In this issue of Blood, Prince et al report that by targeting anticoagulant protein S (PS), they could achieve hemostasis and prevent hemarthrosis in murine models of hemophilia.1
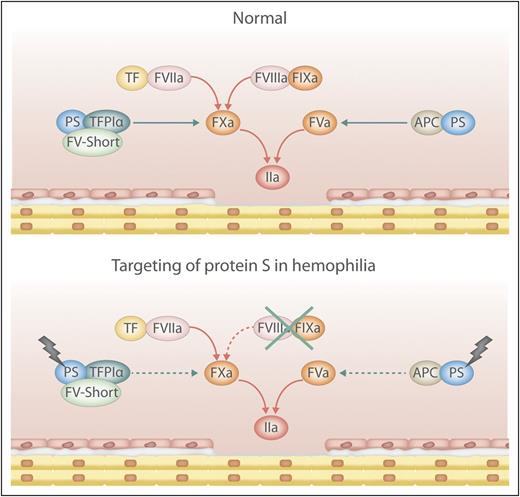
Simplified scheme of pro- and anticoagulant reactions in health and hemophilia with targeted PS. Damage to the endothelium activates coagulation resulting in localized generation of thrombin (IIa). PS-dependent anticoagulant mechanisms control the levels of activated factor X (FXa) and FVa thus balancing the coagulation reactions. By targeting PS in hemophilia the inherent procoagulant-anticoagulant imbalance is corrected and the level of thrombin generated is sufficient for hemostasis. APC, activated protein C; TF, tissue factor; TFPIα, tissue factor pathway inhibitor α. Professional illustration by Luk Cox.
Simplified scheme of pro- and anticoagulant reactions in health and hemophilia with targeted PS. Damage to the endothelium activates coagulation resulting in localized generation of thrombin (IIa). PS-dependent anticoagulant mechanisms control the levels of activated factor X (FXa) and FVa thus balancing the coagulation reactions. By targeting PS in hemophilia the inherent procoagulant-anticoagulant imbalance is corrected and the level of thrombin generated is sufficient for hemostasis. APC, activated protein C; TF, tissue factor; TFPIα, tissue factor pathway inhibitor α. Professional illustration by Luk Cox.
PS knockout mice have uncontrolled disseminated coagulation and embryonic lethality. However, when Prince and colleagues created mice with the combination of PS deficiency and hemophilia A or B, they discovered that these mice were well, with normal viability and no overt coagulopathy. Notably, the mice were protected from acute hemarthrosis, which is a challenging problem in hemophilia. On the basis of the study results, Prince et al propose that targeting PS may be useful for treating hemophilia.
Blood coagulation is activated in response to damage to the vascular wall (see figure). FVIIa binds to the exposed TF, and the FVIIa-TF complex efficiently activates FX. FXa and FVa form the prothrombinase complex that generates thrombin. In addition, FVIIa-TF activates FIX, which together with its cofactor FVIIIa activates FX. The efficiency of coagulation is determined by the amount of FXa and thrombin generated, with thrombin being a multifunctional enzyme that cleaves fibrinogen to fibrin and activates platelets. In tissues with low TF (eg, joints and muscles), insufficient amounts of FXa are generated from FVIIa-TF, and amplification provided by the FIXa-FVIIIa complex is crucial for efficient hemostasis.
The coagulation pathway is controlled by several anticoagulant mechanisms. Each reaction is strictly regulated, and normally there is a delicate balance between pro- and anticoagulant forces. Antithrombin (AT) inhibits several of the enzymes of the pathway (eg, thrombin, FXa, and FIXa).2 The 2 cofactors FVIIIa and FVa are under proteolytic control by APC, the key enzyme of the protein C pathway.3 TFPIα controls the FVIIa-TF complex and FXa.4 PS is particularly interesting because it serves as a cofactor to both TFPIα and APC. The ability of TFPIα to inhibit FXa is efficiently stimulated by the combination of PS and FV-short (a splice variant of FV), the 2 proteins functioning in synergy.3 In the absence of PS, TFPIα is a poor inhibitor of FXa. Likewise, without PS, APC is inefficient at inhibiting FVa and FVIIIa. The physiological importance of PS is illustrated by the severe lethal consumptive coagulopathy that is associated with homozygous PS deficiency in both humans and mice.
Hemophilia A and B are caused by deficiency of FVIII or FIX, respectively. These X-linked bleeding disorders affect males from an early age. Severe hemophilia is associated with lifelong suffering from repeated bleeding episodes, often in joints and muscles. On-demand or prophylactic administration of the missing factor has been the treatment of choice for decades. However, the immune system may recognize the administered factor as foreign and may generate neutralizing antibodies, making the treatment ineffective. This is a challenging clinical situation, and several therapeutic approaches have been tried such as high concentrations of FVIIa, recombinant porcine FVIII, or induction of immune tolerance.5 Recently, other approaches have been tried such as bioengineered zymogen-like FXa, which is resistant to inhibitors and active only when bound to FVa.6 Another novel approach is a bispecific monoclonal antibody that binds to both FIXa and FX thereby mimicking FVIIIa (emicizumab [ACE910]).7 Rebalancing hemostasis in hemophilia by inhibiting anticoagulant mechanisms has also been explored with promising results. This includes RNA silencing of AT (ALN-AT3),2 administration of a monoclonal antibody against TFPIα (concizumab),4 or a bioengineered α1-anti-trypsin (KRK α1AT) that exhibits high efficiency and specificity against APC.8 Gene therapy is an attractive approach, and recent progress holds promise for the future.9 However, whether it will be an option for patients with inhibitors remains to be elucidated.
Inhibition of PS is an attractive approach because PS is required as a cofactor for both TFPIα and APC. Prince et al have tested this by creating hemophilic mice (F8−/− or F9−/−) that are also lacking PS (Pros1−/−). These mice seemed to be completely normal and had no signs of unprovoked consumptive coagulopathy. However, the lack of intrinsic Xase did not prevent lethality in TF-induced thromboembolism and only partially protected Pros1−/− mice against thrombosis in mesenteric arterioles. Moreover, loss of PS limited but did not abrogate tail bleeding in hemophilic mice. Of particular interest was the observation that in both F8−/− and F9−/− mice, the lack of PS or inhibition of PS with antibodies provided full protection against acute hemarthrosis. A potential explanation for these prominent effects may be the demonstrated high expression of TFPIα and PS in the synovium of both hemophilic mice and patients with hemophilia A or B. In thrombin generation assays, the loss of PS was, as expected, associated with decreased anticoagulant effects of TFPIα and APC in both human and mouse plasma.
A critical caveat inherent in the study design relates to species differences. PS in human plasma is present in 2 forms: as free protein and in complex with the complement regulator C4b-binding protein (C4BP), an octopus-like molecule having 7 α-chains and a single PS binding β-chain.10 Mice do not have a C4BP-PS complex because their β-chain gene is converted to a pseudogene. PS is required for secretion of the human β-chain. How silencing of PS will affect C4BP and the complement system in humans remains to be determined. The authors mention another interesting species difference: mice have TFPIα in platelets but not in plasma, whereas human TFPIα circulates in plasma bound to FV/FV-short, which functions in synergy with PS to stimulate TFPIα activity.3 PS has also been demonstrated to be a ligand and activator of a family of tyrosine kinase receptors (TAM receptors) that stimulates phagocytosis of apoptotic cells and regulates immune response.10 How silencing of PS affects the TAM receptor system is an important question that needs to be answered. Despite these caveats, silencing or inhibition of PS is one of several potentially very interesting therapeutic approaches in hemophilia, and the future will reveal which treatment principles will prevail.
Conflict-of-interest disclosure: The author declares no competing financial interests.