Key Points
Deficiency of coagulation factor X in zebrafish results in a severe hemostatic defect that is surprisingly well-tolerated until adulthood.
In vivo analysis of human mutations in zebrafish identifies variants underlying symptomatic factor X deficiency.
Abstract
Deficiency of factor X (F10) in humans is a rare bleeding disorder with a heterogeneous phenotype and limited therapeutic options. Targeted disruption of F10 and other common pathway factors in mice results in embryonic/neonatal lethality with rapid resorption of homozygous mutants, hampering additional studies. Several of these mutants also display yolk sac vascular defects, suggesting a role for thrombin signaling in vessel development. The zebrafish is a vertebrate model that demonstrates conservation of the mammalian hemostatic and vascular systems. We have leveraged these advantages for in-depth study of the role of the coagulation cascade in the developmental regulation of hemostasis and vasculogenesis. In this article, we show that ablation of zebrafish f10 by using genome editing with transcription activator-like effector nucleases results in a major embryonic hemostatic defect. However, widespread hemorrhage and subsequent lethality does not occur until later stages, with absence of any detectable defect in vascular development. We also use f10−/− zebrafish to confirm 5 novel human F10 variants as causative mutations in affected patients, providing a rapid and reliable in vivo model for testing the severity of F10 variants. These findings as well as the prolonged survival of f10−/− mutants will enable us to expand our understanding of the molecular mechanisms of hemostasis, including a platform for screening variants of uncertain significance in patients with F10 deficiency and other coagulation disorders. Further study as to how fish tolerate what is an early lethal mutation in mammals could facilitate improvement of diagnostics and therapeutics for affected patients with bleeding disorders.
Introduction
Coagulation factor X (F10) is a vitamin K–dependent plasma glycoprotein that is one of the pivotal factors in the coagulation cascade. F10 consists of 8 domains, is synthesized in the liver as a single-chain precursor, and circulates in human plasma.1,2 The conversion of F10 from zymogen to its active form (F10a) is triggered by the tenase complex and is important for both the initiation and propagation of coagulation.3-5 Activated F10 participates in the formation of the prothrombinase complex, catalyzing the conversion of prothrombin to thrombin, which is a critical step for normal physiologic hemostasis.6
Deficiency of F10 leads to a rare, inherited autosomal recessive coagulopathy, with the most severe bleeding arising early in life.2,7 It represents 10% of rare bleeding disorders and occurs in 1 per 500 000 to 1 000 000 of the general population.2,7-10 The clinical symptoms of homozygotes range from mild to severe hemorrhage, and currently >100 naturally occurring mutations in F10 have been identified, among which 78% are missense.2,7 Most reported mutations have been found in the N-terminal light chain, particularly the γ-carboxyglutamic acid (Gla) domain, or the C-terminal heavy chain containing the catalytic domain.7 The vast majority of these mutations remain relatively uncharacterized. Current treatment includes the administration of plasma or prothrombin complex concentrates containing F10 and other vitamin K–dependent factors. Additional therapies for the control of bleeding are still needed because the current options are limited.9
Previous studies that use gene targeting in mice have facilitated understanding of F10 and common pathway function, but were impeded by embryonic and neonatal lethality in homozygous mutants.11-14 These studies also suggested a role for thrombin signaling in embryonic vascular development, but this role has been technically difficult to confirm.15 Thus, we have turned to the zebrafish model (Danio rerio) with advantages that include high fecundity, external and transparent development, ease of manipulation and maintenance, accessibility of genetic techniques and imaging, as well as extensive conservation of the hemostatic and vascular systems as we and others have demonstrated.16-30
In this article, we report targeted mutagenesis of zebrafish f10 through genome editing with transcription activator-like effector nucleases (TALENs). We find that loss of F10 results in late-onset lethality by 4 months of age secondary to intracranial and intra-abdominal hemorrhage, without any evidence of a vascular defect. Severe, overt hemorrhage does not occur until ∼1 month of age despite the absence of hemostasis as early as 3 days postfertilization (dpf). We also use our model as an in vivo system to evaluate human F10 variants of uncertain significance (VUS) from 5 patients with symptomatic F10 deficiency whose causative mutations were not yet proven. Our studies demonstrate that loss of zebrafish F10 results in phenotypes similar to those of mice and humans, yet with surprising tolerance to what is a severe insult in mammals. This implies species-specific differences that, if understood, could suggest novel mechanisms for controlling hemorrhage in patients with and without bleeding disorders.
Methods
Zebrafish strains and maintenance
The f10 mutants were generated on a hybrid background of wild-type strains AB and TL (ABxTL). Developmental stages were defined as follows: embryo (0-2 dpf), larva (3-29 dpf), juvenile (30-89 dpf), and adult.31 Pigmentation was inhibited with 0.2 mM phenylthiourea (Sigma-Aldrich).32 All animal experiments were in accordance with guidelines approved by the University of Michigan Animal Care & Use Committee.
Targeted mutagenesis of the f10 locus by using genome-editing nucleases
The f10 locus was identified in the zebrafish genomic sequence assembly,16 and TALEN-mediated genome editing was used to induce mutations in exon 5. TALEN constructs were prepared by using fast ligation-based automatable solid-phase high-throughput assembly33 and were validated as described.22,34 TALEN messenger RNAs (mRNAs) were synthesized and injected into the cytoplasm of 1-cell stage embryos. The resulting F0 population was raised to adulthood and crossed to wild-type fish to confirm germ-line transmission to the F1 generation.
Genotyping of mutant offspring
Staged zebrafish were anesthetized in tricaine (0.16 mg/mL, Western Chemical) and fin-clipped for survival studies35,36 or humanely killed in high-dose tricaine (1.6 mg/mL) followed by genotyping of tail biopsies or whole fish. Genomic DNA was isolated by using lysis buffer (10 mM Tris-Cl, pH 8.0; 2 mM EDTA, 2% Triton X-100, and 100 µg/mL proteinase K) at 55°C overnight.22 Proteinase K was inactivated by heating for 5 minutes at 95°C. Polymerase chain reaction (PCR) was performed by using gene-specific primers (supplemental Table 1, available on the Blood Web site).
Laser-mediated endothelial injury in zebrafish larvae
Laser-mediated injury was performed on the endothelium of the posterior cardinal vein (PCV) of larvae as previously described.22,37,38 Three dpf larvae were anesthetized, embedded in agarose (0.8%), and the PCV endothelium was ablated with a laser at the 5th somite distal to the anal pore (MicroPoint Pulsed Laser System, Andor Technology). The time to complete occlusion was recorded by an observer blinded to genotype for up to 2 minutes. After the ablation, larvae were recovered from agarose and genotyped.
Chemical treatment of embryos
Embryos were incubated in 100 mM ε-aminocaproic acid (Sigma-Aldrich) or 10 µg/mL warfarin (Sigma-Aldrich) dissolved in system water or E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4), respectively, at 1 dpf. The former was evaluated at 3 dpf by laser-mediated endothelial injury, and the latter were stained at 7 dpf using o-dianisidine for the detection of hemoglobin.39,40
Phylogenetic analysis
F10 protein sequences were retrieved from National Center for Biotechnology Information, European Molecular Biology Laboratory, and Ensembl genome databases. Sequence alignments were performed by using ClustalW2, and phylogenetic trees constructed by the neighbor-joining method.
Statistical analysis
Statistical analysis was performed by using the Mann-Whitney U or 2-tailed Student t tests. Survival curves were generated by using Prism (GraphPad Software) and evaluated by log-rank (Mantel-Cox) testing for significance.
Results
Spatiotemporal expression profiles of f10 in developing zebrafish
The predicted protein sequence of zebrafish F10 was compared with various species for phylogenetic analysis. The resulting tree indicated that the zebrafish F10 protein was more closely related to that of chickens than mice or humans (Figure 1A). We analyzed the expression of zebrafish f10 mRNA during embryonic and early larval development by reverse transcription (RT)–PCR and whole-mount in situ hybridization (WISH). Expression of f10 was detected in embryos as early as the 1-cell stage and in larvae at up to 120 hours postfertilization (hpf) (Figure 1B). Transcription of f10 at the 1- and 4-cell stages indicates maternal contribution because zygotic expression does not begin until 2.75 hpf.31 At 4 hpf, both maternal and zygotic expression appeared to be present because f10 was strongly transcribed compared with earlier and later stages (Figure 1B), suggesting crossover from maternal to zygotic f10 transcription. From 24 hpf on, f10 was found to be transcribed at relatively stable levels up to 120 hpf (Figure 1B). WISH and histological analysis of zebrafish larvae at 120 hpf revealed that f10 expression is localized to the brain, jaw, and pharyngeal arches (Figure 1C-F, H-I) in addition to the otic vesicle, liver, and yolk syncytial layer (Figure 1D-E, G, J-L). Our data also showed that f10 was transcribed much more strongly in the liver than other tissues (Figure 1D-L).
Spatiotemporal expression of f10 in the developing zebrafish. (A) Phylogenetic tree of F10 from zebrafish (Danio), mouse (Mus), chicken (Gallus), and human (Homo). (B) RT-PCR from whole embryos demonstrates that zebrafish f10 mRNA is expressed during early embryonic development beginning at the 1-cell stage. Expression is relatively stable from 24 through 120 hpf. The expression of elongation factor 1-α (elfa) was used as an internal control. (C-L) WISH analysis of 120-hpf larvae shows that f10 expression is strong in the liver (D), but relatively weak in the yolk syncytial layer and brain (D-F), otic vesicles (E, G), and arches (H-I) by using an antisense probe (f10-as). (C) A sense control (f10-s) did not show any expression. (J-L) Plastic sections (5 µm) of stained larvae in transverse (J), sagittal (K), and coronal (L) planes are shown. Anterior is toward the left in panels C-H and toward the top in panel I. Scale bars (C-L), 100 µm. b, brain; ch, ceratohyal [2nd visceral pharyngeal arch (VA)]; li, liver; mc, mandibular cartilage (1st VA); ov, otic vesicle; sb, swim bladder; y, yolk; ysl, yolk syncytial layer.
Spatiotemporal expression of f10 in the developing zebrafish. (A) Phylogenetic tree of F10 from zebrafish (Danio), mouse (Mus), chicken (Gallus), and human (Homo). (B) RT-PCR from whole embryos demonstrates that zebrafish f10 mRNA is expressed during early embryonic development beginning at the 1-cell stage. Expression is relatively stable from 24 through 120 hpf. The expression of elongation factor 1-α (elfa) was used as an internal control. (C-L) WISH analysis of 120-hpf larvae shows that f10 expression is strong in the liver (D), but relatively weak in the yolk syncytial layer and brain (D-F), otic vesicles (E, G), and arches (H-I) by using an antisense probe (f10-as). (C) A sense control (f10-s) did not show any expression. (J-L) Plastic sections (5 µm) of stained larvae in transverse (J), sagittal (K), and coronal (L) planes are shown. Anterior is toward the left in panels C-H and toward the top in panel I. Scale bars (C-L), 100 µm. b, brain; ch, ceratohyal [2nd visceral pharyngeal arch (VA)]; li, liver; mc, mandibular cartilage (1st VA); ov, otic vesicle; sb, swim bladder; y, yolk; ysl, yolk syncytial layer.
Targeted inactivation of the f10 gene by using genome-editing nucleases results in spontaneous hemorrhage and lethality in juveniles and adults
To create a model of f10 deficiency, we ablated the zebrafish f10 gene by using genome editing TALENs. We designed a TALEN pair targeting the 5th exon of f10 (Figure 2A), and mRNAs encoding these nucleases were injected into ABxTL wild-type 1-cell embryos. The resulting F0 generation was raised and outcrossed to ABxTL to produce the F1 generation. We identified a mutant line with germ-line transmission of a 17-bp deletion in exon 5, resulting in a frameshift and downstream nonsense mutation (Figure 2B). Quantitative PCR analysis was not able to detect transcription of f10 in 3 dpf homozygous mutant larvae in comparison with f10+/+ and f10+/− siblings (P = .0001; Figure 2C), presumably due to nonsense-mediated decay.41 WISH demonstrated no expression of f10 mRNA in homozygous mutant larvae (Figure 2D). Transcription of other coagulation factors downstream of F10 was distinctly altered in a dose dependent fashion (supplemental Figure 1). This included increases in f10 homozygous mutants of 1.8- and 2.3-fold for fibrinogen α (fga) and antithrombin III (at3) mRNAs, respectively (P < .05; supplemental Figure 1), when compared with wild-type siblings. Prothrombin (f2) transcription was slightly decreased, although the effect was not statistically significant (supplemental Figure 1). To ensure that modest changes in size did not cause these results, we measured 3 dpf larvae, but found no correlation with genotype (supplemental Figure 1D).
Genome editing of f10 using TALENs results in a frameshift and null allele. (A) Schematic diagram of TALENs used for targeted mutagenesis of f10. (B) Targeting of f10 exon 5 using a TALEN resulted in frameshift mutations. The 17-bp deletion mutant used for subsequent studies is shown. (C) Expression of f10 mRNA is reduced in heterozygous and undetectable in homozygous mutants, as evaluated by RT-PCR (each genotype was evaluated in triplicate). (D) Whole-mount in situ hybridization with an antisense probe demonstrates absence of expression in f10−/− mutants. Anterior is toward the left, and dorsal is toward the top. Scale bar, 100 µm. Chr1, chromosome 1; C-term, C-terminal domain; DBD, DNA-binding domain; Fok I, Fok I nuclease; NLS, nuclear localization signal; N-term, N-terminal domain.
Genome editing of f10 using TALENs results in a frameshift and null allele. (A) Schematic diagram of TALENs used for targeted mutagenesis of f10. (B) Targeting of f10 exon 5 using a TALEN resulted in frameshift mutations. The 17-bp deletion mutant used for subsequent studies is shown. (C) Expression of f10 mRNA is reduced in heterozygous and undetectable in homozygous mutants, as evaluated by RT-PCR (each genotype was evaluated in triplicate). (D) Whole-mount in situ hybridization with an antisense probe demonstrates absence of expression in f10−/− mutants. Anterior is toward the left, and dorsal is toward the top. Scale bar, 100 µm. Chr1, chromosome 1; C-term, C-terminal domain; DBD, DNA-binding domain; Fok I, Fok I nuclease; NLS, nuclear localization signal; N-term, N-terminal domain.
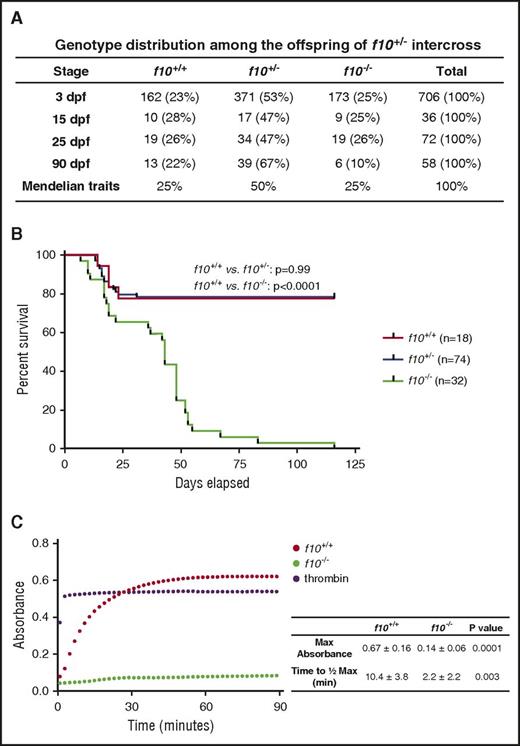
Loss of F10 in mice results in a bimodal phenotype of embryonic and neonatal lethality.14 In a group of closely monitored heterozygous incrosses, we found that there was no specific loss of zebrafish f10−/− mutants during embryonic development and the larval period through ∼16 dpf (Figure 3A). There was a brief period of a 20% general population loss from ∼10 to 16 dpf, which is typical for wild-type zebrafish and was observed across all 3 genotypes (Figure 3B). After that point, there was progressive loss of f10−/− mutants (Figure 3B). By 50 dpf, 75% of f10−/− mutants had expired, and 100% had expired by 4 months of age (Figure 3B). This phenotype has been consistent in our f10 mutant breeding colony since then, although out of a total of 71 homozygous mutants identified thus far, 1 survived for 268 days. To determine whether coagulation was intact in mutants, we performed a thrombin activity assay on plasma at 1 month of age. The results indicate absence of activity specifically in homozygotes, which is suggestive of defective prothrombin activation (Figure 3C).
Complete loss of F10 results in progressive adult lethality. (A) Genotype distributions of offspring from separate f10+/− incrosses evaluated at various stages demonstrate loss of homozygous mutants after 25 dpf. (B) Survival curves of closely monitored clutches of zebrafish offspring from f10+/− incrosses demonstrate progressive loss of 75% of homozygotes by 50 dpf and 100% by 115 dpf. There was no significant loss of heterozygotes (P > .05 by log-rank testing). Larvae were genotyped at 3 dpf and selected individuals were observed daily. There was ∼20% background loss of individual fish across all genotypes up to 20 dpf, which is typical during wild-type fish development. (C) Citrated plasmas from 1-month-old fish were recalcified and incubated with human fibrinogen for 90 minutes and absorbance (405 nm) was measured every 2 minutes. Data shown are the average of 2 experiments (n = 5 total pairs of fish for each genotype). The average time to half-maximal absorbance and maximum absorbance were calculated for each genotype and data were analyzed by using the Student t test. Bovine thrombin was used as a positive control. Max, maximum; min, minutes.
Complete loss of F10 results in progressive adult lethality. (A) Genotype distributions of offspring from separate f10+/− incrosses evaluated at various stages demonstrate loss of homozygous mutants after 25 dpf. (B) Survival curves of closely monitored clutches of zebrafish offspring from f10+/− incrosses demonstrate progressive loss of 75% of homozygotes by 50 dpf and 100% by 115 dpf. There was no significant loss of heterozygotes (P > .05 by log-rank testing). Larvae were genotyped at 3 dpf and selected individuals were observed daily. There was ∼20% background loss of individual fish across all genotypes up to 20 dpf, which is typical during wild-type fish development. (C) Citrated plasmas from 1-month-old fish were recalcified and incubated with human fibrinogen for 90 minutes and absorbance (405 nm) was measured every 2 minutes. Data shown are the average of 2 experiments (n = 5 total pairs of fish for each genotype). The average time to half-maximal absorbance and maximum absorbance were calculated for each genotype and data were analyzed by using the Student t test. Bovine thrombin was used as a positive control. Max, maximum; min, minutes.
Given the embryonic hemorrhage exhibited in the mouse knockout model, we carefully examined zebrafish at early stages of development. A low, but nonsignificant frequency of intracranial hemorrhage was observed in f10−/− larvae at 3 dpf (1.33%, n = 225), whereas no bleeds were observed in their f10+/+ (n = 221) and f10+/− (n = 465) siblings. To prospectively evaluate homozygous mutants, we genotyped f10+/− intercross offspring at 3 dpf, followed by daily visual phenotypic assessments. At 27 dpf, approximately one-third of viable f10−/− fish exhibited massive hemorrhage in the forebrain, midbrain, and hindbrain ventricles (Figure 4A), others displayed bleeding in the eye, muscle, or other tissues, and some appeared normal. Once gross hemorrhage became visible, the majority of f10−/− mutants died within 5 days. The mutants lacking intracranial or grossly visible bleeds tended to survive longer and exhibit intracranial, intra-abdominal, or gill hemorrhage at later stages (eg, 39 dpf; Figure 4A and data not shown). We performed histologic sections in f10 mutants from 27 to 268 dpf (supplemental Table 3), confirming the suspected hemorrhage in the brain, muscles, and abdomen (Figure 4B).
Loss of F10 results in late-onset hemorrhage at multiple sites. (A) Grossly visible hemorrhaging occurred in f10−/− mutant fish as early as 27 dpf, but not in wild-type or heterozygous siblings. Massive hemorrhages were observed in the brain, muscle, gill filaments, and abdomen as shown in viable f10−/− mutants. (B) Hematoxylin and eosin stained histologic sections of f10−/− mutants confirmed substantial intracranial, abdominal, and intramuscular hemorrhage at 27 dpf. Arrows indicate sites of hemorrhage. Locations of magnified insets (×4) are indicated by the smaller boxes in the same panel. Anterior is toward the left, and dorsal is toward the top. Scale bar, 100 µm. ad, abdomen; fb, forebrain; gf, gill filament; hb, hindbrain; mb, midbrain.
Loss of F10 results in late-onset hemorrhage at multiple sites. (A) Grossly visible hemorrhaging occurred in f10−/− mutant fish as early as 27 dpf, but not in wild-type or heterozygous siblings. Massive hemorrhages were observed in the brain, muscle, gill filaments, and abdomen as shown in viable f10−/− mutants. (B) Hematoxylin and eosin stained histologic sections of f10−/− mutants confirmed substantial intracranial, abdominal, and intramuscular hemorrhage at 27 dpf. Arrows indicate sites of hemorrhage. Locations of magnified insets (×4) are indicated by the smaller boxes in the same panel. Anterior is toward the left, and dorsal is toward the top. Scale bar, 100 µm. ad, abdomen; fb, forebrain; gf, gill filament; hb, hindbrain; mb, midbrain.
Deficiency of F10 does not appear to affect vascular development in zebrafish
Loss-of-function studies of the common pathway coagulation factors F2 and F5 in mice have shown embryonic lethality with vascular abnormalities and hemorrhage, whereas fibrinogen knockouts develop normally, suggesting a role for thrombin signaling in vessel development.15 The external growth of zebrafish has been instrumental in the study of angiogenesis and vasculogenesis, thus offering an alternative approach to assess whether F10 plays a role in vascular development.26,30 We evaluated the spatiotemporal expression patterns of the vascular genes ephb2a,42,43 cdh5,44 flk1,45,46 flt4,47 and ephB442 in offspring from f10+/− incrosses by WISH, followed by genotyping. Our results did not show any differences in the qualitative expression patterns between homozygous mutants and sibling controls at 24 hpf (data not shown) when axial and several intersegmental vessels are formed48,49 or at 72 hpf (supplemental Figure 2) during the early stages of the establishment of the vascular network.48 We performed whole transcriptome sequencing on pools of wild-type and homozygous mutant larvae at 72 hpf, but did not find any quantitative differences in these markers (supplemental Table 4). Next, we crossed the f10 mutants into a transgenic line in which the endothelium was labeled with mCherry [Tg(flk1:mCherry/NTR)]. Embryos from an incross of f10+/−; Tg(flk1:mCherry/NTR) fish were evaluated from 24 through 96 hpf. No aberrant sprouting or failure to sprout was observed at 24 hpf, and no signs of abnormal vasculature formation were observed. We measured the intersegmental vessel length from the PCV to the dorsal longitudinal anastomotic vessel and observed no significant differences in homozygous mutants (supplemental Figure 3). Genotyping was performed after phenotypic observation and confirmed the presence of all 3 expected genotypes.
Dysregulated apoptosis has been shown to cause loss of vascular integrity,29 and F10 has been implicated in apoptosis through PAR-1 signaling.50 To detect apoptotic cells, we stained larvae with acridine orange51,52 or probed with an antibody to caspase 3,53 and this was followed by blinded phenotypic observation and subsequent genotyping. We did not observe any differences in f10−/− mutants versus their siblings (supplemental Figure 4), suggesting that deficiency of F10 does not affect cellular apoptosis.
Loss of F10 results in early induced hemostatic defects in larval zebrafish
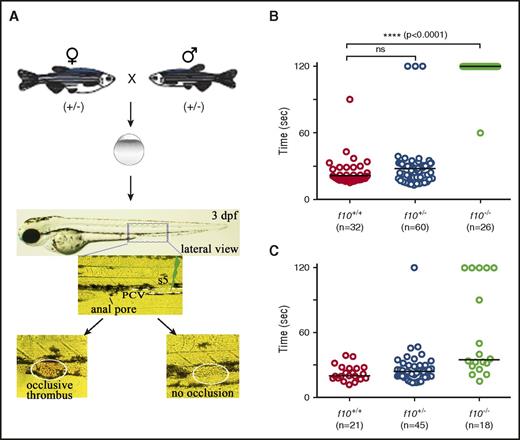
The low frequency of visible hemorrhage in larvae was surprising, especially considering the severe phenotypes observed in F10 knockout mouse embryos and neonates. To address whether F10 plays a role in early zebrafish hemostasis, we evaluated the time to occlusion by using an induced model of venous thrombosis in the PCV of 3 dpf larvae (Figure 5A). We found that occlusion was absent in f10−/− larvae when compared with their f10+/+ and f10+/− siblings, the vast majority of which formed occlusive thrombi (P < .0001; Figure 5B). To confirm that this was specific to loss of F10 rather than off-target mutations due to genome editing, we injected a ubiquitin (ubi) promoter–regulated wild-type f10 cDNA expression construct into 1-cell stage offspring from f10+/− incrosses, and evaluated the time to occlusion in larvae at 3 dpf. Our results showed that 72% of injected f10−/− larvae displayed statistically significant rescue of the ability to form an occlusive thrombus (P = .0001, Mann-Whitney U test; Figure 5C).
Absence of hemostasis in f10 mutant larvae. (A) Schematic diagram of laser-induced endothelial injury of the PCV at the 5th somite (s5) caudal to the anal pore in larvae at 3 dpf. Hemostasis was evaluated by documenting the time to occlusion ≤120 seconds (sec) after laser-induced endothelial injury. (B) The time to occlusion was significantly prolonged in f10−/− larvae in comparison with f10+/+and f10+/− siblings (P < .0001, Mann-Whitney U test). (C) Injection of wild-type zebrafish f10 complementary DNA (cDNA) under control of the ubi promoter into 1-cell–stage embryos resulted in significant rescue of the hemostatic defect in 72% of f10−/− larvae at 3 dpf when compared with uninjected mutants (P < .05). Horizontal bars represent the median time to occlusion. ns, not significant.
Absence of hemostasis in f10 mutant larvae. (A) Schematic diagram of laser-induced endothelial injury of the PCV at the 5th somite (s5) caudal to the anal pore in larvae at 3 dpf. Hemostasis was evaluated by documenting the time to occlusion ≤120 seconds (sec) after laser-induced endothelial injury. (B) The time to occlusion was significantly prolonged in f10−/− larvae in comparison with f10+/+and f10+/− siblings (P < .0001, Mann-Whitney U test). (C) Injection of wild-type zebrafish f10 complementary DNA (cDNA) under control of the ubi promoter into 1-cell–stage embryos resulted in significant rescue of the hemostatic defect in 72% of f10−/− larvae at 3 dpf when compared with uninjected mutants (P < .05). Horizontal bars represent the median time to occlusion. ns, not significant.
Pro- and anticoagulant compounds have no effect on the f10−/− larval hemostatic defect
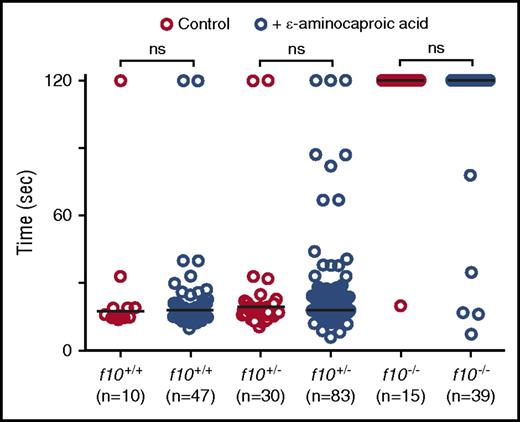
Given the unexpected survival of homozygous mutant fish into adulthood, we hypothesized that there could be alternative pathways for residual thrombin generation and fibrin formation. We therefore evaluated the ability of the fibrinolysis inhibitor, ε-aminocaproic acid, to reverse the lack of induced venous occlusion in f10−/− larvae. We have previously shown that ε-aminocaproic acid reverses the lack of occlusion observed in fibrinogen-deficient at3 mutants.22 We incubated f10+/− incross offspring in ε-aminocaproic acid beginning at 24 hpf and evaluated the time to PCV occlusion at 3 dpf. Our data reveal that although treatment with ε-aminocaproic acid did increase the number of f10−/− larvae with a normal occlusion time (12.8% vs 6.7% in controls), this result was not statistically significant (Figure 6). There were also no differences in time to occlusion for treated and untreated f10+/+ and f10+/− siblings (Figure 6). We have previously shown that warfarin inhibits laser-mediated occlusion in the PCV,22 although the incidence of overt bleeding is minimal. Given the low frequency of spontaneous hemorrhage observed in f10−/− larvae, we speculated that maternally derived F10 might play a role in early mutant embryos and larvae. We treated 1 dpf embryos with warfarin and observed them for evidence of hemorrhage by o-dianisidine staining at 7 dpf and then performed genotyping. Our data revealed no differences in o-dianisidine staining between f10−/− mutants and f10+/− or f10+/+ siblings (supplemental Figure 5).
Treatment with ε-aminocaproic acid does not reverse the hemostatic defect in f10 mutants. Offspring from f10+/− incrosses were treated with ε-aminocaproic acid at 24 hpf and tested for the ability to form a clot in the PCV in response to laser-mediated endothelial injury at 3 dpf, after which genotyping was performed. There was a slight increase in the percentage of occlusion in treated f10−/− larvae (12.8% vs 6.7% in controls). However, this increase was not statistically significant and likely represents background thrombus formation occasionally observed in homozygous mutants. Horizontal bars represent the median time to occlusion. ns, not significant; sec, second.
Treatment with ε-aminocaproic acid does not reverse the hemostatic defect in f10 mutants. Offspring from f10+/− incrosses were treated with ε-aminocaproic acid at 24 hpf and tested for the ability to form a clot in the PCV in response to laser-mediated endothelial injury at 3 dpf, after which genotyping was performed. There was a slight increase in the percentage of occlusion in treated f10−/− larvae (12.8% vs 6.7% in controls). However, this increase was not statistically significant and likely represents background thrombus formation occasionally observed in homozygous mutants. Horizontal bars represent the median time to occlusion. ns, not significant; sec, second.
Identification of causative variants in human F10 deficiency through in vivo evaluation in zebrafish f10−/− mutants
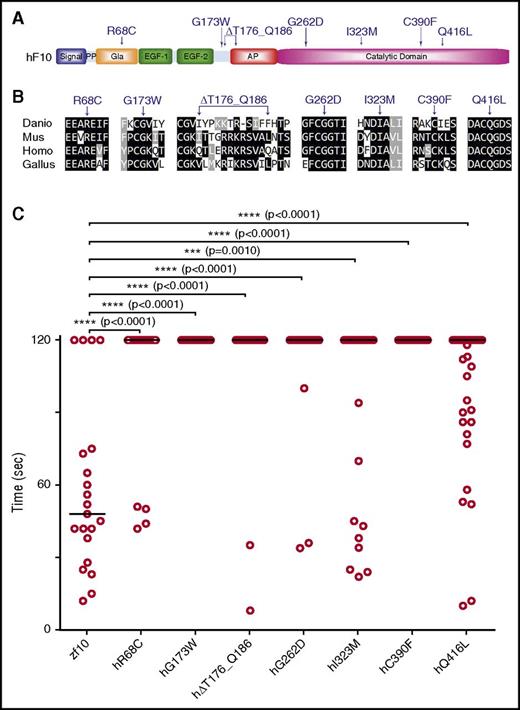
As sequencing costs continue to decline, VUS are increasingly being identified. Although in vitro assays can often discern between functional and silent mutations, a wide variety of alternative techniques may be required, depending on the nature of the mutation, as has been shown with BRCA2.54 As a proof of concept, we tested 2 known F10 mutations in the catalytic domain associated with F10 deficiency, G262D and C390F2 (Figure 7A), sites that are conserved across multiple vertebrate species (Figure 7B). To assess their ability to rescue the lack of thrombotic occlusion in f10−/− larvae, we engineered these variants into the orthologous positions in the zebrafish f10 cDNA expression vector. The resulting mutant plasmids (supplemental Table 2) were injected into 1-cell stage zebrafish embryos from f10+/− incrosses, and then laser-mediated endothelial ablation of the PCV was performed at 3 dpf. No significant rescue was demonstrated, indicating the requirement for function of these residues across species and conservation of the mutant phenotype.
In vivo functional evaluation identifies causative variants for human F10 deficiency. (A) Schematic diagram of the human F10 domain structure and position of known and suspected F10 variants associated with bleeding. (B) Multiple sequence alignment of peptides containing human F10 variants shows conservation across vertebrate species. Protein sequences are from human (Homo; NP_000495.1), mouse (Mus; NP_001229297.1), chicken (Gallus; NP_990353.1), and zebrafish (Danio; NP_958870.2). The altered residues are marked by arrows. (C) Human F10 variants were engineered into the orthologous positions of the zebrafish f10 cDNA and placed under the control of the ubi promoter. The expression vectors were injected into the cytoplasm of 1-cell–stage offspring from f10+/− incrosses. The endothelium of the PCV was injured by laser ablation at 3 dpf, and the time to complete occlusion recorded, after which genotyping was performed. Numbering represents the human amino acid positions. The first 2 variants tested were G262D and C390F, both of which are known to cause clinically significant human F10 deficiency. The subsequent variants, R68C, G173W, ∆T176_Q186, I323M, Q416L, were identified in patients with F10 deficiency and clinically significant bleeding, but are not yet proven to be causative. Although I323M and Q416L showed a trend toward occlusion, none of the variants examined could significantly rescue the hemostatic defect of mutants (P < .001 by Mann-Whitney U test). Horizontal bars represent the median time to occlusion. n ≥ 18 for each variant tested. AP, activation peptide; EGF-1/2, epidermal growth factor-like domains 1/2; Gla, Gla domain; PP, propeptide.
In vivo functional evaluation identifies causative variants for human F10 deficiency. (A) Schematic diagram of the human F10 domain structure and position of known and suspected F10 variants associated with bleeding. (B) Multiple sequence alignment of peptides containing human F10 variants shows conservation across vertebrate species. Protein sequences are from human (Homo; NP_000495.1), mouse (Mus; NP_001229297.1), chicken (Gallus; NP_990353.1), and zebrafish (Danio; NP_958870.2). The altered residues are marked by arrows. (C) Human F10 variants were engineered into the orthologous positions of the zebrafish f10 cDNA and placed under the control of the ubi promoter. The expression vectors were injected into the cytoplasm of 1-cell–stage offspring from f10+/− incrosses. The endothelium of the PCV was injured by laser ablation at 3 dpf, and the time to complete occlusion recorded, after which genotyping was performed. Numbering represents the human amino acid positions. The first 2 variants tested were G262D and C390F, both of which are known to cause clinically significant human F10 deficiency. The subsequent variants, R68C, G173W, ∆T176_Q186, I323M, Q416L, were identified in patients with F10 deficiency and clinically significant bleeding, but are not yet proven to be causative. Although I323M and Q416L showed a trend toward occlusion, none of the variants examined could significantly rescue the hemostatic defect of mutants (P < .001 by Mann-Whitney U test). Horizontal bars represent the median time to occlusion. n ≥ 18 for each variant tested. AP, activation peptide; EGF-1/2, epidermal growth factor-like domains 1/2; Gla, Gla domain; PP, propeptide.
We sequenced F10 in 5 patients with F10 deficiency and identified novel mutations not previously associated with this disorder. These variants are located in various domains of human F10, including the Gla (R68C), activation peptide (G173W, ∆T176_Q186), and catalytic domains (I323M, Q416L) (Figure 7A; supplemental Table 5). The affected positions are conserved across mammalian, avian, and aquatic species (except for the ∆T176_Q186 deletion; Figure 7A) and were engineered into the zebrafish f10 expression vector (supplemental Table 2), after which injection and laser-mediated endothelial injury was performed as described above. Our data show that catalytic domain variants (I323M and Q416L) revealed a trend toward rescue (29% and 36%, respectively), although the results were not statistically significant (Figure 7C). The variants in the Gla domain and activation peptide failed to show any signs of statistically significant rescue of the hemostatic defect (Figure 7C).
Discussion
In this article, we report targeted mutagenesis of zebrafish f10 by using genome-editing TALENs. Based on observations in mice, we expected spontaneous fatal hemorrhage during the embryonic/larval period. Indeed, appropriate hemostatic responses were absent following endothelial injury at 3 dpf. A very low percentage of f10−/− larvae at that stage exhibited hemorrhage, but it was at a background level consistent with our wild-type colony. To our surprise, f10−/− mutants are able to tolerate what should be a severe insult and do not exhibit significant overt bleeding symptoms until 3 to 4 weeks of age, and the majority survive for several months. The visible hemorrhage that eventually ensues predominantly manifests at intracranial and, to a lesser extent, intra-abdominal and intramuscular sites, similar to symptoms observed in humans with severe F10 deficiency.2,7 These phenotypes are the presumed cause of lethality, occurring by 4 months of age, with a steep drop at 1 to 2 months of age. This stark reduction correlates with the onset of visible hemorrhage as well as the transition to adulthood and sexual maturity. This time period includes an increase in aggressive behaviors, which could be responsible for the accelerated loss of homozygous mutants. Knockout mice demonstrate a bimodal phenotype, and approximately one-third of homozygous mutant embryos are lost between days 9.5 and 12.5 in utero, with the remainder succumbing to hemorrhage within 3 weeks postnatally.15 The lack of early onset hemorrhage in our f10 homozygotes might have suggested that the coagulation cascade did not evolve to be active during embryonic/larval development. However, we and others have shown that this is not the case (see Vo et al,21 Liu et al22 , Jagadeeswaran et al,37 and this article). One limitation to the use of zebrafish for modeling human developmental biology is the absence of a placenta. There is a large body of work demonstrating that the coagulation cascade regulates placental development through protease-activated receptors rather than fibrin formation,55,56 which could explain the embryonic lethality observed in F10 knockout mice.
This is the second example of unexpected long-term survival of a coagulation factor knockout in fish when compared with its mammalian counterpart. We have previously shown that targeted disruption of zebrafish at3 results in survival up to 6 months of age,22 despite the fact that complete loss is embryonic lethal in mice14 and has never been described in humans.10 There are several potential explanations for this discrepancy. Our data demonstrate maternal contribution to early embryonic expression of f10, which might provide some protection during development, but is unlikely to account for the observed long-term survival. Another possibility is blood pressure, because ventricular and aortic pressures range from 0.1 to 0.5 mm Hg in larvae and from 0.1 to 2.5 mm Hg in adults.57,58 In mice, embryonic ventricular pressures are up to 10-fold higher59 than fish larvae, and adults exhibit pressures similar to humans. Furthermore, mouse lethality is often in the neonatal period and could be secondary to birth trauma. Alternatively, we speculate that protective species-specific coagulation factors may exist in fish. Teleost fish underwent whole-genome duplication after divergence from mammals ∼320 to 350 million years ago,60 although the majority of coagulation factors have remained single-copy genes.61,62 Neofunctionalization and subfunctionalization of related coagulation factors may have occurred, and elucidation of potential alternative hemostatic pathways could suggest novel therapeutic biologics or drug targets in humans.
Disruption of zebrafish fga has been reported with 40% to 100% survival of homozygous mutants at 4 to 5 months of age,63 concordant with mouse knockout data that show variable adult survival.64 Taken together, the phenotypes of coagulation factor knockouts show similarities between mammals and fish, with loss of fibrinogen better tolerated than disruption of factors within and regulating the common pathway and thrombin generation (ie, at3 and f10). This is consistent with data that thrombin has a multitude of effects beyond conversion of fibrinogen to fibrin.65,66 Previous studies of coagulation factor cross-regulation have primarily focused on protein-protein interactions. We have found that loss of F10 results in upregulation of fga and at3 mRNA expression, although alterations in protein levels or activity have not been confirmed. This finding suggests the possibility of underlying mechanisms that sense either the level of coagulation cascade activity (thrombin activity or fibrin production) or individual factor levels. It is conceivable that such interactions are what contribute to the greater severity seen in the loss of common pathway factors versus fibrinogen.
The phenotypes observed in mouse knockouts of common pathway factors has led to the hypothesis that thrombin signaling is required for vascular development during embryogenesis.11-13,15 F10 knockout mice did not exhibit clear vascular defects, although there was hemorrhaging and rapid embryo resorption that might have prevented visualization of vessel anomalies.14,15 It has been previously shown that pathways regulating vascular development are highly conserved across vertebrate species.26,30 Multiple vascular mutants have been described in zebrafish with intracranial hemorrhage as a primary phenotype secondary to various mechanisms, including defects in vascular development, patterning, integrity, and remodeling.27-29 Our analysis did not reveal any such vascular phenotypes, suggesting that the consequences of F10 loss are solely defects in hemostasis. This implies that thrombin generation is not a key player in zebrafish vasculogenesis or operates through non–F10-dependent pathways. Alternatively, paralogous coagulation factors may have neofunctionalized and assumed new roles in zebrafish vasculogenesis.
Rapid improvements in sequencing technologies continue to spawn new and broader applications to basic, translational, and clinical research.67 One of the major remaining challenges is the interpretation of VUS. For a number of coagulation factor substitutions, in vitro assays miscall functional versus silent mutations (eg, von Willebrand factor68,69 ). As has been shown for BRCA2,54 the availability of alternative methods can aid in clarifying such variants. We have previously used transient transgenesis in mutant zebrafish larvae as an in vivo assay for examination of established human hemostatic variants.22 In this study, we take this a step further and evaluate previously unconfirmed candidate mutations in multiple domains from symptomatic patients with F10 deficiency. The patient phenotypes range from minor to major bleeding, with factor activities from <1% to 5%. Five novel and 2 established substitutions or deletions are unable to rescue the f10−/− hemostatic defect, thus confirming their pathogenicity. This platform will add to our armamentarium for coagulation factor analysis and has the advantage of not being susceptible to in vitro testing artifacts. However, it is important to note that despite the high degree of conservation across vertebrates, there is the possibility that species-specific protein-protein interactions have developed that will limit this approach.
In conclusion, our findings that deficiency of F10 leads to loss of coagulation with massive hemorrhage yet paradoxical embryonic/larval survival provide a powerful alternative to studies in mammals. Overall, our data align with the results of gene targeting in mice and observations in patients. However, the extended survival and external nature of zebrafish embryonic/larval development has already allowed further mechanistic investigation, including data suggesting that thrombin signaling does not regulate vascular development. This model could lead to the development of novel therapeutics through genetic and small-molecule screening70 and is a powerful system for the in vivo functional evaluation of human VUS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Weibin Zhou for technical support and the University of Michigan Sequencing and Microscopy & Image Analysis cores, particularly Shelley Almburg, for imaging support. The authors also thank Colin Kretz and members of the Shavit laboratory for critical review of the manuscript.
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grant R01-HL124232, a Hemophilia of Georgia Clinical Scientist Development Grant, a National Hemophilia Foundation/Novo Nordisk Career Development Award, the Bayer Hemophilia Awards Program (J.A.S.), NIH, National Heart, Lung, and Blood Institute grant T32-HL007622 (M.S.R.), National Institutes of Health (N.C.C.), and NIH, National Institute of General Medical Sciences grant R01-GM088040 (J.K.J.). J.K.J. is the Jim and Ann Orr Massachusetts General Hospital Research Scholar. J.A.S. is the Diane and Larry Johnson Family Scholar of Pediatrics and Communicable Diseases.
Authorship
Contribution: Z.H. designed and performed research, analyzed data, and wrote the manuscript; M.C.H. designed and performed research and analyzed data; Y.L., Z.G.N., D.R., and J.K.J. designed and performed research; N.C.C. provided reagents; M.M. and F.P. designed research; A.N.S., M.S.R., and C.E.R. performed research and analyzed data; and J.A.S. designed and supervised research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: J.A.S. has been a consultant for Bayer, Shire, CSL Behring, Grifols, and Octopharma. F.P. has received honoraria for participating as a speaker at satellite symposia and educational meetings organized by Bayer, Biotest, CSL Behring, Grifols, Novo Nordisk, and Sobi; is the recipient of research grant funding from Alexion, Biotest, Kedrion Biopharma, and Novo Nordisk paid to Fondazione Luigi Villa; and has received consulting fees from Kedrion Biopharma, LFB, and Octapharma. She is member of the Ablynx scientific advisory board. J.K.J. has financial interests in Beacon Genomics, Beam Therapeutics, Editas Medicine, Poseida Therapeutics, and Transposagen Biopharmaceuticals. J.K.J.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. The remaining authors declare no competing financial interests.
The current affiliation for Y.L. is Molecular Innovations, Inc., Novi, MI.
The current affiliation for D.R. is Editas Medicine, Cambridge, MA.
Correspondence: Jordan A. Shavit, Department of Pediatrics, University of Michigan, Room 8301, Medical Science Research Building III, 1150 West Medical Center Dr, Ann Arbor, MI 48109; e-mail: jshavit@umich.edu.

![Figure 1. Spatiotemporal expression of f10 in the developing zebrafish. (A) Phylogenetic tree of F10 from zebrafish (Danio), mouse (Mus), chicken (Gallus), and human (Homo). (B) RT-PCR from whole embryos demonstrates that zebrafish f10 mRNA is expressed during early embryonic development beginning at the 1-cell stage. Expression is relatively stable from 24 through 120 hpf. The expression of elongation factor 1-α (elfa) was used as an internal control. (C-L) WISH analysis of 120-hpf larvae shows that f10 expression is strong in the liver (D), but relatively weak in the yolk syncytial layer and brain (D-F), otic vesicles (E, G), and arches (H-I) by using an antisense probe (f10-as). (C) A sense control (f10-s) did not show any expression. (J-L) Plastic sections (5 µm) of stained larvae in transverse (J), sagittal (K), and coronal (L) planes are shown. Anterior is toward the left in panels C-H and toward the top in panel I. Scale bars (C-L), 100 µm. b, brain; ch, ceratohyal [2nd visceral pharyngeal arch (VA)]; li, liver; mc, mandibular cartilage (1st VA); ov, otic vesicle; sb, swim bladder; y, yolk; ysl, yolk syncytial layer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/5/10.1182_blood-2017-02-765206/4/m_blood765206f1.jpeg?Expires=1765890383&Signature=LOXNVq~M5kQUAoaINmrnbIiC5XaZi64vct~y0IAXVpbonBdBkcufrzVaysuaBMJGywpYkDy2idoYJ6b62O7eF5IgoIpKEvdptNjDvyw1ZDGSHtRU3GUq3yIKTG7uKmWAHdb76JodIGSbaD2o-UvrkKziKPBlhZqQfWYxg0yg2y1gKnDuRTCcRHV56fucjzbkmPb4YZJlGqCwGwlM0hnTQxk7R2dqlsmgDpAdCAAHjpRmoFm6rh9fvNBpFM8iV~XJyQNOgYOaVMwxyEzXXONyRj0aF-ZvCgKhJQ3eZSaz~K9P7j7sn9BK2foZa8-DhI6x-lgVgxoRWx1URVfo~Bp4xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)