Abstract
BACKGROUND:
Proteasome inhibitor (PI) triplet combinations with lenalidomide/dexamethasone (Rd) have demonstrated superior efficacy vs Rd in clinical trials of RRMM pts with 1-3 prior lines of therapy [Stewart NEJM 2015;Moreau NEJM 2015]. The study populations in these recent phase 3 studies applied different eligibility criteria leading to challenges in comparisons across trials. In addition, actual adoption of these regimens in practice may depend on patient, disease and other contextual factors. Effectiveness data for these regimens in clinical practice are needed to complement trial-based results and help inform patient care. The aim of this study wasto evaluate TTNT, a proxy for progression free survival (PFS), and DOT in a cohort of RRMM pts initiating KRd, VRd, or IRd.
METHODS: In this retrospective cohort study, pts who initiated KRd, VRd or IRd (index regimen) in line 2, 3, or 4 were followed between 1/2008 - 12/2016 in Humedica, a large national electronic medical records (EMR) database. Subsequent lines of therapy after 1st line were defined as a switch in regimen, or retreatment after at least a 6 month interval. Maintenance therapy with bortezomib, lenalidomide, or Rd continuation was considered part of 1st line. Baseline data were collected, and where available (30.7% pts), cytogenetic information was extracted using natural language processing from free text in the EMR. High cytogenetic risk was defined as presence of del[17p], t[4;14], t[14;16] and 1q21 gain. Kaplan-Meier analyses were performed to estimate DOT from start to end of index regimen, and separately, the PI component of the index regimen; and TTNT from start of index regimen to start of next line of therapy or death. Observations were censored at time of loss to follow up/end of study period (12/2016).
RESULTS: Among 343 VRd, 139 KRd and 49 IRd pts, median age was: 69, 65 and 73 years (p<.001) and median time since diagnosis was 14.2, 17.7, and 34.8 mos. A higher proportion of pts in the KRd group received a prior transplant (32%), followed by VRd (22%), and IRd (18%), p=.07. Majority of VRd (76%) and KRd (58%) regimens were in 2nd line; IRd was administered predominantly in 3-4th line (59%; p<.001). VRd and KRd groups had a higher incidence of CRAB symptoms (hypercalcemia, renal insufficiency, anemia, bone disease) vs IRd (81% and 86% vs 67%, respectively; p=.01). More pts had high risk cytogenetic disease in the KRd (28%) and IRd (22%) vs VRd (12%) group (p<.001). Charlson Comorbidity Index score was comparable (median: 2 in all 3 groups, p=0.56).
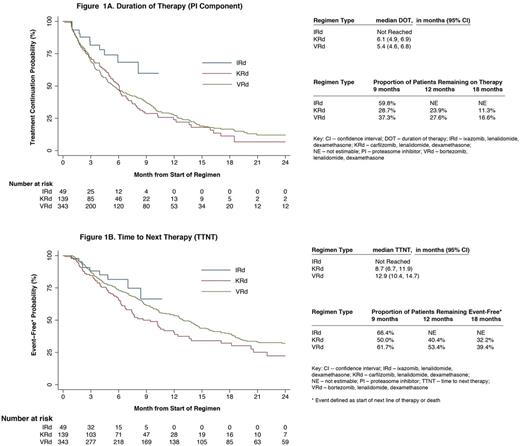
Median follow up was: 17.3 (VRd), 8.3 (KRd) and 5.2 (IRd; p<.001) mos. Median DOT (mos) for PI component alone and for entire regimen was: 5.4 and 8.7 (VRd), 6.1 and 6.3 (KRd) and not reached for both (NR; IRd). Discontinuation rates for PI component of VRd, KRd and IRd at 9 mos were: 63%, 71% and 40%; at 12 mos: 72%, 76% and NR, and at 18 mos: 83%, 89% and NR, respectively. Median TTNT was: 12.9 (VRd), 8.7 (KRd) and NR (IRd) mos (figure).
CONCLUSIONS:
Compared to injectable PI-based Rd triplets, pts selected for treatment with IRd in clinical practice were generally older, less likely to have undergone a transplant, treated in later lines, and had a lower incidence of CRAB symptoms. In unadjusted analyses, the IRd treated pts appear to have lower probability of treatment discontinuation and longer TTNT compared to injectable PI-based Rd triplets. A limitation of this analysis is a shorter follow-up among the IRd and KRd groups due to more recent approvals of these regimens. Longer follow up is needed to confirm these results.
In contrast to the phase 3 clinical trial (median monthly cycles: 22 KRd [Stewart NEJM 2015]), pts treated with KRd in clinical practice were less likely to maintain therapy to the same extent (median DOT: 6.3 mos), with only 11% remaining on carfilzomib at 18 mos. The DOT with VRd was more congruent between trial and clinical practice (median DOT: 8.0 vs 8.7 mos [Richardson Blood 2014]). While the PFS in the KRd clinical trial was 26.3 mos [Stewart NEJM 2015]), the median TTNT in clinical practice was only 8.7 mos. This discrepancy highlights the known limited generalizability of clinical trial findings to pts treated in clinical care [Shermann NEJM 2016]. To increase the external validity of trial findings, future trials must not only enroll pts who are more representative of the target population but also use regimens that are safe and feasible to use for prolonged periods of time in such pts.
Chari: Janssen: Consultancy, Research Funding; Celgene Corporation: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Amgen: Honoraria, Research Funding; Millennium Pharmaceuticals, Inc.: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding. Romanus: Takeda Pharmaceuticals: Employment. Luptakova: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Raju: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Consultancy; Xcenda: Employment. Farrelly: Xcenda: Employment; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Consultancy. Blazer: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Consultancy; Xcenda: Employment. Skacel: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Hari: Sanofi: Honoraria, Research Funding; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy; Janssen: Honoraria; Takeda Pharmaceuticals: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Spectrum: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal