Key Points
Impaired BM EPCs were found in corticosteroid-resistant ITP patients.
Atorvastatin improved BM EPC quantity and function, representing a novel therapy approach for corticosteroid-resistant ITP patients.
Abstract
The pathogenesis of corticosteroid-resistant immune thrombocytopenia (ITP), a clinically challenging condition in which patients exhibit either no response to corticosteroids or are corticosteroid-dependent, remains poorly understood. Murine studies suggest that bone marrow (BM) endothelial progenitor cells (EPCs) play a crucial role in regulating megakaryocytopoiesis. However, little is known regarding the number and function of BM EPCs or how to improve impaired BM EPCs in corticosteroid-resistant ITP patients. In the current case-control study, we evaluated whether the BM EPCs in corticosteroid-resistant ITP differed from those in corticosteroid-sensitive ITP. Moreover, whether atorvastatin could enhance the number and function of BM EPCs derived from corticosteroid-resistant ITP patients was investigated in vitro and in vivo. Reduced and dysfunctional BM EPCs, characterized by decreased capacities of migration and angiogenesis as well as higher levels of reactive oxygen species and apoptosis, were observed in corticosteroid-resistant ITP patients. In vitro treatment with atorvastatin quantitatively and functionally improved BM EPCs derived from corticosteroid-resistant ITP patients by downregulating the p38 MAPK pathway and upregulating the Akt pathway, and rescued the impaired BM EPCs to support megakaryocytopoiesis. Subsequently, a pilot cohort study showed that atorvastatin was safe and effective in corticosteroid-resistant ITP patients. Taken together, these results indicate that reduced and dysfunctional BM EPCs play a role in the pathogenesis of corticosteroid-resistant ITP, and the impaired BM EPCs could be improved by atorvastatin both in vitro and in vivo. Although requiring further validation, our data indicate that atorvastatin represents a promising therapeutic approach for repairing impaired BM EPCs in corticosteroid-resistant ITP patients.
Introduction
Immune thrombocytopenia (ITP), generally characterized by increased peripheral platelet destruction and reduced platelet production, is a common hematological disease.1-6 A panel of the International Working Group (IWG) prefers “immune” to emphasize the immune-mediated mechanism of ITP.7 Moreover, corticosteroids represent the standard first-line therapy, achieving responses in ∼50% to 85% of ITP patients.8-14 However, for the remaining corticosteroid-resistant ITP patients, who exhibit either no response to corticosteroid or are corticosteroid-dependent,7,8,15 the pathogenesis remains poorly understood and the clinical management is challenging.
Thrombopoiesis, which occurs within a specialized bone marrow (BM) microenvironment, is a complex biological process that is initiated with the commitment of hematopoietic stem cells (HSCs) to megakaryocytic progenitors and eventually results in the maturation of megakaryocytes to produce functional platelets.16,17 Abnormalities during any stage of megakaryocytopoiesis might influence platelet production. Endosteal cells, endothelial progenitor cells (EPCs), perivascular cells, and various mature immune cells have been revealed to be key elements of the BM microenvironment.18-23 Emerging evidence from murine studies suggested that BM EPCs might play a crucial role in regulating thrombopoiesis.24-26 We previously reported that the impaired BM EPCs contribute to the occurrence of prolonged isolated thrombocytopenia (PT), which is a serious complication defined as the engraftment of all peripheral blood cell lines other than platelets (with a count <20 × 109/L) or dependence on platelet transfusions after allogeneic HSC transplantation (allo-HSCT).27 Similar to PT, ITP is characterized by low platelet counts and a maturation disorder of megakaryocytes. However, the functional role of BM EPCs in ITP patients is largely unknown. We recently reported that the number of BM EPCs was normal, but an abnormal BM immune microenvironment was observed in corticosteroid-sensitive ITP patients, indicating that dysregulated T-cell responses in the BM microenvironment might play an important role in the pathogenesis of corticosteroid-sensitive ITP.28 Nevertheless, the number and functional role of BM EPCs in corticosteroid-resistant ITP patients have never been reported. Moreover, approaches to improve the dysfunction of BM EPCs in corticosteroid-resistant ITP patients remain unidentified.
Atorvastatin, a synthetic 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, is frequently used in patients with dyslipidemia and associated vascular abnormalities.29,30 Moreover, atorvastatin has been reported to improve the mobilization and function of circulating EPCs in heart disease, diabetes, and other conditions31-33 by downregulating p38 MAPK34,35 or upregulating Akt signaling.36,37 Our recent study suggested that atorvastatin represents a promising therapeutic approach for repairing the impaired BM EPCs in patients with poor graft function (PGF) post allo-HSCT.38 Nevertheless, no previous studies have focused on the roles of atorvastatin in BM EPCs in corticosteroid-resistant ITP patients.
Therefore, the current study was performed to evaluate whether the quantity and function of BM EPCs in corticosteroid-resistant ITP patients differed from those in corticosteroid-sensitive ITP patients. Moreover, we investigated the effects of atorvastatin on the number and function of the cultivated BM EPCs derived from corticosteroid-resistant ITP patients and their underlying molecular mechanisms. Subsequently, a pilot study was performed to evaluate the efficacy and safety of atorvastatin in corticosteroid-resistant ITP patients. Our aim was to provide new insights into the pathogenesis underlying corticosteroid-resistant ITP.
Methods
Patients and controls
In the prospective case-control study, corticosteroid-resistant ITP patients (N = 55) and their matched corticosteroid-sensitive ITP patients (N = 55) were identified from the same cohort of primary ITP patients from 1 September 2016 to 31 August 2017, and from 1 October 2017 to 23 November 2017, at Peking University Institute of Hematology (supplemental Table 1, available on the Blood Web site). The 2 groups of ITP patients were matched for age, sex, and the time at which the BM microenvironment was evaluated after corticosteroid therapy (±3 days; “risk-set sampling”).39
BM samples from transplant donors (N = 30) were considered as healthy controls. The healthy cohort comprised 11 men and 19 women, aged 18 to 55 years (median, 42 years). The study was approved by the ethics committee of Peking University People’s Hospital, and written informed consent was obtained from all subjects, in compliance with the Declaration of Helsinki.
Clinical assessment of the pilot cohort study
From 1 March 2017 to 31 August 2017, after written consent in compliance with the Declaration of Helsinki, 13 of the 18 patients with corticosteroid-resistant ITP,8 who were willing to accept oral treatment with atorvastatin (20 mg per day) and N-acetyl-l-cysteine (NAC; a reactive oxygen species [ROS] scavenger; 400 mg 3 times a day), were enrolled in the pilot study. As shown in supplemental Table 2, patients who were receiving other maintenance regimens, including corticosteroids and danazol, were also eligible if the treatment dose had been stable in the past month and the dose was expected to remain unchanged or taper off at least the first 4 weeks of the study until the initial response was evaluated.
The primary end points of the pilot study were complete response (CR), response, and overall response (OR). Secondary end points were time to response and adverse events (safety). The treatment responses were evaluated weekly after the interventions. The definitions of the treatment responses were as follows: (1) CR was defined as a platelet count of at least 100 × 109/L; (2) response was defined as a platelet count between 30 × 109/L and 100 × 109/L and at least doubling of the baseline count; (3) OR included CR and response; and (4) no response (NR) was defined as a platelet count lower than 30 × 109/L or less than doubling of the baseline count.7
Clinical definitions
According to the IWG guidelines,7,8 primary ITP was defined as an autoimmune disorder characterized by isolated thrombocytopenia (peripheral blood platelet count <100 × 109/L) in the absence of other causes or disorders that might be associated with thrombocytopenia (eg, BM failure or myelodysplastic states, hepatitis C, or Helicobacter pylori infection). Corticosteroid-resistant ITP was defined as no response to at least 1 full-dose corticosteroid, defined as prednisone 1 mg/kg daily for at least 4 weeks then tapered off,7,8,15 to maintain a platelet count at or above 30 × 109/L and/or to avoid bleeding, or depending on the corticosteroid.7,8,15 Corticosteroid-sensitive ITP was defined as maintaining a platelet count at or above 30 × 109/L and/or to avoid bleeding after the initial corticosteroid treatment.7,8
Isolation, cultivation, and characterization of primary BM EPCs
The quantity and function of BM EPCs from the corticosteroid-resistant ITP patients and their matched corticosteroid-sensitive ITP patients were evaluated after 4 weeks of corticosteroid treatment. The numbers of patients performed for the independent experiments were labeled in each figure. In the pilot study, BM EPCs were evaluated pre- and posttreatment only in the patients who were willing to provide BM samples after written consent.
Isolation, cultivation, and characterization of BM EPCs were performed as previously reported.38 In brief, BM mononuclear cells (BMMNCs) were cultivated with EGM-2-MV-SingleQuots (Lonza, Walkersville, MD) and 10% fetal bovine serum (Gibco, Rockville, MD) at 37°C in a humidified incubator with 5% CO2 for 7 days before being used in experiments in 24-well culture plates (Corning Incorporated, Corning, NY) that were precoated within fibronectin (Sigma, St. Louis, MO).
Precultivated and 7-day cultivated BM EPCs were identified by staining with mouse anti-human CD34, vascular endothelial growth factor receptor 2 (VEGFR2; CD309), and CD133 monoclonal antibodies (BD Biosciences, San Jose, CA). The samples were then evaluated using LSRFortessa (Becton Dickinson). Aliquots of isotype-identical antibodies served as negative controls.
DiI–Ac-LDL uptake and FITC–UEA-I binding assay
The cultivated adherent cells were incubated with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil)–acetylated low-density lipoprotein (DiI–Ac-LDL; Life Technologies, Gaithersburg, MD) and fluorescein isothiocyanate (FITC)-labeled Ulex europaeus agglutinin-I (FITC–UEA-I; Sigma).38 To evaluate the numbers of double-positive–stained EPCs per well, 3 random power fields were counted using a fluorescence microscope (Olympus, Tokyo, Japan).
Cell counting, proliferation assay, migration assay, tube-formation assay, and apoptosis analysis of BM EPCs
As previously reported,38,40 the cell counting, cell proliferation assay, migration assay, tube-formation assay, and apoptosis analysis were analyzed in BM EPCs after treatment with: 50 nM, 500 nM, 5000 nM atorvastatin (Sigma); 0.1 mM, 1 mM, 10 mM NAC (Sigma); atorvastatin (500 nM) combined with NAC (1 mM); 10 μM p38 inhibitor (SB203580; Sigma); and 400 nM p38 inhibitor (BIRB796; Selleck Chem, Houston, TX) for 24 hours.
Measurement of intracellular ROS and proteins by flow cytometry
The cells stained with the aforementioned EPC markers were incubated with 10 μM 2′,7′-dichlorofluorescence diacetate (Byotimes, Beijing, China) at 37°C for 20 minutes. The mean fluorescence intensity of intracellular ROS was measured on a LSRFortessa (Becton Dickinson).38
As previously reported,38 cells were incubated with EPC antibodies at 4°C for 20 minutes and then fixed, permeabilized, and incubated with the following antibodies: phospho-p38 (p-p38), phospho–extracellular signal-regulated kinase (p-ERK), phospho-JNK (p-JNK) (Cell Signaling Technology, Danvers, MA), and phospho-Akt (p-Akt; Becton Dickinson). The intracellular protein levels were evaluated by LSRFortessa software (Becton Dickinson) and expressed as the mean fluorescence intensity (means ± standard error of the mean [SEM]).
Western blot analysis
Cultivated EPCs were washed and incubated in radioimmunoprecipitation assay buffer containing proteinase inhibitor and PhosSTOP (Roche, Indianapolis, IN) as described previously.38 The protein levels were determined using a BCA kit (Thermo Scientific, Rockford, IL). The proteins (30-50 µg per lane) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (Millipore, Bedford, MA) membranes. The membranes were then blocked with 5% bovine serum albumin and incubated overnight with antibodies against p-p38 MAPK, total p38, p-ERK, total ERK, p-JNK, total JNK, p-Akt, total Akt, and actin (Cell Signaling Technology) at dilutions specified by the manufacturer’s instructions. Anti-rabbit or anti-mouse secondary antibodies and an ECL chemiluminescence detection system (Pierce Biotechnology Inc, Rockford, IL) were used according to the manufacturer’s instructions to scan and semiquantitatively analyze the proteins.
Histological analysis of the BM microenvironment
BM trephine biopsies (BMBs) were obtained from the posterior superior iliac spine, and fixed with 4% paraformaldehyde and processed for frozen sections as previously described.41 After equilibrium at room temperature (RT) for 30 minutes, nonspecific antibody binding was blocked by incubating the slides with 20% goat serum at RT for 1 hour. Mouse anti-human CD34 (Becton Dickinson) and rabbit anti-human CD133 (Abcam, Cambridge, MA) were incubated at 4°C overnight. Goat anti-rabbit 555 and donkey anti-mouse 488 (Invitrogen, Eugene, OR) were added at RT for 1 hour. 4′,6-Diamidino-2-phenylindole (DAPI) was used to stain the nucleus, and the prepared slides were analyzed using Leica TCS SP8 (Leica Microsystems, Wetzlar, Germany).
Colony-forming unit assays
Colony-forming units (CFUs) were assayed using MethoCult H4434 Classic (Stem Cell Technologies, Vancouver, BC, Canada) as described previously.38,40 After 7 days of cultivation, suspended CD34+ cells were harvested, and 5 × 104 cells were plated and cultured for 14 days. Colony-forming unit erythroid (CFU-E), burst-forming unit erythroid (BFU-E), colony-forming unit–granulocyte-macrophage (CFU-GM), and colony-forming unit–granulocyte, erythroid, macrophage, and megakaryocyte (CFU-GEMM) measurements were scored on an inverted light microscope. Cultures were assayed in duplicate, and the results are expressed as the means ± SEM.
Coculture of BM CD34+ cells with BM EPCs
BM EPCs from patients with corticosteroid-resistant ITP, or corticosteroid-sensitive ITP, were cultivated for 7 days. Then, CD34+ cells (1 × 105 cells per well) were isolated from BMMNCs of healthy donors with a CD34 MicroBead kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and placed in a direct contact culture with 1 mL of StemSpan SFEM (Stem Cell Technologies) containing 100 ng/mL stem cell factor, 100 ng/mL thrombopoietin, and 10 ng/mL interleukin-3 for another 7 days for megakaryocyte differentiation.
Colony-forming unit megakaryocytes (CFU-MKs) were counted with a commercially available kit (MegaCult-C; Stem Cell Technologies) according to the manufacturer’s instructions. Cocultured CD34+ cells were plated in chamber slides (5 × 104 cells per well) and incubated at 37°C in a humidified atmosphere of 5% CO2 for 10 to 12 days. After dehydration, fixation, and immunocytochemical staining with mouse anti–human glycoprotein IIb/IIIa antibody and biotin-conjugated goat anti–mouse immunoglobulin G, megakaryocyte colonies were defined as groups of 3 or more glycoprotein IIb/IIIa+ cells.
Statistical analyses
Statistical analyses were performed using 1-way analysis of variance for comparisons among the groups. Subject variables were compared using the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables. The Wilcoxon test for paired data were used to identify the drug effects. Analyses were performed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA), and P values <.05 were considered significant.
Results
Patient characteristics
As shown in supplemental Table 1, age, sex, white blood cell count, and hemoglobin showed no significant differences between the 2 groups of ITP patients when the BM microenvironment was evaluated. However, the platelet count in corticosteroid-resistant ITP was significantly lower than those in corticosteroid-sensitive ITP. More corticosteroid-resistant ITP patients received danazol and cyclosporine A as prior and concomitant therapies compared with corticosteroid-sensitive ITP patients when the BM microenvironment was evaluated.
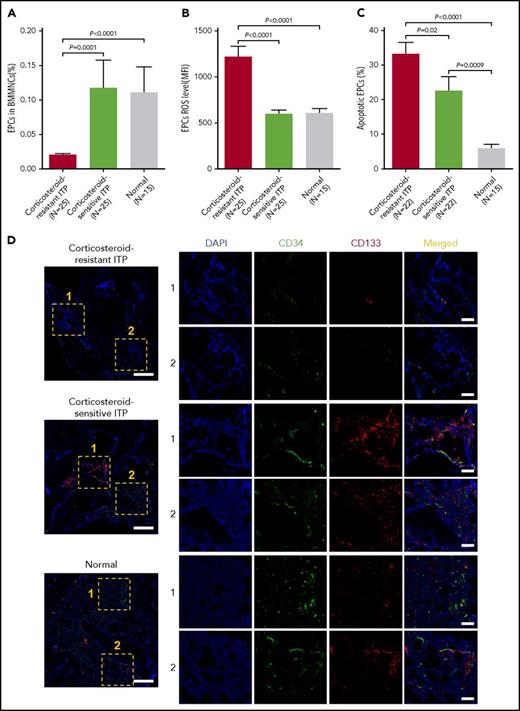
Reduced percentages and increased levels of ROS and apoptosis in precultured BM EPCs from corticosteroid-resistant ITP patients
Baseline percentages of BM EPCs (Figure 1A; 0.02% ± 0.003% vs 0.12% ± 0.04%; P = .0001) from corticosteroid-resistant ITP were significantly lower than those in corticosteroid-sensitive ITP. The levels of ROS (Figure 1B; 1214 ± 122 vs 591.2 ± 45.4; P < .0001) and apoptosis (Figure 1C; 33.1% ± 3.6% vs 22.4% ± 4.3%; P = .02) of precultured BM EPCs from corticosteroid-resistant ITP were significantly higher than those from corticosteroid-sensitive ITP.
Reduced percentages and increased levels of ROS and apoptosis in precultivated BM EPCs from corticosteroid-resistant ITP patients. The (A) percentages and levels of (B) intracellular ROS and (C) apoptosis in precultivated BM EPCs were analyzed by flow cytometry in patients with corticosteroid-resistant ITP and corticosteroid-sensitive ITP and healthy controls (Normal). (D) Mouse anti-human CD34 (green) and rabbit anti-human CD133 (red) were incubated to identify the BM EPCs. DAPI (blue) was used to stain the nucleus. In situ histological analyses of the BMBs showed that the frequency of the double-positive–stained (merged in yellow) EPCs with CD34 and CD133 was significantly reduced in corticosteroid-resistant ITP patients compared with corticosteroid-sensitive ITP patients and healthy controls according to immunofluorescent staining (scale bars, 50 μm). Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure. MFI, mean fluorescence intensity.
Reduced percentages and increased levels of ROS and apoptosis in precultivated BM EPCs from corticosteroid-resistant ITP patients. The (A) percentages and levels of (B) intracellular ROS and (C) apoptosis in precultivated BM EPCs were analyzed by flow cytometry in patients with corticosteroid-resistant ITP and corticosteroid-sensitive ITP and healthy controls (Normal). (D) Mouse anti-human CD34 (green) and rabbit anti-human CD133 (red) were incubated to identify the BM EPCs. DAPI (blue) was used to stain the nucleus. In situ histological analyses of the BMBs showed that the frequency of the double-positive–stained (merged in yellow) EPCs with CD34 and CD133 was significantly reduced in corticosteroid-resistant ITP patients compared with corticosteroid-sensitive ITP patients and healthy controls according to immunofluorescent staining (scale bars, 50 μm). Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure. MFI, mean fluorescence intensity.
Consistent with our flow cytometry data, in situ immunofluorescent staining with CD34 and CD133 revealed that the quantification of BM CD34+CD133+ EPCs was significantly reduced in corticosteroid-resistant ITP compared with those in the corticosteroid-sensitive ITP and the healthy controls (Figure 1D).
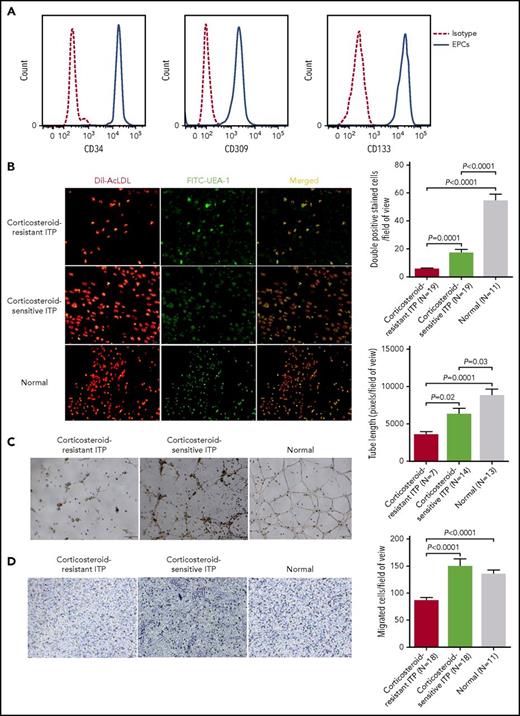
Characterization of primary BM EPCs
At day 7 of cultivation, the typical EPC phenotype38 was confirmed by demonstrating the positive expression of CD34, CD309, and CD133 by flow cytometry (Figure 2A). Moreover, the spindle-shaped and elongated BM adherent cells were further functionally characterized as EPCs by their capacities of DiI–Ac-LDL uptake and FITC–UEA-I binding (Figure 2B).
Characterization of cultivated BM EPCs and dysfunctional BM EPCs in corticosteroid-resistant ITP patients compared with corticosteroid-sensitive ITP patients and healthy controls. (A) The typical EPC phenotype of cultivated BM EPCs was confirmed by demonstrating the positive expression of CD34, CD309, and CD133 at day 7 in culture by flow cytometry (full line). Aliquots of isotype-identical antibodies served as negative controls (dotted line). (B) Representative images (left panel) for cultivated BM EPCs at day 7 in culture among patients with corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls (Normal). Typical BM EPCs were characterized by double-positive–stained (merged in yellow) with DiI–Ac-LDL (red) and FITC–UEA-I (green) (original magnification ×10). Quantification (right panel) of double-positive–stained cells (merged in yellow) with DiI–Ac-LDL (red) and FITC–UEA-I (green) at day 7 in culture among the corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy control groups (original magnification ×10). The data were expressed as the means ± SEM. (C) Tube formation was inspected on an inverted light microscope. The relative tube length per field of view was analyzed by Image Proplus by counting 3 random fields per sample. Representative images (left panel) and quantification (right panel) of tube formation (pixels of tubes per field of view) by cultivated BM EPCs at day 7 in culture (original magnification ×4). (D) The migrated cells (blue) were fixed and stained with crystal violet. Cell images were obtained on a phase-contrast microscope (Olympus) and counted in 3 random fields per sample. Representative images (left panel) and quantification (right panel) of the transwell migration assays of cultivated BM EPCs at day 7 in culture (original magnification ×10). The numbers of migrated BM EPCs per field of view were compared among patients with corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls. Three power fields were randomly counted and averaged per sample. Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure.
Characterization of cultivated BM EPCs and dysfunctional BM EPCs in corticosteroid-resistant ITP patients compared with corticosteroid-sensitive ITP patients and healthy controls. (A) The typical EPC phenotype of cultivated BM EPCs was confirmed by demonstrating the positive expression of CD34, CD309, and CD133 at day 7 in culture by flow cytometry (full line). Aliquots of isotype-identical antibodies served as negative controls (dotted line). (B) Representative images (left panel) for cultivated BM EPCs at day 7 in culture among patients with corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls (Normal). Typical BM EPCs were characterized by double-positive–stained (merged in yellow) with DiI–Ac-LDL (red) and FITC–UEA-I (green) (original magnification ×10). Quantification (right panel) of double-positive–stained cells (merged in yellow) with DiI–Ac-LDL (red) and FITC–UEA-I (green) at day 7 in culture among the corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy control groups (original magnification ×10). The data were expressed as the means ± SEM. (C) Tube formation was inspected on an inverted light microscope. The relative tube length per field of view was analyzed by Image Proplus by counting 3 random fields per sample. Representative images (left panel) and quantification (right panel) of tube formation (pixels of tubes per field of view) by cultivated BM EPCs at day 7 in culture (original magnification ×4). (D) The migrated cells (blue) were fixed and stained with crystal violet. Cell images were obtained on a phase-contrast microscope (Olympus) and counted in 3 random fields per sample. Representative images (left panel) and quantification (right panel) of the transwell migration assays of cultivated BM EPCs at day 7 in culture (original magnification ×10). The numbers of migrated BM EPCs per field of view were compared among patients with corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls. Three power fields were randomly counted and averaged per sample. Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure.
Impaired quantity and function in 7-day cultivated BM EPCs from corticosteroid-resistant ITP patients
To evaluate the quantity and function of primary BM EPCs, cell count, double-positive staining of DiI–Ac-LDL and FITC–UEA-I, and angiogenic potential (including migration and tube formation) were analyzed.
At day 7 of cultivation, double-positive–stained BM EPCs from corticosteroid-resistant ITP patients were significantly lower than those in corticosteroid-sensitive ITP (Figure 2B; 5.5 ± 0.9 vs 17 ± 2.6; P = .0001).
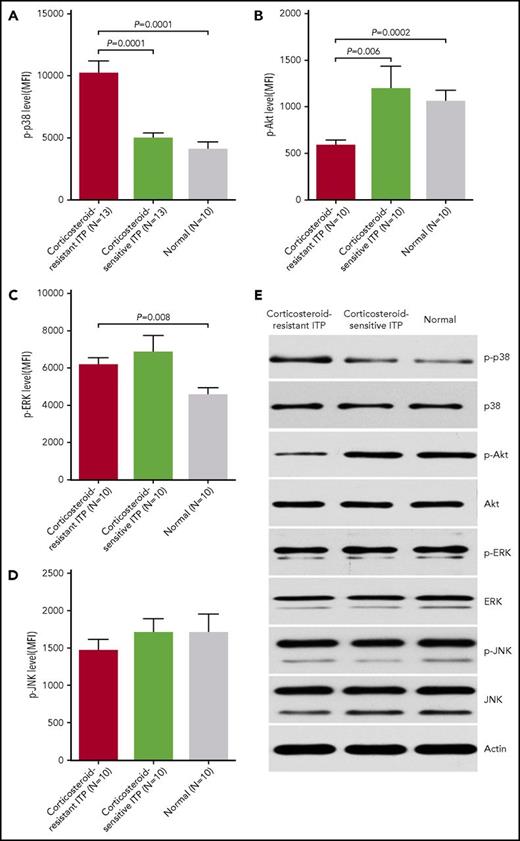
Elevated p-p38 MAPK and decreased p-Akt in BM EPCs from corticosteroid-resistant ITP patients
Notably higher levels of p-p38 (Figure 3A; 10 182 ± 1022 vs 4917 ± 504.5; P = .0001) and lower levels of p-Akt (Figure 3B; 581.4 ± 60.7 vs 1188 ± 247.8; P = .006) were detected in BM EPCs from corticosteroid-resistant ITP patients than in those from corticosteroid-sensitive ITP patients. In contrast, the levels of intracellular p-ERK (Figure 3C; 6141 ± 416.8 vs 6805 ± 943.9; P = .57) and p-JNK (Figure 3D; 1456 ± 162.3 vs 1703 ± 192.5; P = .57) were not significantly different between corticosteroid-resistant ITP and corticosteroid-sensitive ITP. Similarly, the basal expression patterns of these intracellular proteins detected by flow cytometry were further validated in cultivated BM EPCs by western blot (Figure 3E).
Elevated p-p38 and decreased p-Akt in BM EPCs from corticosteroid-resistant ITP patients. Flow cytometry revealed elevated (A) p-p38 and decreased (B) p-Akt, whereas no significant differences in (C) p-ERK or (D) p-JNK expression in precultivated BM EPCs among patients with corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls (Normal) were observed. The data were expressed as the MFI (means ± SEM). (E) Representative western blots of p-p38, total p38, p-Akt, total Akt, p-ERK, total ERK, p-JNK, total JNK, and actin in cultivated BM EPCs at day 7 in culture among patients with corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls. Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure.
Elevated p-p38 and decreased p-Akt in BM EPCs from corticosteroid-resistant ITP patients. Flow cytometry revealed elevated (A) p-p38 and decreased (B) p-Akt, whereas no significant differences in (C) p-ERK or (D) p-JNK expression in precultivated BM EPCs among patients with corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls (Normal) were observed. The data were expressed as the MFI (means ± SEM). (E) Representative western blots of p-p38, total p38, p-Akt, total Akt, p-ERK, total ERK, p-JNK, total JNK, and actin in cultivated BM EPCs at day 7 in culture among patients with corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls. Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure.
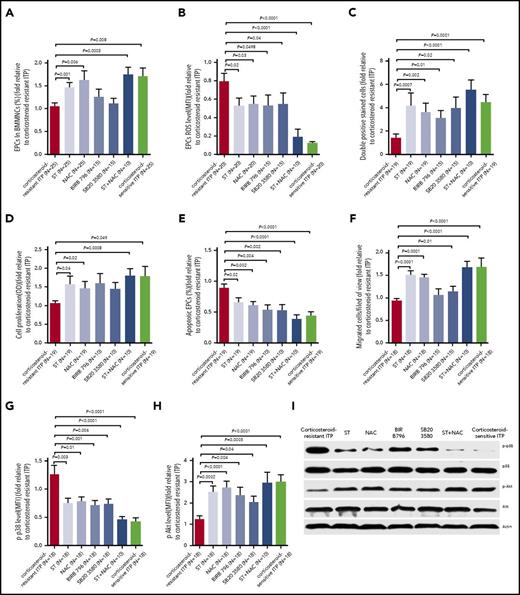
Atorvastatin and NAC produced superior improvement of the number and function of BM EPCs from corticosteroid-resistant ITP patients compared with those from corticosteroid-sensitive ITP patients
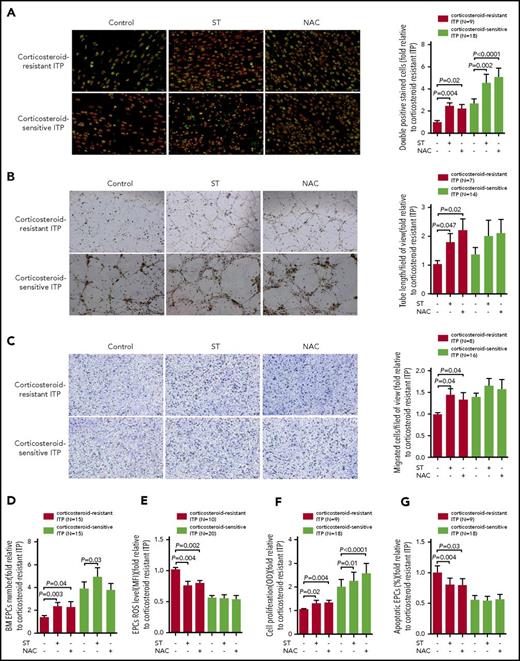
We first investigated the dose-response effect and found that atorvastatin (500 nM) and NAC (1 mM) demonstrated superior effects on the cultivated BM EPCs from corticosteroid-resistant ITP patients (supplemental Figure 1).
Atorvastatin (500 nM) or NAC (1 mM) significantly increased the quantity of BM EPCs, including the double-positive–stained cells (Figure 4A; 2.5-fold ± 0.3-fold, P = .004; 2.2-fold ± 0.4-fold, P = .02) and total number (Figure 4D; 2.3-fold ± 0.4-fold, P = .003; 2.3-fold ± 0.5-fold, P = .04) of BM EPCs, in corticosteroid-resistant ITP compared with untreated cells. Moreover, atorvastatin or NAC ameliorated the function of BM EPCs in corticosteroid-resistant ITP patients, including tube-formation capability (Figure 4B; 1.8-fold ± 0.3-fold, P = .047; 2.2-fold ± 0.4-fold, P = .02), migration capacity (Figure 4C; 1.4-fold ± 0.2-fold, P = .04; 1.3-fold ± 0.2-fold, P = .04), and proliferation rate (Figure 4F; 1.3-fold ± 0.1-fold, P = .02; 1.3-fold ± 0.1-fold, P = .004). Reduced levels of intracellular ROS (Figure 4E; 0.8-fold ± 0.1-fold, P = .004; 0.8-fold ± 0.1-fold, P = .002) and apoptosis (Figure 4G; 0.8-fold ± 0.1-fold, P = .004; 0.8-fold ± 0.1-fold, P = .03) were observed in atorvastatin or NAC treatment groups.
In vitro treatment with atorvastatin and NAC demonstrated superior effects on the number, proliferation, apoptosis, and ROS levels of cultivated BM EPCs from corticosteroid-resistant ITP patients compared with corticosteroid-sensitive ITP patients. The cultivated BM EPCs from corticosteroid-resistant ITP and corticosteroid-sensitive ITP patients were incubated with atorvastatin (ST; 500 nM) or NAC (1 mM). The effects of the different treatments on representative images (left panel) and quantification (right panel) of (A) double-positive–stained (merged in yellow) with DiI–Ac-LDL (red) and FITC–UEA-I (green) (per well) (original magnification ×10), (B) tube formation inspected on an inverted light microscope (original magnification ×4), and (C) the migrated cells (blue) which were fixed and stained with crystal violet (original magnification ×10) of cultivated BM EPCs from corticosteroid-resistant ITP patients and corticosteroid-sensitive ITP patients were compared at day 7 in culture. In addition, the (D) cell number (per well), (E) ROS levels, (F) cell proliferation, and (G) apoptosis of cultivated BM EPCs from corticosteroid-resistant ITP patients and corticosteroid-sensitive ITP patients were compared at day 7 in culture. The data were expressed as fold-change relative to corticosteroid-resistant ITP. The Wilcoxon test for paired data was used to identify the drug effects. All P values <.05 were considered significant and were provided in the figure. OD, optical density.
In vitro treatment with atorvastatin and NAC demonstrated superior effects on the number, proliferation, apoptosis, and ROS levels of cultivated BM EPCs from corticosteroid-resistant ITP patients compared with corticosteroid-sensitive ITP patients. The cultivated BM EPCs from corticosteroid-resistant ITP and corticosteroid-sensitive ITP patients were incubated with atorvastatin (ST; 500 nM) or NAC (1 mM). The effects of the different treatments on representative images (left panel) and quantification (right panel) of (A) double-positive–stained (merged in yellow) with DiI–Ac-LDL (red) and FITC–UEA-I (green) (per well) (original magnification ×10), (B) tube formation inspected on an inverted light microscope (original magnification ×4), and (C) the migrated cells (blue) which were fixed and stained with crystal violet (original magnification ×10) of cultivated BM EPCs from corticosteroid-resistant ITP patients and corticosteroid-sensitive ITP patients were compared at day 7 in culture. In addition, the (D) cell number (per well), (E) ROS levels, (F) cell proliferation, and (G) apoptosis of cultivated BM EPCs from corticosteroid-resistant ITP patients and corticosteroid-sensitive ITP patients were compared at day 7 in culture. The data were expressed as fold-change relative to corticosteroid-resistant ITP. The Wilcoxon test for paired data was used to identify the drug effects. All P values <.05 were considered significant and were provided in the figure. OD, optical density.
Furthermore, in vitro treatment with atorvastatin or NAC slightly influenced the number and function of BM EPCs from corticosteroid-sensitive ITP patients (Figure 4). These data suggest that BM EPCs from corticosteroid-resistant ITP patients are more sensitive to atorvastatin or NAC than those from corticosteroid-sensitive ITP patients.
Atorvastatin, NAC, and p38 inhibitors similarly affected the number and function of BM EPCs in corticosteroid-resistant ITP patients
Treatment with NAC and p38 inhibitors (SB203580 and BIRB796) increased the percentages (Figure 5A; 1.6-fold ± 0.2-fold, P = .006; 1.1-fold ± 0.1-fold, P = .5; 1.3-fold ± 0.2-fold, P = .3), double-positive–stained cells (Figure 5C; 3.6-fold ± 0.8-fold, P = .002; 3.9-fold ± 1.0-fold, P = .02; 3.1-fold ± 0.6-fold, P = .01), proliferation (Figure 5D; 1.5-fold ± 0.2-fold, P = .02; 1.4-fold ± 0.2-fold, P = .08; 1.6-fold ± 0.3-fold, P = .06), and migration (Figure 5F; 1.4-fold ± 0.1-fold, P = .0001; 1.1-fold ± 0.1-fold, P = .01; 1.1-fold ± 0.1-fold, P = .09) of BM EPCs in corticosteroid-resistant ITP patients. NAC and p38 inhibitors reduced ROS (Figure 5B; 0.5-fold ± 0.1-fold; P = .03; 0.5-fold ± 0.1-fold; P = .04; 0.5-fold ± 0.1-fold, P = .0498) and apoptosis levels (Figure 5E; 0.6-fold ± 0.1-fold, P = .002; 0.5-fold ± 0.1-fold, P = .002; 0.5-fold ± 0.1-fold, P = .004) of BM EPCs from corticosteroid-resistant ITP. These findings indicate that inhibiting ROS or p38 activation enhances the number and function of BM EPCs in corticosteroid-resistant ITP patients, similar to atorvastatin treatment.
Atorvastatin and NAC enhanced the quantity and function of cultivated BM EPCs from corticosteroid-resistant ITP patients via reducing p-p38 MAPK activity and inducing p-Akt activity. The cultivated BM EPCs from corticosteroid-resistant ITP patients were incubated with atorvastatin (ST; 500 nM), NAC (1 mM), SB203580 (10 μM), BIRB796 (400 nM), or ST (500 nM) combined with NAC (1 mM). The effects of the different treatments on (A) percentages (per well), (B) ROS levels, (C) double-positive–stained cells, (D) cell proliferation, (E) apoptosis, and (F) migration of cultivated BM EPCs from corticosteroid-resistant ITP patients were compared at day 7 in culture. (G) p-p38 and (H) p-Akt expression were analyzed by flow cytometry in the cultivated BM EPCs at day 7 in culture. (I) Representative western blots of p-p38, total p38, p-Akt, total Akt, and actin in the cultivated BM EPCs from corticosteroid-resistant ITP patients at day 7 in culture among the different treatments. The data were expressed as fold-change relative to corticosteroid-resistant ITP. Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure. BIRB796 and SB203580 indicate 2 kinds of p38 inhibitors.
Atorvastatin and NAC enhanced the quantity and function of cultivated BM EPCs from corticosteroid-resistant ITP patients via reducing p-p38 MAPK activity and inducing p-Akt activity. The cultivated BM EPCs from corticosteroid-resistant ITP patients were incubated with atorvastatin (ST; 500 nM), NAC (1 mM), SB203580 (10 μM), BIRB796 (400 nM), or ST (500 nM) combined with NAC (1 mM). The effects of the different treatments on (A) percentages (per well), (B) ROS levels, (C) double-positive–stained cells, (D) cell proliferation, (E) apoptosis, and (F) migration of cultivated BM EPCs from corticosteroid-resistant ITP patients were compared at day 7 in culture. (G) p-p38 and (H) p-Akt expression were analyzed by flow cytometry in the cultivated BM EPCs at day 7 in culture. (I) Representative western blots of p-p38, total p38, p-Akt, total Akt, and actin in the cultivated BM EPCs from corticosteroid-resistant ITP patients at day 7 in culture among the different treatments. The data were expressed as fold-change relative to corticosteroid-resistant ITP. Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure. BIRB796 and SB203580 indicate 2 kinds of p38 inhibitors.
Atorvastatin and NAC reduced p-p38 MAPK activity and enhanced p-Akt activity in BM EPCs from corticosteroid-resistant ITP patients
The higher p-p38 expression in BM EPCs from corticosteroid-resistant ITP patients decreased significantly following treatment with atorvastatin, NAC, p38 inhibitors, or atorvastatin/NAC cotreatment (Figure 5G; 0.8-fold ± 0.1-fold, P = .003; 0.8-fold ± 0.1-fold, P = .01; 0.8-fold ± 0.1-fold, P = .006; 0.7-fold ± 0.1-fold, P = .001; 0.5-fold ± 0.1-fold, P < .0001), whereas p-Akt was increased significantly in the different treatment groups (Figure 5H; 2.5-fold ± 0.3-fold, P = .0002; 2.7-fold ± 0.3-fold, P < .0001; 2.0-fold ± 0.3-fold, P = .04; 2.4-fold ± 0.4-fold, P = .004; 2.9-fold ± 0.5-fold, P = .0005), which is consistent with the western blot analysis results (Figure 5I).
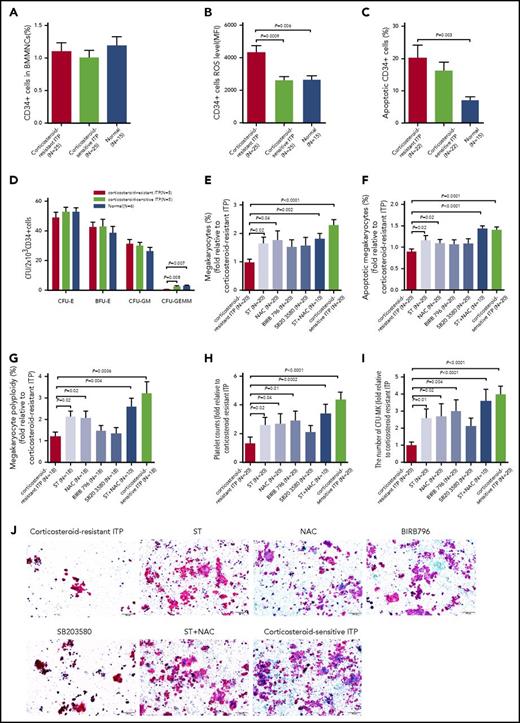
Increased ROS levels and defective CFU-plating efficiency in BM CD34+ cells from corticosteroid-resistant ITP patients
The levels of intracellular ROS in primary BM CD34+ cells derived from corticosteroid-resistant ITP patients were significantly higher than those in corticosteroid-sensitive ITP patients (Figure 6B; 4350 ± 415.3 vs 2590 ± 238; P = .0009), whereas no significant differences in the cell counts (Figure 6A) or apoptosis rates (Figure 6C) of BM CD34+ cells were found between the 2 groups.
Atorvastatin and NAC improved megakaryocytopoiesis in BM CD34+cells cocultured with BM EPCs from corticosteroid-resistant ITP patients. Even though the (A) percentages of BM CD34+ cells among corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls showed no significant differences, the (B) intracellular ROS levels and (C) apoptosis rates of BM CD34+ cells were increased in the corticosteroid-resistant ITP group. (D) The CFU-plating efficiency of BM CD34+ cells from corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls was analyzed. The megakaryocyte production, maturation, platelet release, and CFU-MK–plating efficiencies in BM CD34+ cells were analyzed after coculture with the differently treated BM EPCs from corticosteroid-resistant ITP. The (E) percentages of megakaryocytes, (F) megakaryocyte apoptosis, (G) megakaryocyte ploidy distribution, (H) platelet release, and (I-J) CFU-MK counts were analyzed after 12 days of coculture. CFU-MKs were stained with mouse anti–human GPIIb/IIIa antibody and biotin-conjugated goat anti–mouse IgG, then counterstained with Evans Blue. CFU-MK colonies were defined as groups of 3 or more GPIIb/IIIa-positive cells (pink membranes with blue nuclei) (scale bars, 200 μm). The data were expressed as fold change relative to corticosteroid-resistant ITP. Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure. ST, atorvastatin.
Atorvastatin and NAC improved megakaryocytopoiesis in BM CD34+cells cocultured with BM EPCs from corticosteroid-resistant ITP patients. Even though the (A) percentages of BM CD34+ cells among corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls showed no significant differences, the (B) intracellular ROS levels and (C) apoptosis rates of BM CD34+ cells were increased in the corticosteroid-resistant ITP group. (D) The CFU-plating efficiency of BM CD34+ cells from corticosteroid-resistant ITP, corticosteroid-sensitive ITP, and healthy controls was analyzed. The megakaryocyte production, maturation, platelet release, and CFU-MK–plating efficiencies in BM CD34+ cells were analyzed after coculture with the differently treated BM EPCs from corticosteroid-resistant ITP. The (E) percentages of megakaryocytes, (F) megakaryocyte apoptosis, (G) megakaryocyte ploidy distribution, (H) platelet release, and (I-J) CFU-MK counts were analyzed after 12 days of coculture. CFU-MKs were stained with mouse anti–human GPIIb/IIIa antibody and biotin-conjugated goat anti–mouse IgG, then counterstained with Evans Blue. CFU-MK colonies were defined as groups of 3 or more GPIIb/IIIa-positive cells (pink membranes with blue nuclei) (scale bars, 200 μm). The data were expressed as fold change relative to corticosteroid-resistant ITP. Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure. ST, atorvastatin.
As shown in Figure 6D, the BM CD34+ cells from corticosteroid-resistant ITP patients had a deficit in CFU-GEMM (0.6 ± 0.3 vs 2.7 ± 0.3; P = .008) outgrowth at a baseline level compared with corticosteroid-sensitive ITP patients, whereas the CFU-E, BFU-E, and CFU-GM outgrowth showed no significant differences.
Atorvastatin improved CFU-MK–plating efficiency in BM CD34+ cells and promoted megakaryocytopoiesis when cocultured with BM EPCs from corticosteroid-resistant ITP patients
Treatment with atorvastatin, NAC, p38 inhibitors, or atorvastatin/NAC cotreatment improved the percentages of megakaryocytes (Figure 6E; 1.6-fold ± 0.2-fold, P = .02; 1.8-fold ± 0.3-fold, P = .04; 1.6-fold ± 0.3-fold, P = .27; 1.5-fold ± 0.3-fold, P = 0.23; 1.8-fold ± 0.2-fold, P = .002), megakaryocyte apoptosis (Figure 6F; 1.2-fold ± 0.1-fold, P = .02; 1.1-fold ± 0.1-fold, P = .02; 1.1-fold ± 0.1-fold, P = .1; 1.1-fold ± 0.1-fold, P = 0.16; 1.4-fold ± 0.1-fold, P < .0001), megakaryocyte ploidy distribution (Figure 6G; 2.1-fold ± 0.3-fold, P = .02; 2.2-fold ± 0.4-fold, P = .02; 1.3-fold ± 0.3-fold, P = 0.87; 1.5-fold ± 0.3-fold, P = .61; 2.6-fold ± 0.4-fold, P = .004). and platelet release (Figure 6H; 2.6-fold ± 0.6-fold, P = .02; 2.7-fold ± 0.7-fold, P = .04; 2.1-fold ± 0.5-fold, P = .35; 2.9-fold ± 0.7-fold, P = .01; 3.4-fold ± 0.6-fold, P = .0002) of BM EPCs from corticosteroid-resistant ITP patients to support CD34+ hematopoietic progenitors from healthy donors. Moreover, the CFU-MK–plating efficiency (Figure 6I; 2.6-fold ± 0.6-fold, P = .01; 2.7-fold ± 0.7-fold, P = .02; 2.1-fold ± 0.5-fold, P = 0.23; 3.0-fold ± 0.6-fold, P = .004; 3.6-fold ± 0.7-fold, P < .0001) from the intervention groups were significantly higher than those in the vehicle-treated control group. These data suggest that atorvastatin, NAC, or p38 inhibitors could improve the ability of the impaired BM EPCs from corticosteroid-resistant ITP patients to support HSC differentiation into megakaryocytes and ultimately to platelet production, as determined by the CFU-MK–plating efficiency in vitro. Notably, atorvastatin and NAC provided markedly better repair capacity to BM EPCs from corticosteroid-resistant ITP patients than did the p38 inhibitors.
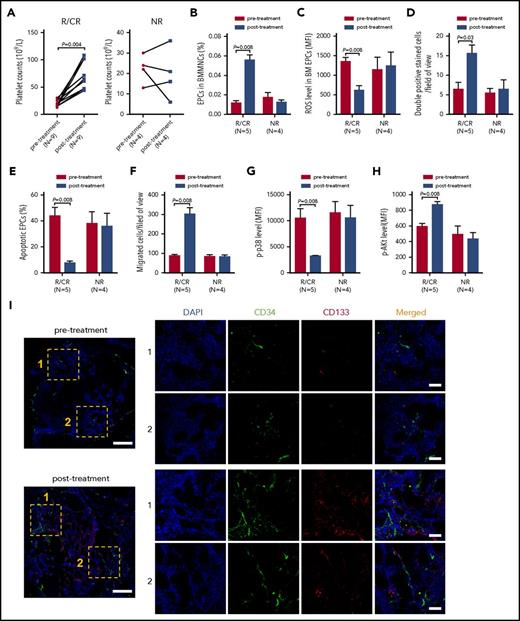
Clinical responses of atorvastatin and NAC in corticosteroid-resistant ITP patients
The CR, response, and OR rates of atorvastatin and NAC treatment were 23.1% (3 of 13), 46.2% (6 of 13), and 69.2% (9 of 13) in corticosteroid-resistant ITP patients. In patients who achieved CR/R, the median time to response was 25 days (7-51 days), and no apparent adverse events were observed. The platelet counts in corticosteroid-resistant ITP patients who achieved CR/response (N = 9) were significantly increased posttreatment compared with pretreatment (Figure 7A; 61 × 109/L vs 21 × 109/L; P = .004).
In vivo treatment with atorvastatin and NAC improved the number and function of either precultivated or cultivated BM EPCs from corticosteroid-resistant ITP patients. The effects of atorvastatin and NAC treatment on the (A) platelet counts, (B) percentages, (C) ROS levels, (D) double-positive–stained cells (per well), (E) apoptosis rates, (F) migration, (G) p-p38, and (H) p-Akt of BM EPCs were compared pre- and posttreatment from corticosteroid-resistant ITP patients who achieved response (R) or CR (left panel) and NR (right panel). (I) Mouse anti-human CD34 (green) and rabbit anti-human CD133 (red) were incubated to identify the BM EPCs. DAPI (blue) was used to stain the nucleus. In situ histological analyses of the BMBs showed that the frequency of the double-positive–stained (merged in yellow) EPCs with CD34 (green) and CD133 (red) was increased in corticosteroid-resistant ITP patients posttreatment compared with pretreatment according to immunofluorescent staining (scale bars, 50 μm). Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure. ST, atorvastatin.
In vivo treatment with atorvastatin and NAC improved the number and function of either precultivated or cultivated BM EPCs from corticosteroid-resistant ITP patients. The effects of atorvastatin and NAC treatment on the (A) platelet counts, (B) percentages, (C) ROS levels, (D) double-positive–stained cells (per well), (E) apoptosis rates, (F) migration, (G) p-p38, and (H) p-Akt of BM EPCs were compared pre- and posttreatment from corticosteroid-resistant ITP patients who achieved response (R) or CR (left panel) and NR (right panel). (I) Mouse anti-human CD34 (green) and rabbit anti-human CD133 (red) were incubated to identify the BM EPCs. DAPI (blue) was used to stain the nucleus. In situ histological analyses of the BMBs showed that the frequency of the double-positive–stained (merged in yellow) EPCs with CD34 (green) and CD133 (red) was increased in corticosteroid-resistant ITP patients posttreatment compared with pretreatment according to immunofluorescent staining (scale bars, 50 μm). Subject variables were compared using the Mann-Whitney U test for continuous variables. All P values <.05 were considered significant and were provided in the figure. ST, atorvastatin.
Compared with the baseline levels before atorvastatin and NAC treatment, the quantity and function of BM EPCs and CD34+ cells were improved to some degree in the patients who showed CR/response after treatment when evaluated by flow cytometry and immunofluorescence (Figure 7B-I). By contrast, no significant improvement was found in the NR patients posttreatment when compared with their baseline levels pretreatment (Figure 7B-H).
Discussion
The current study provided the first demonstration that reduced and dysfunctional BM EPCs, characterized by decreased migration and angiogenesis, as well as higher levels of ROS and apoptosis, were involved in the pathogenesis of corticosteroid-resistant ITP. Moreover, atorvastatin quantitatively and functionally improved BM EPCs derived from corticosteroid-resistant ITP patients by downregulating p38 MAPK and upregulating Akt pathway; atorvastatin also partially rescued the impaired BM EPCs to support megakaryocytopoiesis. Although requiring further validation, our preliminary data suggest that atorvastatin is a promising therapeutic strategy in corticosteroid-resistant ITP patients.
Abnormal megakaryocyte maturation and apoptosis in BM and increased peripheral platelet destruction have been reported to be involved in the occurrence of primary ITP.1,28,44-49 BM EPCs, which have been identified as a critical element of the BM microenvironment, possess the capacity to support hematopoiesis and megakaryocytopoiesis.25,50 In this regard, murine studies suggested that the effective cross-talk between EPCs and megakaryocytes regulates megakaryocyte maturation and thrombopoiesis in BM microenvironment.25,50 Moreover, disruption of the BM EPCs or interference with megakaryocyte motility was found to inhibit thrombopoiesis, both under physiological conditions and after myelosuppression.24,26 However, no previous study has focused on the number and functional role of BM EPCs in corticosteroid-resistant ITP patients.
BM EPCs were found quantitatively and functionally impaired in corticosteroid-resistant ITP patients, which is consistent with the defective BM EPCs in PT patients post allo-HSCT.27 In contrast, Song et al reported that the frequency of BM EPCs was normal in corticosteroid-sensitive ITP patients, whereas dysregulated T-cell responses in the BM microenvironment were involved in corticosteroid-sensitive ITP.28 These findings might explain why corticosteroid-resistant ITP patients exhibit either no response to corticosteroids or are corticosteroid-dependent, indicating that immune-mediated platelet destruction is not the sole pathogenic mechanism. Considering the predominant role of EPCs in supporting megakaryocytopoiesis in the BM microenvironment,24,25 as well as the current study, it is conceivable that impaired BM EPCs might hamper the differentiation progress from HSCs to megakaryocytes, resulting in the occurrence of corticosteroid-resistant ITP, which is different from the immune-mediated mechanism in corticosteroid-sensitive ITP.28,38 Although more corticosteroid-resistant ITP patients received danazol or cyclosporine A as prior and concomitant therapies, no harmful effects were reported about danazol and cyclosporine A on EPC biology.51,52 Therefore, we speculated that the BM EPCs differences between the 2 groups of ITP patients might be due to their disease biology rather than, for example, treatment effects. Consequently, therapeutic strategies to enhance the prognosis of corticosteroid-resistant ITP patients through improving BM EPCs are urgently needed.
Direct infusion of circulating EPCs can improve endothelial repair, significantly increase BM HSCs, and ultimately accelerate hematopoietic and immune reconstitution after allo-HSCT in mice.53 However, the critical limitation for the therapeutic application of EPC infusion in humans is their low number in circulation. In contrast, atorvastatin showed minimal toxic effects, can be easily administered orally, and is also affordable. We recently reported that atorvastatin represents a promising therapeutic approach for repairing impaired BM EPCs in PGF patients through downregulating the p38 MAPK pathway. Different from our previous study in PGF,38 atorvastatin not only quantitatively and functionally improved BM EPCs from corticosteroid-resistant ITP patients by downregulating p38 MAPK and upregulating Akt pathway, but also partially rescued the impaired ability of BM EPCs to support megakaryocytopoiesis. Although the underlying molecular mechanisms are needed to further explore, the repair of endogenous BM EPCs via exogenous agents represents a potential therapeutic approach in corticosteroid-resistant ITP patients through regulating megakaryocytopoiesis.
We are aware, however, that the pathogenesis of corticosteroid-resistant ITP is heterogeneous. It would be interesting and informative to further investigate whether the impaired BM EPCs directly affect megakaryocytopoiesis or act through their immunoregulatory effects in the occurrence of corticosteroid-resistant ITP. Moreover, atorvastatin might have effects on many other cells in corticosteroid-resistant ITP patients, which needs to be further clarified.
In summary, these results indicate that reduced and dysfunctional BM EPCs play a role in the pathogenesis of corticosteroid-resistant ITP, and the impaired BM EPCs could be improved by atorvastatin. Our preliminary data indicate that atorvastatin represents a promising therapeutic approach for repairing impaired BM EPCs in corticosteroid-resistant ITP patients. We are aware, however, that further prospective large-scale randomized clinical trials, particularly in other ethnicities, are needed to validate our findings in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the core facilities at Peking University Institute of Hematology for sample collection. The authors thank Cai-Wen Duan from Shanghai Jiao Tong University School of Medicine for excellent assistance with the immunofluorescent staining. American Journal Experts (www.journalexperts.com) provided editorial assistance to the authors during the preparation of the manuscript.
This work was supported by the National Natural Science Foundation of China (grant nos. 81570127, 81370638, and 81530046), the Science and Technology Project of the Guangdong Province of China (2016B030230003), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81621001), and the National Key Research and Development Program (2017YFA0104500).
Authorship
Contribution: X.-J.H. and Y.K. designed the study and supervised the manuscript preparation; Y.K., X.-N.C., and X.-H.Z. performed the research; Y.K. and X.-N.C. analyzed the data and wrote the manuscript; M.-M.S., Y.-Y.L., Y.W., L.-P.X., and Y.-J.C. participated in the collection of patients’ data; and all authors agreed to submit the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Jun Huang, Peking University People's Hospital, Peking University Institute of Hematology, No. 11 Xizhimen South St, Beijing, 100044, China; e-mail: xjhrm@medmail.com.cn.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal