Key Points
Patients with AL amyloidosis and low dFLC burden (<50 mg/L) have less severe heart involvement and better survival.
These patients are evaluable for hematologic response with adapted criteria predicting improvement of overall and renal survival.
Abstract
The validated criteria of hematologic response in light-chain (AL) amyloidosis are based on the measurement of circulating free light chains (FLCs). Patients with a difference between involved and uninvolved FLC (dFLC) <50 mg/L cannot be assessed for response and are excluded from clinical trials. We compared the clinical characteristics and outcome of 203 newly diagnosed patients with dFLC <50 mg/L (low dFLC) with 866 patients with measurable dFLC (high dFLC) evaluated between 2004 and 2015. Heart involvement was significantly less common and less advanced in the low-dFLC group (43% vs 83% and Mayo stage III 45% vs 15%, both P < .001), whereas renal involvement was more frequent (77% vs 63%, P < .001) and more severe (renal stage III 26% vs 18%, P = .001). Overall survival (OS) was significantly better in the low-dFLC group (median 117 vs 21 months, P < .001), whereas no difference was seen in renal survival (RS). Within each Mayo stage, patients with low dFLC had a longer survival. In the low-dFLC group, complete response was associated with a significant advantage in OS (median not reached vs 117 months, P = .005) and with a better RS. A reduction in dFLC after therapy of <10 mg/L was associated with a better OS and RS in patients with at least a dFLC >20 mg/L baseline. Nineteen percent of newly diagnosed patients with AL amyloidosis have low dFLC and had a better outcome. Hematologic response assessed with adapted criteria predicts OS and RS in these patients, who can thus be assessed for response and included in clinical trials with appropriate stratification.
Introduction
Immunoglobulin light-chain (AL) amyloidosis is caused by a misfolded light chain that deposits in tissues and causes organ dysfunction if not effectively treated. Recent evidence indicates that amyloidogenic light chains are responsible of direct proteotoxicity in this disease.1 Although alternative approaches are being designed, the mainstay of treatment is chemotherapy targeting the plasma cell producing the amyloid-forming light chain.2,3 The possibility of quantifying circulating free light chains (FLCs) represented a major step forward in the management of this disease. Measurement of FLCs is now part of the diagnostic workup4-6 and has been included in the prognostic staging system.7 When chemotherapy succeeds in reducing the concentration of FLCs, cardiac and renal dysfunction improves in a substantial proportion of patients, translating into prolonged survival and lower risk of progression to dialysis.8,9 The difference between involved (amyloidogenic) and uninvolved FLC (dFLC) is used in response assessment.10 Current validated criteria for hematologic response are based on changes in dFLC.9,11 In particular, 4 levels of response were identified: amyloid complete response (CR; normal FLC ratio and negative serum and urine immunofixation), very good partial response (VGPR; difference between involved and uninvolved FLCs [dFLC] <40 mg/L), PR (dFLC decrease >50%), and no response.11 To allow the assessment of VGPR, accounting for the imprecision of the FLC assay,12 it is agreed that baseline dFLC needs to be ≥50 mg/L, and subjects with lower dFLC concentrations are generally excluded from clinical trials.13 However, it has been reported that FLC burden is associated with poor outcome,14 and exclusion of patients with low dFLC concentrations could affect the outcome of clinical trials. In the present study, we elucidated the clinical characteristics of patients whose dFLC is <50 mg/L and explored alternative approaches to assess response to treatment in this group. A parallel study was performed by the Heidelberg group.15
Methods
The study population is composed of 1069 consecutive, newly diagnosed patients with AL amyloidosis evaluated between 2004 and 2015 at the Pavia Amyloidosis Research and Treatment Center and prospectively followed for response and survival. All patients gave written informed consent as approved by the institutional ethics committee for their clinical data to be used for research purposes, in accordance with the Declaration of Helsinki. All the patients had biopsy-proven diagnosis of AL amyloidosis, and the deposits were characterized as AL type by immunoelectron microscopy16 or proteomics17 analysis according to the time of diagnosis. A monoclonal light chain of the same isotype as that found in the deposits needed to be detected in serum and/or urine by immunofixation and or circulating FLC quantitation.4,5 Subjects with lytic bone lesions were excluded. Baseline evaluation included a complete physical examination, assessment of amyloid organ involvement, echocardiography, and measurement of cardiac biomarkers, serum creatinine concentration, and of 24-hour urinary protein excretion. Organ involvement was defined according to the 2005 International Society of Amyloidosis criteria.18,19 Hematologic CR was assessed according to the 2012 International Society of Amyloidosis criteria at 3 and 6 months after treatment initiation.11 In particular, CR is defined as obtaining a negative serum and urine immunofixation result and a normal FLC ratio. The analysis of response was by intent to treat, and the patients who died before the evaluation of response were considered nonresponders. The cardiac biomarkers amino-terminal pronatriuretic peptide type B (NT-pro-BNP, cutoff 332 ng/L) and troponin I (cutoff 0.1 ng/mL) were combined according to the Mayo 2004 staging system for prognostic stratification.20 Stage I, II, and III patients had none, 1, or both markers above the cutoffs. Stage III patients whose NT-pro-BNP was >8500 ng/L were classified as stage IIIb.21 Twenty-four hour proteinuria (cutoff >5 g per 24 hours) and estimated glomerular filtration rate (eGFR; cutoff < 50 mL/min per 1.73 m2) were used for the assessment of risk for dialysis, according to the renal staging system.9 Renal stage I, II, and III patients had none, one or both unfavorable markers. Organ response was assessed according to the current consensus criteria.9,11 Cardiac response or progression required a decrease or increase in NT-pro-BNP >30% and >300 ng/L. Baseline NT-pro-BNP had to be >650 ng/L to be evaluable. Renal response required a decrease in proteinuria >30% or below 0.5 g per 24 hours in the absence of renal progression. Patients who died without requiring dialysis were considered censored for the purpose of the analysis of renal survival. A ≥25% eGFR decrease was considered as the criterion for renal progression. The present study was performed in parallel with a German study (companion manuscript submitted). Hematologic response criteria designed for patients with low-dFLC burden were tested in the German cohort (testing cohort) and validated in the Italian cohort (validation cohort).
Serum FLC concentration was measured with a latex enhanced immunoassay (The Binding Site, Birmingham, United Kingdom)22 on a Behring BN II (Dade Behring, Deerfield, IL) nephelometer. The reference intervals for κ and λ FLC are 3.3 to 19.4 mg/L and 5.7 to 26.3 mg/L, respectively, and the κ/λ ratio diagnostic range is 0.26 to 1.65.
Continuous data were described using median and interquartile range. Fischer’s exact test or χ2 test was used to test differences in continuous variables between groups, as appropriate. Survival was analyzed using the Kaplan-Meier method to estimate the distribution of survival as a function of the follow-up duration while censoring those not known to be deceased at last known follow-up. Differences in survival were tested for significance according to the log-rank test. Multivariate analysis of factors affecting survival was carried out using the Cox proportional hazard models. All analyses were performed using MedCalc Statistical Software version 16.8 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org).
Results
A total of 1069 previously untreated patients were diagnosed with AL amyloidosis at our center between 2004 and 2015 and were included in the study. Patients’ characteristics are reported in Table 1. We compared the 203 subjects (19%) who had a baseline dFLC <50 mg/L (low-dFLC group) with the remaining 866 patients (high-dFLC group). In the low-dFLC group, all patients had a monoclonal protein (MP) in serum and/or in urine by immunofixation at the time of diagnosis, and in 53 patients (26%), we found an abnormal κ/λ ratio. The proportion of patients with an intact MP was significantly higher in the low-dFLC group than in the high-dFLC group (66% vs 47%, P < .001). In patients with a low dFLC concentration, heart involvement was less frequent (43% vs 83%, P < .001) and less severe (stage III 15% vs 45%, P < .001), whereas renal involvement was more frequent (77% vs 63%, P < .001), and a higher proportion of patients in the low-dFLC cohort were classified as renal stage III (26% vs 18%, P < .001). A significantly lower proportion of patients in the low-dFLC cohort had an advanced New York Heart Association class (class III, 19% vs 40%, P < .001). The performance status according to Eastern Cooperative Oncology Group was >2 in 33% vs 55% in the low- and high-dFLC cohorts, respectively (P < .001). The median bone marrow plasma cell infiltrate was lower in the low-dFLC group (8% vs 12%, P < .001), and only 35 patients (17%) had a bone marrow plasma cell infiltrate >10%, compared with 37% of patients in the high-dFLC group (P < .001).
Patient characteristics
| Variable . | Low-dFLC group (203 patients) . | High-dFLC group (866 patients) . | P . |
|---|---|---|---|
| Age (y) | 66 (40-85) | 65 (30-87) | .090 |
| Male sex | 114 (56) | 501 (58) | .193 |
| Organ involvement | |||
| Heart | 87 (43) | 723 (83) | <.001 |
| Kidney | 157 (77) | 547 (63) | <.001 |
| Liver | 28 (14) | 121 (14) | .565 |
| PNS | 27 (13) | 100 (12) | .219 |
| >2 organs | 30 (15) | 229 (26) | <.001 |
| Cardiac stages | |||
| I | 75 (37) | 112 (13) | <.001 |
| II | 98 (48) | 364 (42) | .062 |
| III | 30 (15) | 390 (45) | <.001 |
| PS-ECOG ≥2 | 68 (33) | 480 (55) | <.001 |
| NYHA class 3 | 39 (19) | 351 (40) | <.001 |
| Renal stage | |||
| I | 41 (26) | 176 (33) | .056 |
| II | 75 (48) | 260 (49) | .482 |
| III | 40 (26) | 96 (18) | .024 |
| eGFR <30 mL/min per 1.73 m2 | 29 (14) | 119 (14) | .398 |
| eGFR <15 mL/min per 1.73 m2 | 7 (3) | 32 (3) | .449 |
| Dialysis at diagnosis | 1 (0.4) | 10 (1) | .224 |
| Involved light-chain type | |||
| κ | 38 (19) | 206 (24) | .070 |
| λ | 165 (81) | 660 (76) | .130 |
| Intact MP | 134 (66) | 411 (47) | <.001 |
| Intact MP concentration, g/L | 11 (3-34) | 11 (1-42) | .183 |
| Bone marrow plasma cells, % | 8 (5-15) | 12 (8-20) | <.001 |
| dFLC >20 mg/L | 117 (59) | — | — |
| Treatment type | |||
| MDex | 67 (33) | 289 (33) | .495 |
| CyBorD | 54 (26) | 191 (22) | .098 |
| BMDex | 20 (10) | 110 (13) | .158 |
| Thalidomide-based regimen | 13 (6) | 71 (8) | .242 |
| ASCT | 6 (3) | 15 (2) | .193 |
| Others | 43 (22) | 190 (22) | .411 |
| Variable . | Low-dFLC group (203 patients) . | High-dFLC group (866 patients) . | P . |
|---|---|---|---|
| Age (y) | 66 (40-85) | 65 (30-87) | .090 |
| Male sex | 114 (56) | 501 (58) | .193 |
| Organ involvement | |||
| Heart | 87 (43) | 723 (83) | <.001 |
| Kidney | 157 (77) | 547 (63) | <.001 |
| Liver | 28 (14) | 121 (14) | .565 |
| PNS | 27 (13) | 100 (12) | .219 |
| >2 organs | 30 (15) | 229 (26) | <.001 |
| Cardiac stages | |||
| I | 75 (37) | 112 (13) | <.001 |
| II | 98 (48) | 364 (42) | .062 |
| III | 30 (15) | 390 (45) | <.001 |
| PS-ECOG ≥2 | 68 (33) | 480 (55) | <.001 |
| NYHA class 3 | 39 (19) | 351 (40) | <.001 |
| Renal stage | |||
| I | 41 (26) | 176 (33) | .056 |
| II | 75 (48) | 260 (49) | .482 |
| III | 40 (26) | 96 (18) | .024 |
| eGFR <30 mL/min per 1.73 m2 | 29 (14) | 119 (14) | .398 |
| eGFR <15 mL/min per 1.73 m2 | 7 (3) | 32 (3) | .449 |
| Dialysis at diagnosis | 1 (0.4) | 10 (1) | .224 |
| Involved light-chain type | |||
| κ | 38 (19) | 206 (24) | .070 |
| λ | 165 (81) | 660 (76) | .130 |
| Intact MP | 134 (66) | 411 (47) | <.001 |
| Intact MP concentration, g/L | 11 (3-34) | 11 (1-42) | .183 |
| Bone marrow plasma cells, % | 8 (5-15) | 12 (8-20) | <.001 |
| dFLC >20 mg/L | 117 (59) | — | — |
| Treatment type | |||
| MDex | 67 (33) | 289 (33) | .495 |
| CyBorD | 54 (26) | 191 (22) | .098 |
| BMDex | 20 (10) | 110 (13) | .158 |
| Thalidomide-based regimen | 13 (6) | 71 (8) | .242 |
| ASCT | 6 (3) | 15 (2) | .193 |
| Others | 43 (22) | 190 (22) | .411 |
Values are reported as median (range) number (percentage) of patients. High dFLC is >50 mg/L, and low-dFLC is <50 mg/L. “Thalidomide-based-regimen” indicates cyclophosphamide, thalidomide, and dexamethasone or thalidomide and dexamethasone. “Others” indicates cyclophosphamide and dexamethasone, high-dose dexamethasone, melphalan and prednisone, cyclophosphamide-lenalidomide-dexamethasone, lenalidomide, and dexamethasone.
ASCT, autologous stem cell transplant; B, bortezomib; CyBorD, cyclophosphamide, bortezomib and dexamethasone; MDex, melphalan-dexamethasone; MP, monoclonal protein; NYHA, New York Heart Association; PNS, peripheral nervous system; PS-ECOG, performance status of the Eastern Cooperative Oncology Group.
Survival outcome
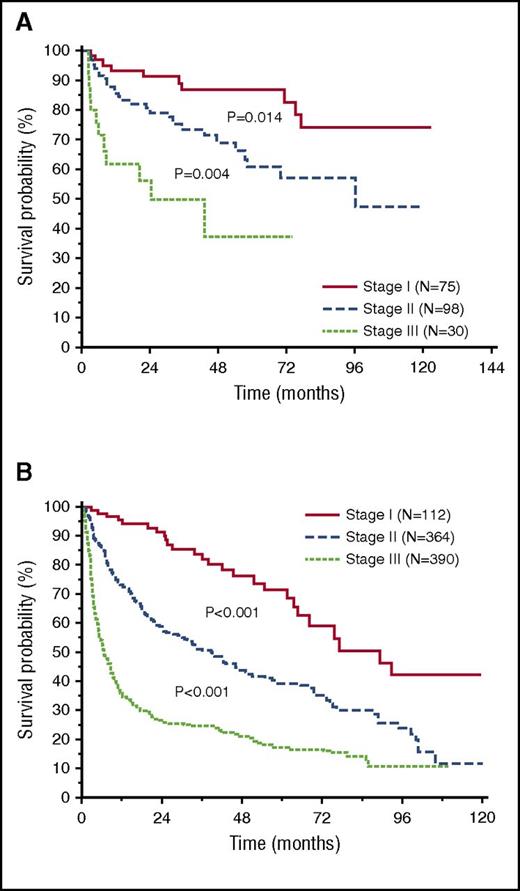
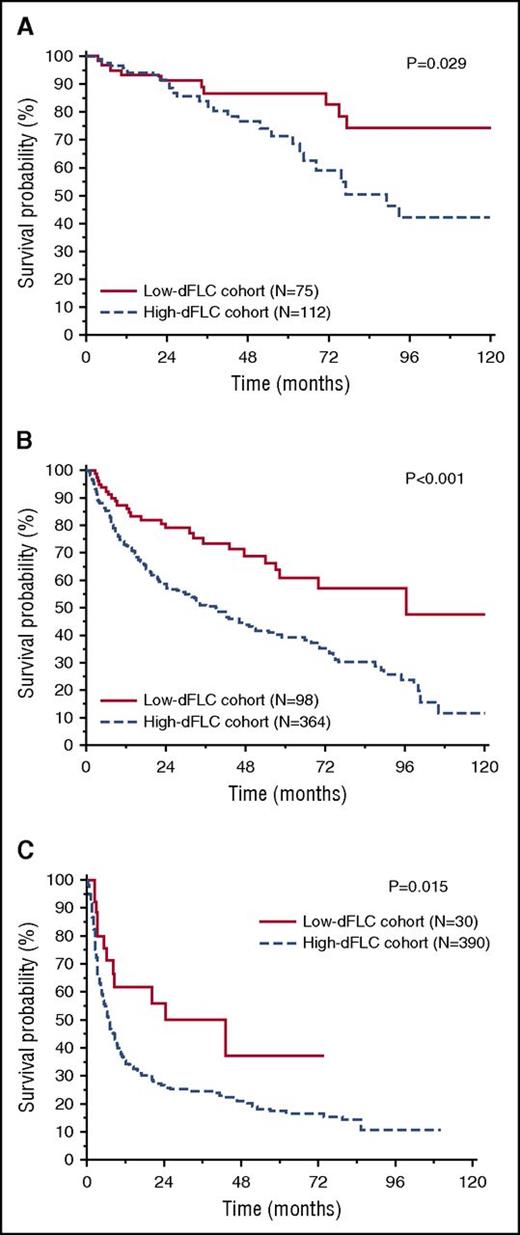
After a median follow-up of living patients of 28 months, 554 patients (52%) died in the entire cohort, 56 (27%) in the low-dFLC group, and 498 (57%) in the high-dFLC group. In the entire cohort, low-dFLC patients had a significantly better overall survival (median survival 118 vs 21 months, P < .001; Figure 1). The survival advantage of low-dFLC patients was observed in patients with and without heart involvement (median survival not reached vs 75 months, P = .011, and median survival 81 vs 14 months, P < .001, respectively). When considering separately the subsets of patients treated with melphalan and dexamethasone and with bortezomib-based regimens, low-dFLC subjects had a significantly better overall survival independent of treatment type (P < .001 in both the analysis; supplemental Figure 1A-B, available on the Blood Web site). The Mayo 2004 staging system was able to clearly discriminate 3 groups of patients with significant different survival in both the low- and high-dFLC groups (Figure 2A-B). However, within each Mayo stage, patients with low dFLC had a longer survival (88% vs 78% at 3 years in stage I, P = .029; 69% vs 45% in stage II, P < .001; and median survival 43 vs 11 months in stage III, P = .015; Figure 3A-C). A multivariate model that included dFLC and Mayo stage showed that the cutoff of 50 mg/L was an independent predictor of overall survival (Table 2).
Overall survival according to dFLC 50 mg/L and the first line of therapy in the entire cohort of patients (P < .001). In the low-dFLC cohort (N = 203), median survival was 118 months. In the high-dFLC cohort (N = 866), median survival was 21 months.
Overall survival according to dFLC 50 mg/L and the first line of therapy in the entire cohort of patients (P < .001). In the low-dFLC cohort (N = 203), median survival was 118 months. In the high-dFLC cohort (N = 866), median survival was 21 months.
Overall survival according to Mayo stage 2004. (A) In the low-dFLC cohort, median survival was not reached (stage I, N = 75), 96 months (stage II, N = 98), or 43 months (stage III, N = 30). (B) In the high-dFLC cohort, median survival was 89 months (stage I, N = 112), 38 months (stage II, N = 364), or 6.6 months (stage III, N = 390).
Overall survival according to Mayo stage 2004. (A) In the low-dFLC cohort, median survival was not reached (stage I, N = 75), 96 months (stage II, N = 98), or 43 months (stage III, N = 30). (B) In the high-dFLC cohort, median survival was 89 months (stage I, N = 112), 38 months (stage II, N = 364), or 6.6 months (stage III, N = 390).
Overall survival according to dFLC 50 mg/L in the different cardiac stages. (A) Overall survival according to dFLC 50 mg/L in patients in Mayo stage I (P = .029). In the low-dFLC cohort (N = 75), median survival was not reached. In the high-dFLC cohort (N = 112), median survival was 89 months. (B) Overall survival according to dFLC 50 mg/L in Mayo stage II patients (P < .001). In the low-dFLC cohort (N = 98), median survival was 96 months. In the high-dFLC cohort (N = 364), median survival was 38 months. (C) Overall survival according to dFLC 50 mg/L in stage III patients (P = .015). In the low-dFLC cohort (N = 30), median survival was 43 months. In the high-dFLC cohort (N = 390), median survival was 6.6 months.
Overall survival according to dFLC 50 mg/L in the different cardiac stages. (A) Overall survival according to dFLC 50 mg/L in patients in Mayo stage I (P = .029). In the low-dFLC cohort (N = 75), median survival was not reached. In the high-dFLC cohort (N = 112), median survival was 89 months. (B) Overall survival according to dFLC 50 mg/L in Mayo stage II patients (P < .001). In the low-dFLC cohort (N = 98), median survival was 96 months. In the high-dFLC cohort (N = 364), median survival was 38 months. (C) Overall survival according to dFLC 50 mg/L in stage III patients (P = .015). In the low-dFLC cohort (N = 30), median survival was 43 months. In the high-dFLC cohort (N = 390), median survival was 6.6 months.
Multivariable proportional hazards models incorporating dFLC
| . | Overall survival (985 cases, 503 events) . | P . | Renal survival* (670 cases, 90 events) . | P . |
|---|---|---|---|---|
| dFLC <50 mg/L | 0.44 (0.31-0.64) | <.001 | 0.83 (0.54-1.28) | .403 |
| Mayo stage II vs I | 2.67 (1.86-3.82) | <.001 | Not included | — |
| Mayo stage III vs I | 5.56 (3.84-8.03) | <.001 | Not included | — |
| Renal stage II vs I | Not included | — | 16.9 (2.33-122.2) | <.001 |
| Renal stage III vs I | Not included | — | 38.6 (5.30-280.1) | <.001 |
| . | Overall survival (985 cases, 503 events) . | P . | Renal survival* (670 cases, 90 events) . | P . |
|---|---|---|---|---|
| dFLC <50 mg/L | 0.44 (0.31-0.64) | <.001 | 0.83 (0.54-1.28) | .403 |
| Mayo stage II vs I | 2.67 (1.86-3.82) | <.001 | Not included | — |
| Mayo stage III vs I | 5.56 (3.84-8.03) | <.001 | Not included | — |
| Renal stage II vs I | Not included | — | 16.9 (2.33-122.2) | <.001 |
| Renal stage III vs I | Not included | — | 38.6 (5.30-280.1) | <.001 |
Mayo stage is based on NT-pro-BNP >332 ng/L and troponin I >0.1 ng/mL. Renal stage is based on proteinuria 5 g per 24 hours and estimated glomerular filtration rate 50 mL/min per 1.73 m2.
dFLC, difference between involved and uninvolved free light chains.
Only in patients with renal involvement.
In the whole cohort, a total of 107 (10%) patients required dialysis. No difference in renal survival was observed between high- and low-dFLC groups, both in the overall population (at 3 years 37% vs 25%, P = .856) and in the subset of patients with renal involvement (at 3 years 34% vs 22%, P = .860). Renal stage was able to sharply discriminate 3 groups with significantly different renal survival both in the high- and low-dFLC groups (supplemental Figure 2A-B). Within each renal stage, the dFLC 50 mg/L cutoff did not predict renal survival.
Response assessment
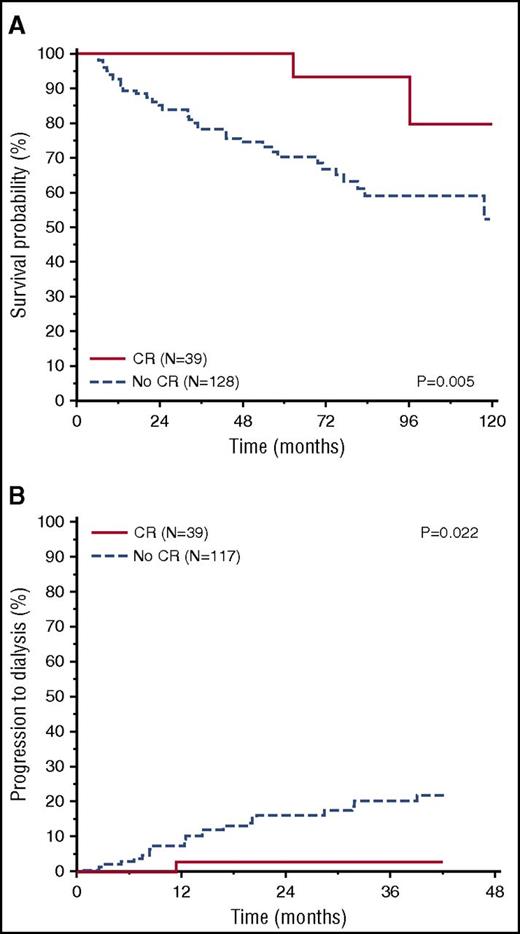
Response to therapy was assessed in the whole cohort at 6 months from treatment initiation and also at 3 months in a subset of patients (115, 56%) with available data. In Table 3, we report hematologic and organ response at 6 months in the low-dFLC cohort. Complete response was obtained in 39 patients (19%) in the low-dFLC group and 82 patients (10%) in the high-dFLC group (P = .002). In this group, VGPR was achieved in 184 patients (21%) and PR in 124 patients (14%). In patients with low dFLC, achieving a CR 6 months after first-line therapy was associated with a survival advantage (median not reached vs 118 months, P = .005; Figure 4A). CR after therapy was also associated with a significant improvement in renal outcome (97% vs 82% at 2 years, P = .022; Figure 4B). Only 9 out of 115 patients obtained a CR after 3 months of therapy, preventing assessment of the impact of early CR on outcome.
Hematologic and organ response assessment at 6 months in the low-dFLC cohort in the subset of patients with baseline dFLC <20 mg/L
| . | Hematologic response, n (%) . | Cardiac response, n (%) . | Renal response, n (%) . | Overall survival at 5 y, % . | Renal survival at 3 y, % . |
|---|---|---|---|---|---|
| CR | 20 (21) | 4/7 (57) | 3/16 (18) | 93 | 97 |
| Low dFLC PR | 38 (39) | 6/17 (35) | 5/30 (16) | 93 | 96 |
| No response | 38 (60) | 4/21 (19) | 1/39 (2) | 57 | 76 |
| . | Hematologic response, n (%) . | Cardiac response, n (%) . | Renal response, n (%) . | Overall survival at 5 y, % . | Renal survival at 3 y, % . |
|---|---|---|---|---|---|
| CR | 20 (21) | 4/7 (57) | 3/16 (18) | 93 | 97 |
| Low dFLC PR | 38 (39) | 6/17 (35) | 5/30 (16) | 93 | 96 |
| No response | 38 (60) | 4/21 (19) | 1/39 (2) | 57 | 76 |
Cardiac response is defined as a decrease in NT-pro-BNP >30% and >300 ng/L (in patients with a baseline NT-pro-BNP >650 ng/L). Renal response required a decrease in proteinuria >30% or <0.5 g per 24 hours in the absence of renal progression (decrease in estimated glomerular filtration rate ≥25%). Low dFLC PR indicates a reduction of dFLC <10 mg/L.
Overall survival and renal survival according to CR in low-dFLC patients. (A) Overall survival according to CR (P = .005); landmark analysis at 6 months (CR, N = 39, median survival not reached; no CR, N = 128, median survival = 117 months). (B) Renal survival according to CR (P = .022) (CR, N = 39, renal survival at 2 years = 97%; no CR, N = 117, renal survival at 2 years = 82%).
Overall survival and renal survival according to CR in low-dFLC patients. (A) Overall survival according to CR (P = .005); landmark analysis at 6 months (CR, N = 39, median survival not reached; no CR, N = 128, median survival = 117 months). (B) Renal survival according to CR (P = .022) (CR, N = 39, renal survival at 2 years = 97%; no CR, N = 117, renal survival at 2 years = 82%).
Different cutoffs of dFLC after treatment were evaluated in order to identify possible predictors of overall survival or renal outcome in the low-dFLC cohort. In the parallel German cohort,15 a reduction of dFLC <10 mg/L in patients whose baseline dFLC was >20 mg/L was found able to improve outcome. This cutoff emerged from the analysis that was performed in 783 newly diagnosed patients reported by Dittrich et al.15 In this series, the presence of renal response was associated with a reduced level of dFLC at 3 months, and the cutoff of dFLC 10 mg/L was defined. We called this criterion low-dFLC response, and it was tested in the Heidelberg series (testing cohort). We validated this criterion in our cohort (validation cohort) on the 117 (59%) patients in whom baseline dFLC was >20 mg/L at baseline. Sixty-nine patients were evaluable for response at 3 months and 97 patients at 6 months. At 3 months, obtaining a reduction of dFLC <10 mg/L was associated with a significant survival advantage (median survival not reached vs 118 months, P = .049; Figure 5A) and with a better renal outcome (at 3 years 100% vs 73%, P = .036; Figure 5B). At 6 months, a low-dFLC response was also associated with a significant survival advantage (median survival not reached vs 118 months, P = .008; Figure 5C) and with a better renal outcome (at 3 years 96% vs 74%, P = .025; Figure 5D). We compared survival of patients with low-dFLC response (that not obtained CR) with patients who did not obtain a response. We found a significant survival advantage for responders (median survival not reached vs 83 months, P = .022; data not shown).
Overall survival and renal survival according to low-dFLC response in patients of the low-dFLC cohort and a baseline value of dFLC <20 mg/L. (A) Overall survival according to low-dFLC response, 3-month landmark analysis (N = 69, P = .049). In patients who obtained a low-dFLC response, median survival was not reached. In patients who did not obtain a low-dFLC response, median survival was 118 months. (B) Renal survival according to low-dFLC response, 3-month landmark analysis (N = 68, P = .036). In patients who obtained a low-dFLC response, renal survival at 3 years was 100%. In patients who did not obtain a low-dFLC response, renal survival at 3 years was 73%. (C) Overall survival according to the criteria of low-dFLC response, 6-month landmark analysis (N = 97, P = .008). In patients who obtained a low-dFLC response, median survival was not reached. In patients who did not obtain a low-dFLC response, median survival was 118 months. (D) Renal survival according to low-dFLC response, 6-month landmark analysis (N = 94, P = .025). In patients who obtained a low-dFLC response, renal survival at 3 years was 96%. In patients who did not obtain a low-dFLC response, renal survival at 3 years was 74%.
Overall survival and renal survival according to low-dFLC response in patients of the low-dFLC cohort and a baseline value of dFLC <20 mg/L. (A) Overall survival according to low-dFLC response, 3-month landmark analysis (N = 69, P = .049). In patients who obtained a low-dFLC response, median survival was not reached. In patients who did not obtain a low-dFLC response, median survival was 118 months. (B) Renal survival according to low-dFLC response, 3-month landmark analysis (N = 68, P = .036). In patients who obtained a low-dFLC response, renal survival at 3 years was 100%. In patients who did not obtain a low-dFLC response, renal survival at 3 years was 73%. (C) Overall survival according to the criteria of low-dFLC response, 6-month landmark analysis (N = 97, P = .008). In patients who obtained a low-dFLC response, median survival was not reached. In patients who did not obtain a low-dFLC response, median survival was 118 months. (D) Renal survival according to low-dFLC response, 6-month landmark analysis (N = 94, P = .025). In patients who obtained a low-dFLC response, renal survival at 3 years was 96%. In patients who did not obtain a low-dFLC response, renal survival at 3 years was 74%.
Discussion
Our study showed that patients with low-dFLC burden represent a distinct subgroup characterized by less frequent and less severe cardiac involvement and prolonged survival. This survival advantage, however, was not only due to less severe heart dysfunction, because it was observed across all cardiac stages, indicating that a low dFLC level represents a distinct independent factor associated with good prognosis. This is consistent with previous observations of the prognostic relevance of FLC concentration and bone marrow plasma cell burden23 and, together with recent observations of the impact of chromosomal abnormalities on treatment outcome, further emphasizes the importance of clonal characteristics as prognostic markers in AL amyloidosis.24-26 In particular, as previously published by the Mayo group,7 dFLC is an independent prognostic marker along with cardiac biomarkers, and we showed that is independent in a multivariate analysis with advanced cardiac staging with a much lower cutoff. Taken together, these data and our observation indicate that specific characteristics of the amyloid plasma cell clone can result in a more aggressive and less easily treatable presentation. The parallel German study gave very similar results, further strengthening this observation. Thus, the exclusion of patients with a dFLC <50 mg/L from clinical trials not only has an impact on patient accrual but also results in a selection bias, potentially leading to underestimating treatment efficacy by excluding a significant proportion (∼20%) of low-risk patients.
The reason for the exclusion of patients with a low dFLC concentration from clinical trials is the inapplicability of the validated criteria for a partial response (PR) (50% reduction in dFLC) and VGPR (dFLC reduction <40 mg/L). In the present study, we evaluated and validated novel specific response criteria for patients whose baseline dFLC concentration is <50 mg/L. CR retains its ability to predict overall survival and renal outcome, as in the overall population of patients with AL amyloidosis. Moreover, we validated the observation made in the parallel study of the Heidelberg group that patients whose dFLC decrease <10 mg/L at 3 and/or 6 months after treatment initiation have a longer overall survival and are less likely to require dialysis. This can be proposed as the criterion for PR in patients with baseline dFLC between 20 and 50 mg/L, allowing inclusion in clinical trials of almost 60% of patients who are now excluded due to a low dFLC. Interestingly, a significant survival advantage of patients who obtained a low-dFLC response was also seen with the exclusion of patients who obtained a CR.
Even more importantly, the observation that an FLC decrease within the reference range retains a remarkable prognostic significance in AL amyloidosis emphasizes the need for more specific tools for FLC measurement. For example, the assessment of urinary MP is not considered for the evaluation of response to therapy per current criteria in AL amyloidosis. In this series, we have no data on urinary MP assessment, but in our center, a prospective study is ongoing to evaluate the role of this measurement for the assessment of response.27 Novel mass spectrometry–based assays will hopefully address this need in the near future.28-31 In addition, further studies are warranted to assess the ability of multiparameter flow-cytometer studies32,33 in the assessment of minimal residual disease in AL amyloidosis.
In conclusion, our study showed that patients with AL amyloidosis and low dFLC burden have distinct clinical features and a better clinical outcome. These patients should be included in clinical trials with appropriate stratification, and response to treatment should be assessed with the novel specific hematologic response criteria validated in the 2 parallel German and Italian studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (Special Program Molecular Clinical Oncology 5 per mille 9965) and Fondazione Cariplo (Structure-function relation of amyloid: understanding the molecular bases of protein misfolding diseases to design new treatments 2013-0964 and Molecular mechanisms of Ig toxicity in age-related plasma cell dyscrasias 2015-0591). G.P. is supported, in part, by the Bart Barlogie Young Investigator Award from the International Myeloma Society (IMS). The authors acknowledge the study coordinator and data manager, Anna Carnevale Baraglia.
Authorship
Contribution: P.M. and G.P. designed the study, evaluated patients, collected data, analyzed data, wrote the manuscript, and gave final approval; G.M. designed the study, evaluated patients, critically reviewed the manuscript, and gave final approval; and M.B., F.R., and A.F. evaluated patients, collected data, critically reviewed the manuscript, and gave final approval.
Conflict-of-interest disclosure: G.P. received honoraria from Janssen-Cilag, honoraria and travel support from Prothena, and travel support from Celgene. G.M. is consultant for Takeda, Pfizer, Janssen, Prothena, and Ionis. The remaining authors declare no competing financial interests.
Correspondence: Giampaolo Merlini, Amyloidosis Research and Treatment Center, Foundation IRCCS Policlinico San Matteo, Viale Golgi, 19, 27100 Pavia, Italy; e-mail: gmerlini@unipv.it.