Abstract
Despite the fact that autoantibodies to a variety of antigens have been described in patients with the antiphospholipid syndrome (APS), it is now generally accepted that β2glycoprotein I (β2GPI) is a primary target of pathogenic autoantibodies in APS patients. Unraveling the immunodominant epitope of β2GPI at the molecular/conformational level may help to understand the disease and develop specific therapies. β2GPI is an abundant plasma protein that, upon interaction with negative phospholipids, has been shown to undergo a conformational change resulting in the exposure of a pathogenic epitope on domain I. Although the conformation of β2GPI has been previously reported by small angle X-ray scattering (SAXS), we are the first to show the interaction with autoimmune anti-β2GPI antibodies. We previously showed that antibodies towards the cryptic domain I epitope are highly associated with both thrombosis and pregnancy morbidity. Previous studies indicated that amino acids R39-R43 and the interlinking region between domains I and II are part of this cryptic epitope. However, these studies used point-mutated variants of β2GPI, and the results could be influenced by structural changes compared to native β2GPI.
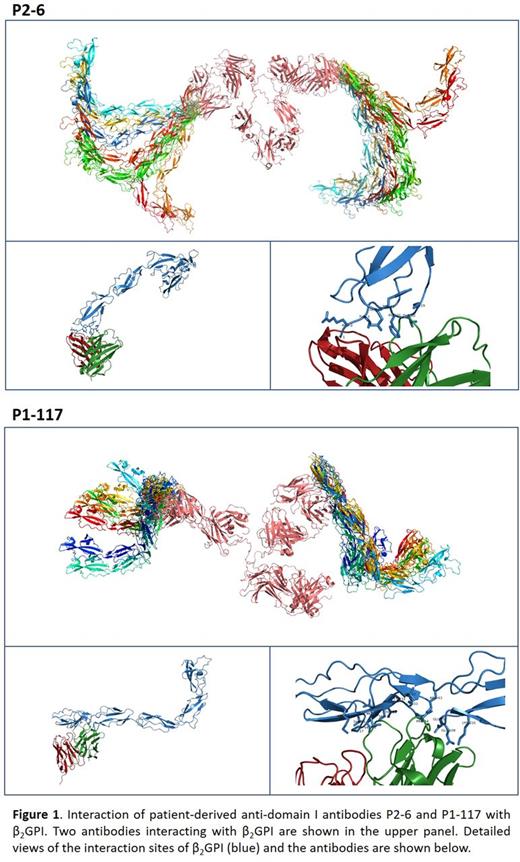
In this study, we visualized the conformation of β2GPI in solution by SAXS and its interaction with two anti-domain I antibodies, obtained via B cell isolation from two APS patients. Antibody P2-6 previously showed affinity for domain I regardless of the conformation of β2GPI, whereas antibody P1-117 only recognized domain I of β2GPI upon binding to anionic phospholipids. Via point-mutated variants of β2GPI we previously demonstrated that P1-117 is reactive against the cryptic domain I epitope including epitope G40-R43. In a first set of SAXS experiments, we visualized plasma purified β2GPI in its native conformation. We found that, due to the flexibility of domain I-III of β2GPI, β2GPI is able to adapt into several different conformations ranging from a sort of circular shape into a S shape, confirming results of previous studies. Upon increasing the pH, the conformation of β2GPI changed into a more erect fish-hook conformation resembling the crystal structure of β2GPI. The three-dimensional structures of both antibodies were determined by SAXS and allowed to react with β2GPI in the open conformation (Figure 1). Antibody P2-6 interacted mainly with the amino terminal end of domain I of β2GPI which is available in both the native and open conformations of β2GPI. In contrast, antibody P1-117 interacted with a broader part of the protein, identifying a discontinuous and conformational epitope covering domain I, the interlinking region and a small part of domain II. Importantly, (part of) the epitope is not available in the native closed conformation of β2GPI due to its interaction with domain V (circular conformation) or due to a carbohydrate chain positioned on the interface of domain I-II (S shaped conformation). These data confirm the importance of the conformation of β2GPI coated on the solid phase of diagnostic anti-β2GPI assays to avoid false negative classification of APS patients.
In conclusion, our findings contribute to elucidating the conformation of β2GPI in solution and clearly indicate that pathogenic autoantibodies bind a discontinuous conformational epitope. Identification of this conformational epitope will allow optimization of peptide-based therapies and also epitope-specific diagnostic assays that will enable the stratification of patients according to their risk on thrombosis/pregnancy morbidity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal