Can the detection of measurable residual disease (MRD) become the standard approach for acute lymphoblastic leukemia (ALL)? In this issue of Blood,Wood et al1 use a highly sensitive sequencing approach that is an improvement on conventional methods by both performance characteristics and potential for standardization.
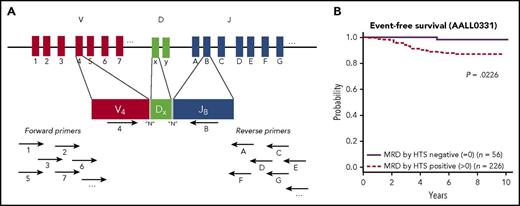
(A) Cartoon of the sequencing strategy, demonstrating targeting the IgH gene rearrangements. Multiple primers complementary to various V and J sequences are simultaneously used, and in this example, primers specific to V4 and JB bind and can form a PCR VDJ rearrangement product. The amplified rearrangements are sequenced and bioinformatics approaches are used to identify the dominant clones that will subsequently be tracked for the MRD assay. (B) Kaplan-Meier estimates of EFS for standard-risk group patients who were MRD positive by HTS at any level vs MRD negative. See Figure 4A in the article by Wood et al that begins on page 1350.
(A) Cartoon of the sequencing strategy, demonstrating targeting the IgH gene rearrangements. Multiple primers complementary to various V and J sequences are simultaneously used, and in this example, primers specific to V4 and JB bind and can form a PCR VDJ rearrangement product. The amplified rearrangements are sequenced and bioinformatics approaches are used to identify the dominant clones that will subsequently be tracked for the MRD assay. (B) Kaplan-Meier estimates of EFS for standard-risk group patients who were MRD positive by HTS at any level vs MRD negative. See Figure 4A in the article by Wood et al that begins on page 1350.
The presence of MRD in the context of a morphological remission is associated with a higher relapse rate than MRD-negative cases in all leukemia subtypes of leukemia and across most (if not all) treatment modalities. Indeed, in chronic myeloid leukemia (CML), the measurement of BCR-ABL messenger RNA has become the standard of care for assessing disease burden and treatment response both in the community and in clinical trials.2
Although CML is the poster child of MRD direct treatment strategies, it can be argued that the data for MRD and response in ALL is even stronger. MRD has been associated with poorer event-free survival (EFS) and overall survival (OS) in both pediatric and adult ALL, though the reported experience in the pediatric arena dwarfs that in the adult setting.3,4 Several pediatric studies in Europe and the United States have risk stratification treatment options based on postinduction and consolidation MRD status.5,6 A recently completed National Cancer Institute–sponsored large meta-analysis of MRD in ALL found that the impact of MRD on overall and EFS was amazingly stable over types of populations (pediatric vs adult), subtypes of ALL (B, T, and Ph+), and assays used to detect MRD (flow cytometry vs polymerase chain reaction [PCR]; see below).7 In all these situations, the hazard ratio of MRD negativity ranged from ∼0.2 to 0.3.
If MRD is so important, then why is it not the standard of care? Simply said, it is not easy to do it well. MRD can be measured in several ways, including PCR of the immunoglobulin heavy chain (IgH) VDJ and/or T cell receptor (TCR) gene rearrangements or leukemia-specific fusion transcripts (eg, BCR-ABL), or by multiparametric flow cytometry (FC). These methods can have laboratory-to-laboratory variations in performance characteristics (eg, the sensitivity of detecting a leukemic cell in a background of normal cells) and can be very labor intensive (gene rearrangement PCR) or equipment intensive (FC). Consequently, standardization has lagged, making it difficult to use in clinical trials across academic centers, let alone in the community setting, and regulatory agencies have been (perhaps understandably) slow to adopt MRD in ALL as a surrogate in trials of new drug agents.
The study by Wood et al suggests a path forward to improve the sensitivity of MRD detection while offering potential standardization, utilizing a deep sequencing methodology of IgH and TCR rearrangements. Basically, a high-throughput sequencing (HTS) method has been devised with simultaneous use of a multiplex of upstream and downstream primers to sequence across all IgH and TCR rearrangements (see figure, panel A). This is in contrast to the conventional PCR method, which takes several steps to go from amplification of the patient specific rearrangement to the generation of patient specific primers and probes used in the subsequent MRD detection. Several small retrospective studies have suggested that the HTS method is more sensitive than state-of-the-art flow cytometry by an order of magnitude (or so).8-10
In the Wood et al study, HTS and FC for MRD detection at the end of induction chemotherapy were directly compared in 619 standard- or high-risk pediatric patients with newly diagnosed B-lymphoblastic leukemia (B-ALL). HTS detected a clonal rearrangement in 95% of samples, and 75% of these cases were found to have MRD detected after induction. Analyses revealed several important findings. (1) At the lower-level detection threshold of 0.01% (the lower-end cutoff value used for the FC assays in these trials), HTS and FC detection showed similar 5-year EFS and OS for patients with and without MRD. (2) In 55 cases (∼10% of cases), MRD was detectable by HTS, but not by FC. The contrast (HTS negative, FC positive) occurred more rarely (17 cases). The EFS was best for patients without MRD by FC and HTS, worse for patients with MRD only by HTS (suggesting the better sensitivity picked up new cases of clinically relevant MRD), and worst for patients MRD positive by both methods. (3) Twenty percent of standard-risk patients were free of MRD by the more sensitive HTS negative method, and these patients had a spectacular 5-year EFS (98%) and OS (100%) compared with patients without MRD by HTS (see figure, panel B).
Why is this study important? The clear relationship between MRD and outcome has implications for clinical trial design (for risk stratification or an early surrogate end point for drug investigation), and in clinical practice, it can identify patients who might benefit from an alternative approach (a clinical trial or allogeneic transplantation), perhaps sparing allografting for those without MRD. The HTS method can be packaged into kit form, with centralized testing and quality control that can lead to the broad democratization of the powerful methodology. Lastly, while conventional wisdom dictates that MRD in ALL is best done from the bone marrow, the increased sensitivity of the HTS assay may make frequent peripheral blood testing as useful as intermittent bone marrow sampling. This will obviously need to be tested, but perhaps in ALL, routine bone marrow procedures may become a thing of the past, like in CML.
MRD should not be thought as a “biomarker” of ALL; rather, MRD is the disease. However, no assay is perfect. Although MRD is a direct measure of disease burden and treatment response in ALL, there may be sanctuary sites (in the brain or testes, for example) that also contribute to relapse and yet may not be measurable by marrow or blood MRD analyses. Nonetheless, the advent of sensitive, standardized, and quantifiable assays makes MRD a rich, continuous measure of disease burden rather than a dichotomous measure. MRD thus has evolved from minimal residual disease to measurable residual disease.
Conflict-of-interest disclosure: J.P.R. is a member of Adaptive’s scientific advisory board and has collaborated with Adaptive on MRD studies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal