in B-lymphoma cells (supplemental Figure 5A), implicating that the degradation of Carabin by proteasome depends on hydroxylation. Consistently, we found that Carabin could be ubiquitinated (Figure 3F). To determine whether the ubiquitination of Carabin is dependent on its hydroxylation, we set up an assay in which the purified GST-Carabin was incubated with His-ubiquitin protein and HEK293T cell lysate in the absence or presence of 2-oxoglutarate and ferrous sulfate of hydroxylase. As shown in Figure 3G, we found that the ubiquitination of GST-Carabin was obviously enhanced in the presence of substrates for hydroxylase. Moreover, compared with GST-tagged WT Carabin and P116A, there was a significant decrease in the polyubiquitination of GST-tagged P306A in cell lysates (Figure 3H; supplemental Figure 5B). These results suggest that hydroxylation of Carabin leads to its ubiquitinated and proteolysis.
To examine the effect of hydroxylation of Carabin in cell proliferation, SU-DHL-6 cells were transfected with the plasmids expressing the WT Carabin, P116A, or P306A mutants. As expected, although overexpression of WT Carabin and P116A retarded cell proliferation, overexpression of P306A caused significantly enhanced inhibition of cell proliferation (Figure 3I). This inhibition is consistent with the downregulation of phosphorylation of Erk1/2 downstream of Carabin (Figure 3J).
P4HA2 promotes B-cell lymphoma tumorigenesis
BCR signaling is not only essential for normal B-cell development but also plays a key role in the pathogenesis of B-cell malignancies. We thus assessed the effect of BCR signaling on P4HA2 expression using a BCR-signaling agonist phorbol 12-myristate 13-acetate (PMA). As shown in supplemental Figure 6A-B, P4HA2 was upregulated at both the protein and mRNA levels in B-lymphoma cells in response to PMA treatment. We did not find obvious change in the expression of P4HA1, although previous studies showed that both P4HA1 and P4HA2 were elevated in breast cancers,23,24 suggesting that P4HA2 might play a unique role in the B-cell lymphoma. Consistently, decreased accumulation of Carabin protein (but not its mRNAs) and increased phosphorylation of Erk1/2 were observed in B-lymphoma cells when the BCR-signaling pathway was activated by PMA or lipopolysaccharide (LPS) (supplemental Figure 6C-D). Similar results were also obtained when B-lymphoma cells were treated with LPS. Interestingly, P4HA2 was barely detected in B cells sorted from peripheral blood mononuclear cells (PBMCs) but dramatically accumulated after activating BCR signaling, suggesting that P4HA2 may function as a feedback regulator of BCR signaling (supplemental Figure 6E-F).
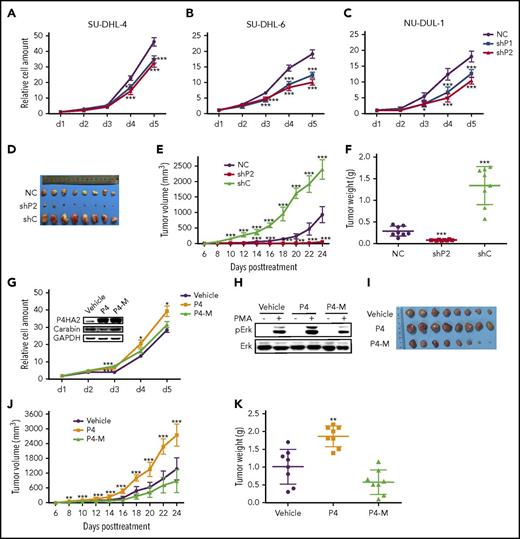
To assess the role of P4HA2 in B lymphoma, we knocked down the expression of P4HA2 using shRNAs. The proliferation of B-lymphoma cells was found to be significantly inhibited (Figure 4A-C) with a concomitant decrease in Erk1/2 kinase activity (supplemental Figure 7). We further assessed the effect of P4HA2 knockdown on cell proliferation in xenograft models of B lymphoma. SU-DHL-4 cells stably transfected with shRNA targeting P4HA2 (shP2) were subcutaneously injected into nude mice. Compared with those injected with the control cells (NC and shC), knockdown of P4HA2 dramatically inhibited tumor growth in nude mice (Figure 4D-F). In contrast, overexpression of P4HA2 (P4), not P4HA2 dominant-negative mutant (P4-M), accelerated cell proliferation of B lymphoma and enhanced the activity of Erk1/2 (Figure 4G-H) in SU-DHL-4 cells and in xenograft animal models (Figure 4I-K). P4-M overexpression in mice resulted in a decrease in tumor sizes and weight in comparison with the control. These results suggest that P4HA2 plays important roles in lymphoma growth both in vitro and in vivo. Furthermore, the hydroxylase activity of P4HA2 is required for promoting the growth of B-cell lymphoma.
P4HA2 promotes the growth of B lymphoma. Suppression of P4HA2 inhibits proliferation of B-lymphoma cells. Growth assays were carried out with SU-DHL-4 (A), SU-DHL-6 (B), and NU-DUL-1 (C) cells of P4HA2 knockdown. Points, mean (n = 6); bar, SD; ***P < .001. (D-F) Suppression of P4HA2 inhibits proliferation of B-lymphoma cells in vivo. The resected tumors (D) from individual nude mice. Eight mice were used in each group. The tumor volume (E) of the xenografts. Points, mean (n = 8); bar, SD; ***P < .001. The tumor weight (F) of the xenografts after dissection. Scatter plot, mean (n = 8); bars, SD; ***P < .001. (G) P4HA2 improves B-lymphoma cell proliferation depending on its enzyme activity. SU-DHL-4 cells were stably transfected with P4HA2 (P4), enzyme inactivation (P4-M), and the control (Vehicle) by lentivirus, respectively. Cell growth assay was performed as above. Points, mean (n = 6); bar, SD; *P < .05; ***P < .001. (H) P4HA2 facilitates Erk1/2 phosphorylation depending on its enzyme activity. Phosphorylation of Erk was detected after PMA stimulating as depicted in Figure 3J. (I-K) P4HA2 promotes B-lymphoma cell proliferation in vivo depending on its enzyme activity. The resected tumors (I) from individual nude mice. The tumor volume (J) of the xenografts. Points, mean (n = 8); bar, SD; ***P < .001. The tumor weight (K) of the xenografts after dissection. Scatter plot, mean (n = 8); bars, SD; ***P < .001.
P4HA2 promotes the growth of B lymphoma. Suppression of P4HA2 inhibits proliferation of B-lymphoma cells. Growth assays were carried out with SU-DHL-4 (A), SU-DHL-6 (B), and NU-DUL-1 (C) cells of P4HA2 knockdown. Points, mean (n = 6); bar, SD; ***P < .001. (D-F) Suppression of P4HA2 inhibits proliferation of B-lymphoma cells in vivo. The resected tumors (D) from individual nude mice. Eight mice were used in each group. The tumor volume (E) of the xenografts. Points, mean (n = 8); bar, SD; ***P < .001. The tumor weight (F) of the xenografts after dissection. Scatter plot, mean (n = 8); bars, SD; ***P < .001. (G) P4HA2 improves B-lymphoma cell proliferation depending on its enzyme activity. SU-DHL-4 cells were stably transfected with P4HA2 (P4), enzyme inactivation (P4-M), and the control (Vehicle) by lentivirus, respectively. Cell growth assay was performed as above. Points, mean (n = 6); bar, SD; *P < .05; ***P < .001. (H) P4HA2 facilitates Erk1/2 phosphorylation depending on its enzyme activity. Phosphorylation of Erk was detected after PMA stimulating as depicted in Figure 3J. (I-K) P4HA2 promotes B-lymphoma cell proliferation in vivo depending on its enzyme activity. The resected tumors (I) from individual nude mice. The tumor volume (J) of the xenografts. Points, mean (n = 8); bar, SD; ***P < .001. The tumor weight (K) of the xenografts after dissection. Scatter plot, mean (n = 8); bars, SD; ***P < .001.
P4HA2 accelerates the growth of B-cell lymphoma through hydroxylation and degradation of Carabin
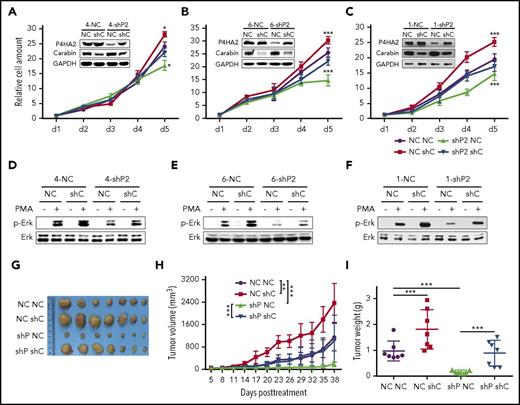
P4HA2, like other proline hydroxylases, may have multiple substrates in vivo. To determine whether the promotion of B-cell lymphoma growth by P4HA2 is mediated primarily through its action on Carabin, we examined the effects of knockdown of both P4HA2 and Carabin on proliferation of lymphoma cells. We found that the growth rate of lymphoma cells with concomitant knockdown of both P4HA2 and Carabin was almost comparable to that of the wild-type cells (Figure 5A-C), and the level of Erk1/2 phosphorylation (Figure 5D-F) and xenograft growth in vivo showed little change after knockdown of both P4HA2 and Carabin (Figure 5G-I). Intriguingly, compared with that in the wild-type cells, expression of P4HA2 in B-lymphoma cells was moderately elevated when Carabin was knocked down, suggesting that there may be a feedback regulatory loop between Carabin and P4HA2. These results indicate that P4HA2 promotes the growth of B-cell lymphoma mainly through its hydroxylase activity toward Carabin.
P4HA2 improves B lymphoma depending on Carabin. Knockdown of Carabin abolishes the inhibition of B-lymphoma cell growth by suppression of P4HA2. SU-DHL-4 (A), SU-DHL-6 (B), and NU-DUL-1 (C) cells transduced with P4HA2 shP2 or NC control were induced to express Carabin shC or NC control. Then, a cell growth assay was performed. Points, mean (n = 6); bar, SD; *P < .05; ***P < .001. Knockdown of Carabin abolishes the inhibition of Erk1/2 kinase activity by suppression of P4HA2. SU-DHL-4 (D), SU-DHL-6 (E), and NU-DUL-1 (F) cells were stimulated with PMA (40 ng/mL) for 30 minutes (+) or not (−), and then analyzed by immunoblotting for the indicated proteins. (G-I) P4HA2 promotes B-lymphoma cell proliferation in vivo depending on Carabin. The resected tumors (G) from individual nude mice. The tumor volume (H) of the xenografts. Points, mean (n = 7); bar, SD; *P < .01; ***P < .001. The tumor weight (I) of the xenografts after dissection. Scatter plot, mean (n = 7); bars, SD; ***P < .001.
P4HA2 improves B lymphoma depending on Carabin. Knockdown of Carabin abolishes the inhibition of B-lymphoma cell growth by suppression of P4HA2. SU-DHL-4 (A), SU-DHL-6 (B), and NU-DUL-1 (C) cells transduced with P4HA2 shP2 or NC control were induced to express Carabin shC or NC control. Then, a cell growth assay was performed. Points, mean (n = 6); bar, SD; *P < .05; ***P < .001. Knockdown of Carabin abolishes the inhibition of Erk1/2 kinase activity by suppression of P4HA2. SU-DHL-4 (D), SU-DHL-6 (E), and NU-DUL-1 (F) cells were stimulated with PMA (40 ng/mL) for 30 minutes (+) or not (−), and then analyzed by immunoblotting for the indicated proteins. (G-I) P4HA2 promotes B-lymphoma cell proliferation in vivo depending on Carabin. The resected tumors (G) from individual nude mice. The tumor volume (H) of the xenografts. Points, mean (n = 7); bar, SD; *P < .01; ***P < .001. The tumor weight (I) of the xenografts after dissection. Scatter plot, mean (n = 7); bars, SD; ***P < .001.
P4HA2 expression is negatively correlated with the survival of DLBCL patients
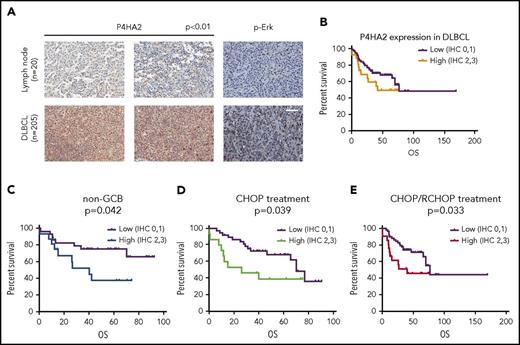
To investigate the role of P4HA2 in human DLBCLs, we analyzed the expression of P4HA2 and compared it with that of Carabin in the same DLBCL tissues (Figure 1A; supplemental Table 1) by western blotting (supplemental Figure 8). In >50% cases (DLBCL 1, 2, 6, 7, 9, 12, 13, 14, 15, 16, and 18; control 2, 3, and 4), there is a negative correlation between P4HA2 and Carabin. Although most cells are B-lymphoma cells in DLBCL tissue, the DLBCL tissues might contain contaminating fibroblasts that express high levels of P4HA2. Thus, we further analyzed P4HA2 expression in DLBCL tissues by IHC. We surgically collected 205 DLBCL specimens and 20 reactive hyperplasias of lymph nodes and performed an IHC assay to evaluate the clinical relevance of P4HA2. We found that the expression of P4HA2 was almost undetectable in B lymphocytes of all 20 reactive lymph nodes. In contrast, positive staining of P4HA2 was detected in over half of (120 of 205, 59.7%) malignant B lymphomas (Figure 6A). To examine the correlation between P4HA2 and p-Erk, we analyzed p-Erk levels in the same cases (Figure 6A). There is a positive relationship between these 2 proteins (P = .021; Table 1). In addition to p-Erk, as shown in Table 1, P4HA2 was highly expressed in non-GCB, which is an aggressive malignant subtype of DLBCL, rather than GCB DLBCL subtype (P = .014). In addition, a high level of expression of P4HA2 was correlated with high-risk International Prognostic Index (IPI) (P = .006).
P4HA2 promotes DLBCL progression. (A) Upregulation of P4HA2 in DLBCL. Immunohistochemical analysis of P4HA2 and p-Erk in 205 DLBCL tissue samples and 20 reactive hyperplastic lymph node control samples. Hematoxylin and eosin stain; scale bar, 25 µM. (B) The OS with different expression levels of P4HA2 in DLBCL. Kaplan-Meier OS analysis of DLBCL based on low (n = 77) and high (n = 26) expression of P4HA2. Low, low expression of P4HA2, IHC 0-1; high, high expression of P4HA2, IHC 2-3. (C-E) Prognostic values of P4HA2 expression using Kaplan-Meier analysis. The OS of non-GCB subtype DLBCL (C), CHOP treatment (D), or CHOP/R-CHOP (E) treatment DLBCL based on different expression levels of P4HA2.
P4HA2 promotes DLBCL progression. (A) Upregulation of P4HA2 in DLBCL. Immunohistochemical analysis of P4HA2 and p-Erk in 205 DLBCL tissue samples and 20 reactive hyperplastic lymph node control samples. Hematoxylin and eosin stain; scale bar, 25 µM. (B) The OS with different expression levels of P4HA2 in DLBCL. Kaplan-Meier OS analysis of DLBCL based on low (n = 77) and high (n = 26) expression of P4HA2. Low, low expression of P4HA2, IHC 0-1; high, high expression of P4HA2, IHC 2-3. (C-E) Prognostic values of P4HA2 expression using Kaplan-Meier analysis. The OS of non-GCB subtype DLBCL (C), CHOP treatment (D), or CHOP/R-CHOP (E) treatment DLBCL based on different expression levels of P4HA2.
Correlation of the clinicopathological findings with P4HA2 expression
| Characteristic . | P4HA2 expression in DLBCL . | ||
|---|---|---|---|
| Low (0, 1) . | High (2, 3) . | P . | |
| Age, y | |||
| ≤54 | 69 | 17 | .295 |
| >54 | 85 | 30 | |
| Sex | |||
| Female | 60 | 19 | .649 |
| Male | 96 | 26 | |
| DLBCL subtypes | |||
| GCB | 88 | 16 | .014 |
| Non-GCB | 68 | 29 | |
| p-Erk | |||
| Low (0-1) | 101 | 22 | .021 |
| High (2-3) | 48 | 23 | |
| IPI risk group | |||
| Low (0-1) | 86 | 15 | .006 |
| High (2-5) | 55 | 26 | |
| Characteristic . | P4HA2 expression in DLBCL . | ||
|---|---|---|---|
| Low (0, 1) . | High (2, 3) . | P . | |
| Age, y | |||
| ≤54 | 69 | 17 | .295 |
| >54 | 85 | 30 | |
| Sex | |||
| Female | 60 | 19 | .649 |
| Male | 96 | 26 | |
| DLBCL subtypes | |||
| GCB | 88 | 16 | .014 |
| Non-GCB | 68 | 29 | |
| p-Erk | |||
| Low (0-1) | 101 | 22 | .021 |
| High (2-3) | 48 | 23 | |
| IPI risk group | |||
| Low (0-1) | 86 | 15 | .006 |
| High (2-5) | 55 | 26 | |
Bold P values represent significant difference.
The standard treatment of DLBCL patients has been a combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with or without rituximab (R-CHOP/CHOP).4,33,34 The IPI was developed to identify patients at high risk of relapse.35 The IPI system has been validated with R-CHOP therapy in which the highest risk group has a 4-year progression-free survival (PFS) of 53% and overall survival (OS) of 55%.36 To assess the clinical significance of P4HA2 expression in DLBCL, we conducted the OS analysis to examine the correlation of the expression level of P4HA2 with the survival rate. There was marked difference in survival rate of all patients between the low and high P4HA2 expression groups, though the P value was not significant (Figure 6B; supplemental Table 2). Strikingly, high expression of P4HA2 is closely associated with reduced OS in non-GCB (P = .042), CHOP treatment (P = .039), and CHOP/R-CHOP treatment (P = .033) patients (Figure 6C-E; Table 2).
OS in all DLBCL
| Clinical features . | P4HA2 expression . | MST (95% CI) . | Log-rank P . |
|---|---|---|---|
| ≤54 y old | .201 | ||
| Low | |||
| High | 42.0 | ||
| >54 y old | .598 | ||
| Low | 77.1 (61.0-93.2) | ||
| High | |||
| Male | .921 | ||
| Low | 70.3 (49.4-91.2) | ||
| High | |||
| Female | .052 | ||
| Low | |||
| High | 42.0 (4.1-79.9) | ||
| Non-GCB | .042 | ||
| Low | 70.3 (63.1-77.5) | ||
| High | |||
| GCB | .820 | ||
| Low | |||
| High | 40.1 (15.9-64.3) | ||
| IPI low (0-1) | .317 | ||
| n = 53 | Low | ||
| High | |||
| IPI high (2-5) | .521 | ||
| n = 44 | Low | 46.8 | |
| High | 20.7 | ||
| CHOP | .039 | ||
| n = 55 | Low | 70.3 | |
| High | 26.2 | ||
| R-CHOP | .529 | ||
| n = 27 | Low | ||
| High | |||
| CHOP/R-CHOP | .033 | ||
| n = 82 | Low | 77.1 | |
| High | 44.1 |
| Clinical features . | P4HA2 expression . | MST (95% CI) . | Log-rank P . |
|---|---|---|---|
| ≤54 y old | .201 | ||
| Low | |||
| High | 42.0 | ||
| >54 y old | .598 | ||
| Low | 77.1 (61.0-93.2) | ||
| High | |||
| Male | .921 | ||
| Low | 70.3 (49.4-91.2) | ||
| High | |||
| Female | .052 | ||
| Low | |||
| High | 42.0 (4.1-79.9) | ||
| Non-GCB | .042 | ||
| Low | 70.3 (63.1-77.5) | ||
| High | |||
| GCB | .820 | ||
| Low | |||
| High | 40.1 (15.9-64.3) | ||
| IPI low (0-1) | .317 | ||
| n = 53 | Low | ||
| High | |||
| IPI high (2-5) | .521 | ||
| n = 44 | Low | 46.8 | |
| High | 20.7 | ||
| CHOP | .039 | ||
| n = 55 | Low | 70.3 | |
| High | 26.2 | ||
| R-CHOP | .529 | ||
| n = 27 | Low | ||
| High | |||
| CHOP/R-CHOP | .033 | ||
| n = 82 | Low | 77.1 | |
| High | 44.1 |
Bold P values represent significant difference.
CI, confidence interval; MST, mean survival time.
These clinicopathological findings suggest that patients with high P4HA2 expression may not mitigate the disease even after the standard treatments and further support that P4HA2 is a key determinant promoting the growth of B-cell lymphoma.
Discussion
The ras family of proto-oncogenes plays an essential role in tumorigenesis.37,38 Downstream of the BCR-signaling pathway, gain-of-function mutations or dysregulation in the Ras/Erk pathway are frequently reported in different types of B lymphoma.39 Understanding the regulation of key players in the Ras-Erk cascade might provide potential new targets for treating malignancy of B lymphoma. In this study, we found that Carabin, an endogenous inhibitor of Ras and calcineurin, plays a pivotal role in controlling the growth of B-cell lymphoma (Figure 1B-D), and we confirmed the findings in vivo using an animal xenograft model (Figure 1E-H). Moreover, the negative effect of Carabin on the Ras/Erk-signaling pathway is in agreement with Carabin’s effects on tumor cell proliferation (Figure 1H; supplemental Figure 1A-B). Our observations support broader roles of Carabin in renal cancer, T and B lymphocytes as well as cardiac hypertrophy. We also found that the protein levels of Carabin are downregulated in a number of B-cell lymphoma samples from patients (Figure 1A; supplemental Figure 1A), but mRNA transcripts are not (data not shown). Furthermore, when mature B cells or B lymphoma were stimulated with PMA or LPS, Carabin levels are regulated only at the protein level (supplemental Figure 6C-D), suggesting that downregulation of Carabin involves likely posttranscriptional regulation.
Using a TAP purification method, we identified P4HA2 as a new binding protein and negative regulator of Carabin (Figure 2A-D; supplemental Figure 3A). We demonstrated that P4HA2 hydroxylates Carabin on Pro306, leading to its ubiquitination and proteasomal degradation (Figure 3A-D). This mode of regulation is similar to that for HIF1α, which undergoes proline hydroxylation by PHD under normoxic conditions. In previous studies, P4Hs have been reported to promote extracellular matrix (ECM) signaling and were suggested to be potential targets for fibrosis. They have also been shown to be involved in invasion and metastasis of breast cancers recently with no appreciable effects on the proliferation of breast cancer cells.23,24 In this study, we unraveled a novel function of P4HA2 in B-cell lymphoma (Figure 4A-F). Inverse correlation of Carabin and P4HA2 in different cell lines is consistent with the degradation of Carabin through hydroxylation by P4HA2. P4HA2 is ubiquitously expressed in most human tissues, but the basal expression level of P4HA2 in naive T and B cells is extremely low and could not be detected by any commercial antibodies. Upon activation, P4HA2, but not P4HA1, is upregulated in activated B cells (supplemental Figure 6A-B,E-F) and T cells (data not shown). In contrast, we could not detect Carabin in other cell types or tissue except blood cells such as T and B cells. The context-dependent effect of P4HA2 on proliferation might be explained by the tissue-restricted expression of Carabin and P4HA2. Moreover, the effects of P4HA2 knockdown on proliferation and Erk1/2 activity were partially reversed by concomitant suppression of Carabin (Figure 5A-F), indicating that P4HA2’s effects in lymphoma indeed depend on Carabin.
Consistent with the prevention of tumor growth upon knockdown of P4HA2, the promotion of lymphoma tumorigenesis by overexpression of P4HA2 depends on its hydroxylase activity and substrate Carabin (Figures 4I-K and 5G-I). As shown in Figure 7, we propose that Carabin is hydroxylated by P4HA2 in B-cell lymphoma, thereby degrading Carabin and increasing Erk activity in tumor cells. We verified the negative correlation between P4HA2 and Carabin in DLBCL tissues by western blotting. Due to the limitation of Carabin antibodies for immunostaining (we have tested several commercial or our own antibodies), we could not further precisely assess Carabin expression in different cell types by IHC in the same DLBCL tissues. However, considering that most cells are B-lymphoma cells in DLBCL tissue, degradation of Carabin should be caused by overexpression of P4HA2. As expected, we confirmed positive correlation of P4HA2 and p-Erk in DLBCL patient samples by IHC staining (Figure 6A; Table 1). P4HA2 expression increased in malignant B lymphocytes of DLBCL tissue samples, especially in non-GCB DLBCL (Figure 6A, Table 1). Non-GCB DLBCL is a more malignant subtype than GCB and higher expression of P4HA2 in non-GCB patients was associated with a shorter OS (Figure 6C, Table 1). Furthermore, P4HA2 associates with IPI risk, suggesting that P4HA2 might also contribute to B-cell lymphoma malignant transformation. Importantly, higher expression levels of P4HA2 also correlate with reduced OS in CHOP or CHOP/R-CHOP patients (Figure 6C-E). With the standard treatment of DLBCL with CHOP/R-CHOP, over 30% of patients fail to respond to currently available regimens or relapse with drug resistance. The cause for this incomplete treatment remains unknown. Our results suggest that upregulation of P4HA2 in DLBCL is 1 cause, and P4HA2 may serve as a new biomarker for DLBCL diagnosis and an effective target for anti–B-cell lymphoma drug discovery. Our results also suggest that inhibitors of P4HA2 may be uniquely effective in B-lymphoma patients who do not respond to the current CHOP and R-CHOP regimen, pointing to P4HA2 as a promising target for developing novel drugs to treat B-cell lymphoma.
The model diagram of P4HA2 regulating Carabin in B-lymphoma cells. The picture depicts a working model of how Carabin is modified by P4HA2 in B-cell lymphoma tumorigenesis, thereby degrading Carabin and increasing Erk activity in tumor cells.
The model diagram of P4HA2 regulating Carabin in B-lymphoma cells. The picture depicts a working model of how Carabin is modified by P4HA2 in B-cell lymphoma tumorigenesis, thereby degrading Carabin and increasing Erk activity in tumor cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lei Shen and Shuai Wu for help sorting PBMCs and thank Sarah Head for careful and critical reading of their manuscript.
This work was supported by National Natural Science Foundation of China grants (31270830 and 21572038 [Y.D.]; 81502394 [W.J.]; 31370814 [M.T.]; 81470353 and 81272630 [X.Z.]), the National Basic Research Program of China (973 Program; 2014CBA02004 [M.T.]), the Flight Attendant Medical Research Institute and P30 CA006973 from the National Institutes of Health National Cancer Institute (J.O.L./Willian Nelson), the Development Fund for Shanghai Talents (Y.D.), Fund of State Key Laboratory of Bioorganic and Natural Products Chemistry (Y.D.), and Fund of State Key Laboratory of Drug Research, Chinese Academy of Sciences (SIMM1601KF-08) (Y.D.).
Authorship
Contribution: Y.D. and W.J. designed and oversaw the project; W.J., K.L., R.T., Z.C., Q.L., M.B., and Y.D. performed experiments and collected data; W.W. and X.Z. provided tumor samples and performed the IHC assay; X.C. and M.T. performed the MS assay; J.S., Y.Z., Y.C., and B.L. provided human blood and performed the PBMC sorting assay; S.S. and L.J. provided technical support; and W.J., Z.L., D.K.M., J.O.L., and Y.D. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yongjun Dang, Key Laboratory of Metabolism and Molecular Medicine, The Ministry of Education, Department of Biochemistry and Molecular Biology, Shanghai Medical College, Fudan University, Shanghai 200032, China; e-mail: yongjundang@fudan.edu.cn.
REFERENCES
Author notes
W.J., X.Z., and Z.L. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal