Key Points
ZFP521 regulates HSC self-renewal and differentiation.
ZFP521 facilitates leukemogenesis in an MLL-AF9–mediated leukemia model.
Abstract
The concept that tumor-initiating cells can co-opt the self-renewal program of endogenous stem cells as a means of enforcing their unlimited proliferative potential is widely accepted, yet identification of specific factors that regulate self-renewal of normal and cancer stem cells remains limited. Using a comparative transcriptomic approach, we identify ZNF521/Zfp521 as a conserved hematopoietic stem cell (HSC)–enriched transcription factor in human and murine hematopoiesis whose function in HSC biology remains elusive. Competitive serial transplantation assays using Zfp521-deficient mice revealed that ZFP521 regulates HSC self-renewal and differentiation. In contrast, ectopic expression of ZFP521 in HSCs led to a robust maintenance of progenitor activity in vitro. Transcriptional analysis of human acute myeloid leukemia (AML) patient samples revealed that ZNF521 is highly and specifically upregulated in AMLs with MLL translocations. Using an MLL-AF9 murine leukemia model and serial transplantation studies, we show that ZFP521 is not required for leukemogenesis, although its absence leads to a significant delay in leukemia onset. Furthermore, knockdown of ZNF521 reduced proliferation in human leukemia cell lines possessing MLL-AF9 translocations. Taken together, these results identify ZNF521/ZFP521 as a critical regulator of HSC function, which facilitates MLL-AF9–mediated leukemic disease in mice.
Introduction
Hematopoietic stem cells (HSCs) self-renew and give rise to all blood lineages, enabling lifelong blood cell production. How HSCs balance self-renewal and differentiation remains elusive. A better understanding into how these processes are regulated could inform strategies to improve the clinical utility of HSCs and may provide insight into the basis of hematopoietic malignancy.
Zinc-finger protein 521 (human, ZNF521; mouse, Zfp521) encodes a zinc-finger domain–bearing transcription factor that is widely expressed across many tissues,1 where it has been shown to regulate self-renewal and differentiation. In mesenchymal stem cells, ZFP521 regulates differentiation by repressing RUNX2, EBF1, and ZFP423,2-4 and modulates apoptosis via BCL-2.5 In embryonic stem cells, ectopic expression of ZNF521/ZFP521 directs differentiation toward a self-renewing neural progenitor cell fate.6,7 In the blood, short hairpin RNA (shRNA)-mediated approaches have shown that ZNF521/ZFP521 represses B-cell and erythroid differentiation in vitro8,9 and regulates in vivo progenitor cell function.10 While ZNF521/Zfp521 has been shown to be expressed within primitive hematopoietic compartments,10-12 the functions of this gene within HSCs have yet to be fully characterized.
Herein, we identified ZNF521/Zfp521 as a conserved HSC-enriched gene within the human and murine hematopoietic systems, and using loss-of-function and gain-of-function approaches, we demonstrate that it regulates HSC self-renewal and differentiation. We show that ZNF521 is specifically upregulated in acute myeloid leukemias (AMLs) possessing MLL translocations and that it modulates proliferation in human MLL-AF9 leukemic cell lines and facilitates MLL-AF9 driven leukemogenesis in mice. These results establish ZNF521/ZFP521 as a regulator of HSC biology that also plays a role in MLL-AF9–mediated leukemic disease in mice.
Study design
Identification of HSC-enriched genes
Microarray datasets curated from the Gene Expression Omnibus were normalized and used to identify HSC-enriched genes in comparison with downstream progenitor and effector cells using a 5.0 fold-change (FC) cutoff and P < .005.
Competitive transplantation
A total of 2 × 106 whole fetal liver cells from 2 biological wild-type (WT) replicates and 2 biological Zfp521-deficient replicates were separately transplanted against 1 × 106 bone marrow competitor cells into lethally irradiated congenic recipients (5 recipients per biological group; 10 recipients per genotype). Serial transplantation was performed by transplanting 1 × 106 donor-derived cells against 2 × 105 competitor cells (10 recipients per genotype). For statistical analysis of each group at all time points, the mean, standard error of the mean (SEM), and P values (2 sided) were determined by GraphPad Prism software using an unpaired t test for the entire WT (Zfp521+/+) or knockout (KO; Zfp521−/−) group.
CFC assays
A total of 2.5 × 102 murine HSCs (lineage−c-Kit+Sca-1+CD34−Flk2−) were transduced with doxycycline-inducible ZsGreen (control), ZFP521, or ZFP521(ΔNuRD) encoding lentivirus and plated into methylcellulose supplemented with doxycycline. Colonies were scored 10 to 12 days postculture. Serial colony-forming cell (CFC) assays were performed by replating 1 × 104 cells per well (6-well plate).
Induction of leukemia
c-Kit+ fetal liver cells from 2 Zfp521+/+ and 2 Zfp521−/− mice were pooled and transduced with MigR1-MLL-AF9-GFP retrovirus,13 cultured for 3 days, and injected into sublethally irradiated congenic recipients.
See supplemental Methods (available on the Blood Web site) for full details.
Results and discussion
ZNF521/Zfp521 is an HSC-enriched transcription factor in human and murine hematopoiesis
We sought to identify and characterize novel HSC-specific transcription factors. Utilizing a comparative transcriptomics approach, we analyzed the transcriptional profiles of human and murine HSCs in comparison with their downstream progenitor and effector cells (supplemental Table 1), identifying 364 human and 172 murine HSC-enriched genes with a FC > 5 and P < .005 (supplemental Tables 2 and 3). Of these identified genes, 26 genes were HSC enriched in both species (supplemental Figure 1A; supplemental Table 4), although most were also expressed to varying degrees in downstream cells (supplemental Figure 1B-C). The conserved genes included 6 transcription factors (HLF, MECOM, MEIS1, PRDM16, HOXA9, and ZNF521) that have been previously identified in murine or human hematopoietic stem and progenitor subsets.10,14-18 Of these, all but ZNF521/Zfp521 have been experimentally validated as regulators of HSC potential.14,19-23 Therefore, we focused on characterizing the role of Zfp521 in HSC biology. ZNF521/Zfp521 encodes a Kruppel-like zinc-finger domain–containing factor, which contains 30 C2H2 zinc-finger DNA-binding domains, and an N-terminal nucleosome remodeling deacetylase (NuRD) complex–binding domain (supplemental Figure 1D).9,11
ZFP521 regulates murine HSC self-renewal and differentiation
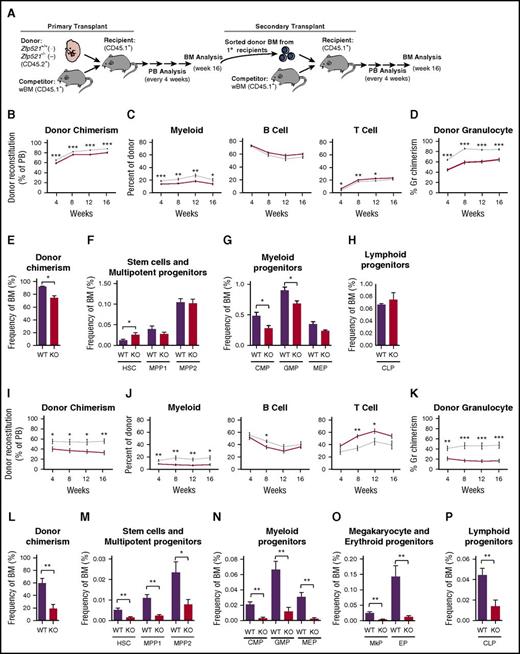
To assess the role of ZFP521 in HSCs, we competitively transplanted whole fetal liver cells from Zfp521+/+ (WT) or Zfp521−/− (KO)24 E17.5 embryos against 1 × 106 whole bone marrow cells (CD45.1+) into lethally irradiated congenic recipients (Figure 1A). Fetal liver was used, because Zfp521-deficient mice die postnatally.24 Zfp521 deficiency did not affect total fetal liver cell numbers or cell frequencies of stem and progenitor subsets (data not shown). Zfp521 deficiency led to a small but significant decrease in total donor chimerism at 16 weeks posttransplantation, which was underwritten by diminished myeloid reconstitution (Figure 1B-C). Long-term lymphoid reconstitution was unaffected, although marginal yet statistically significant differences were observed at earlier time points (Figure 1C). Diminished donor granulocyte chimerism, which is used as a surrogate for ongoing HSC potential,25 suggested a potential deficit in HSC function in the absence of ZFP521 (Figure 1D). To explore this further, we analyzed the bone marrow compartment of recipient mice at 16 weeks posttransplantation. While loss of Zfp521 resulted in a significant decrease in total bone marrow chimerism (Figure 1E), it also unexpectedly resulted in an increase in HSC frequency (Figure 1F). Analysis of progenitor cell subsets revealed significant reductions in both common myeloid progenitor (CMP) and granulocyte-macrophage progenitor (GMP) cell populations (Figure 1G), consistent with the decreased myeloid cell output observed in the periphery. In line with the observed preservation of long-term B- and T-cell potential, no differences were observed in common lymphoid progenitor (CLP) frequency (Figure 1H).
ZFP521 regulates murine HSC self-renewal and differentiation. (A) Schematic representation of serial competitive transplantation studies. (B-D) Peripheral blood analysis of primary transplant recipients (10 recipients per genotype) of WT (Zfp521+/+) and Zfp521 KO (Zfp521−/−) whole fetal liver cells showing (B) total donor chimerism; (C) donor contribution to myeloid, B, and T cells; and (D) granulocyte chimerism. (E-H) Bone marrow analysis at 16 weeks posttransplantation showing (E) total chimerism and frequencies of (F) HSCs, MPPs (MPP1 and MPP2), (G) CMPs, GMPs, megakaryocyte-erythroid progenitors (MEPs), and (H) CLPs. Peripheral blood analysis of secondary transplant recipients (10 recipients per genotype) showing (I) total donor chimerism; (J) donor contribution to myeloid, B, and T cells; and (K) granulocyte chimerism. (L-P) Bone marrow analysis at 16 weeks posttransplantation showing (L) total chimerism and frequencies of (M) HSCs, MPP1, and MPP2; (N) CMPs, GMPs, and MEPs, (O) megakaryocyte progenitors, and erythroid progenitors, and (P) CLPs. Unpaired t test: *P < .05, **P < .01, ***P < .001. BM, bone marrow; EP, erythroid progenitor; Gr, granulocyte; MkP, megakaryocyte progenitor; PB, peripheral blood; wBM, whole bone marrow.
ZFP521 regulates murine HSC self-renewal and differentiation. (A) Schematic representation of serial competitive transplantation studies. (B-D) Peripheral blood analysis of primary transplant recipients (10 recipients per genotype) of WT (Zfp521+/+) and Zfp521 KO (Zfp521−/−) whole fetal liver cells showing (B) total donor chimerism; (C) donor contribution to myeloid, B, and T cells; and (D) granulocyte chimerism. (E-H) Bone marrow analysis at 16 weeks posttransplantation showing (E) total chimerism and frequencies of (F) HSCs, MPPs (MPP1 and MPP2), (G) CMPs, GMPs, megakaryocyte-erythroid progenitors (MEPs), and (H) CLPs. Peripheral blood analysis of secondary transplant recipients (10 recipients per genotype) showing (I) total donor chimerism; (J) donor contribution to myeloid, B, and T cells; and (K) granulocyte chimerism. (L-P) Bone marrow analysis at 16 weeks posttransplantation showing (L) total chimerism and frequencies of (M) HSCs, MPP1, and MPP2; (N) CMPs, GMPs, and MEPs, (O) megakaryocyte progenitors, and erythroid progenitors, and (P) CLPs. Unpaired t test: *P < .05, **P < .01, ***P < .001. BM, bone marrow; EP, erythroid progenitor; Gr, granulocyte; MkP, megakaryocyte progenitor; PB, peripheral blood; wBM, whole bone marrow.
Serial transplantation revealed that Zfp521−/− HSCs exhibited diminished long-term repopulating activity (Figure 1I) that was more exacerbated than that of primary transplants (Figure 1B). Lineage analysis revealed that T-cell reconstitution was significantly elevated at 8 and 12 weeks posttransplantation, but not at 16 weeks posttransplantation (Figure 1J). While it has previously been reported that ZNF521/Zfp521 shRNA-mediated knockdown in hematopoietic progenitors increased B-cell differentiation in vitro,8 transplantation of Zfp521-deficient donor cells resulted in B-cell reconstitution that was only modestly elevated in primary hosts and decreased in secondary hosts, respectively (Figure 1C,J). By contrast, myeloid cell and granulocyte differentiation was significantly diminished in the absence of Zfp521 in the secondary recipients (Figure 1J-K), further suggestive of a deficit in HSC function following Zfp521 loss.
Bone marrow analysis of secondary recipients revealed decreased total chimerism at 4 months posttransplantation (Figure 1L). This was underwritten by a marked decrease in HSC frequency, as well as diminished frequencies of all downstream progenitors analyzed, including multipotent progenitors (MPPs) 1 and 2, oligopotent myeloid (CMP, GMP, and MEP) and lymphoid (CLP) progenitors, and lineage-restricted megakaryocyte and erythroid progenitors (Figure 1M-P). Cumulatively, these results strongly suggest that ZFP521 regulates HSC self-renewal.
ZFP521 regulates HSC self-renewal ex vivo
To further investigate the role of ZFP521 in murine HSCs, we used a doxycycline-inducible lentivirus system14,15 to assess how enforced expression of ZFP521 affects HSC potential in CFC serial replating assays. HSCs from transgenic mice expressing the reverse tet-transactivator at the Rosa26 locus26 were transduced with lentiviruses encoding ZFP521, ZFP521 lacking the NuRD domain (ZFP521(ΔNuRD)), or ZsGreen (control), followed by plating in methylcellulose supplemented with doxycycline. HSCs transduced with each of the constructs produced similar numbers of colonies with comparable composition in the primary plating (Figure 2A). However, while the CFC potential of control-transduced cells was rapidly exhausted in secondary and tertiary replating as expected, ZFP521-transduced cells continued to generate high numbers of colonies (Figure 2A). Interestingly, ZFP521(ΔNuRD)-transduced cells generated colonies through tertiary plating; however, fewer colonies were formed, indicating that the serial replating activity of ZFP521 was, at least in part, dependent on its NuRD domain (Figure 2A). Consistent with this, ZFP521 transduced HSCs maintained in culture (4 weeks) showed a significantly higher percentage of cells in the S/G2/M phase of the cell cycle compared with control and ZFP521(ΔNuRD)-transduced cells (Figure 2B). These results may also reflect the previously observed prosurvival activity of ZFP521.5,27,28 Histological staining showed that ZFP521-transduced cells contain multiple myeloid cell types post-culturing, including macrophages, granulocytes and less differentiated progenitor-like cells, whereas the control and ZFP521(ΔNuRD)-transduced cultures predominantly contained macrophages (Figure 2C). Taken together, these results demonstrate that ectopic expression of ZFP521 imparts enhanced self-renewal activity in vitro underwritten by increased cell cycling and maintenance of progenitor activity.
ZFP521 regulates self-renewal ex vivo and facilitates MLL-AF9–mediated AML leukemia in mice. (A) Serial replating assays of HSCs transduced with lentiviruses encoding ZsGreen (control), ZFP521, or ZFP521(ΔNuRD). (B) Representative histograms (left) and quantitation (right) of DNA content per cell cycle from 3 independent experiments. The percentage of cells in S/G2/M phase of the cell cycle is presented as mean ± SEM. Unpaired t test: ***P < .001. (C) Wright-Giemsa staining (G, granulocyte; M, macrophage; PL, progenitor-like cell) of cultured HSC-derived cells ectopically expressing ZsGreen (control), ZFP521, or ZFP521(ΔNuRD). Scale bar represents 50 µm. (D) Heatmap showing relative expression (red [high expression] to blue [low expression]) of 26 conserved HSC-enriched genes, including the 6 indicated transcription factors within patient samples from 4 AML cytogenetic subtypes. See supplemental Table 4 for a complete gene list. (E-F) Kaplan-Meier survival curves following (E) primary and (F) secondary transplantation of MLL-AF9–transduced cells on a WT or Zfp521-deficient (KO) background, with the number (n) of mouse recipients indicated per group. Log-rank (Mantel-Cox) testing was conducted for statistical analyses; *P < .05, **P < .01. (G) Serial plating of bone marrow–derived MLL-AF9–transduced cells. Number of colonies at each passage is presented as mean ± SEM of 3 independent experiments. Unpaired t test: *P < .05, **P < .01. (H) Relative cell growth of MLL-AF9–positive (NOMO-1 and THP-1) or non–MLL-AF9 AML cells (OCI-AML3, HL-60, and U937) following doxycycline (+DOX)–induced shRNA-mediated ZNF521 knockdown (shZNF521) or scramble shRNA (shCONTROL) compared with the uninduced (−DOX) condition. Relative cell growth (+DOX/−DOX) values are presented as mean ± SEM of 3 independent experiments. Unpaired t test: *P < .05, **P < .01.
ZFP521 regulates self-renewal ex vivo and facilitates MLL-AF9–mediated AML leukemia in mice. (A) Serial replating assays of HSCs transduced with lentiviruses encoding ZsGreen (control), ZFP521, or ZFP521(ΔNuRD). (B) Representative histograms (left) and quantitation (right) of DNA content per cell cycle from 3 independent experiments. The percentage of cells in S/G2/M phase of the cell cycle is presented as mean ± SEM. Unpaired t test: ***P < .001. (C) Wright-Giemsa staining (G, granulocyte; M, macrophage; PL, progenitor-like cell) of cultured HSC-derived cells ectopically expressing ZsGreen (control), ZFP521, or ZFP521(ΔNuRD). Scale bar represents 50 µm. (D) Heatmap showing relative expression (red [high expression] to blue [low expression]) of 26 conserved HSC-enriched genes, including the 6 indicated transcription factors within patient samples from 4 AML cytogenetic subtypes. See supplemental Table 4 for a complete gene list. (E-F) Kaplan-Meier survival curves following (E) primary and (F) secondary transplantation of MLL-AF9–transduced cells on a WT or Zfp521-deficient (KO) background, with the number (n) of mouse recipients indicated per group. Log-rank (Mantel-Cox) testing was conducted for statistical analyses; *P < .05, **P < .01. (G) Serial plating of bone marrow–derived MLL-AF9–transduced cells. Number of colonies at each passage is presented as mean ± SEM of 3 independent experiments. Unpaired t test: *P < .05, **P < .01. (H) Relative cell growth of MLL-AF9–positive (NOMO-1 and THP-1) or non–MLL-AF9 AML cells (OCI-AML3, HL-60, and U937) following doxycycline (+DOX)–induced shRNA-mediated ZNF521 knockdown (shZNF521) or scramble shRNA (shCONTROL) compared with the uninduced (−DOX) condition. Relative cell growth (+DOX/−DOX) values are presented as mean ± SEM of 3 independent experiments. Unpaired t test: *P < .05, **P < .01.
ZFP521 facilitates MLL-AF9–mediated leukemia in mice
Given that self-renewal is a defining property of hematopoietic malignancies, we examined the expression of ZNF521 and the other conserved HSC-enriched genes within 4 patient-derived AML subsets possessing different cytogenetic lesions. Interestingly, whereas ZNF521 was not expressed in AMLs associated with t(15;17) or t(8;21) translocations, it was robustly upregulated in AMLs possessing MLL t(11q23) translocations (Figure 2D), as has been previously suggested.11,29,30 MLL translocations in leukemia are often associated with poor prognosis and underlie not only 70% to 80% of infant (<1 year) acute lymphoblastic leukemias but also 50% to 60% of infant AMLs.31-33 To assess the importance of ZNF521/ZFP521 in the context of MLL-AF9–mediated leukemia, we transduced WT and Zfp521-deficient c-Kit+ fetal liver cells with MigR1-MLL-AF9-GFP retrovirus13 and transplanted them into congenic recipients (supplemental Figure 2A). Zfp521 deficiency led to significantly delayed mortality in primary transplant recipients (Figure 2E), although all mice eventually succumbed to leukemic disease regardless of genotype (supplemental Figure 2B). A significant delay in mortality was again observed upon secondary transplantation, indicating that ZFP521 loss delays leukemic onset, although it was not strictly required for leukemogenesis or leukemic self-renewal (Figure 2F). Moreover, MLL-AF9–expressing primary bone marrow cells on a Zfp521-deficient background displayed reduced colony-forming ability following serial plating in methylcellulose (Figure 2G). Consistent with this, MLL-AF9–expressing cells taken from the periphery of primary transplant recipients showed slightly reduced blast-like cells, increased neutrophil terminal differentiation, and increased apoptosis on the Zfp521-deficient background (supplemental Figure 2C-D).
To further evaluate the role of ZNF521 in human MLL-AF9–positive AML cells, we generated stable doxycycline-inducible shRNA NOMO-1 and THP-1 cell lines using a previously characterized ZNF521-specific shRNA (shZNF521).8 These cell lines express high levels of ZNF521 transcript compared with non–MLL-AF9 AML cell lines (OCI-AML3, HL-60, and U937) (supplemental Figure 2E). Transient knockdown of ZNF521 significantly reduced cell growth of both NOMO-1 and THP-1 cell lines compared with scramble control shRNA (shCONTROL) (Figure 2H), which was associated with an increase in the proportion of cells in the G0/G1 phase of the cell cycle (supplemental Figure 2F). By contrast, the non-MLL-AF9 cell lines were unaffected by ZNF521 knockdown (Figure 2H). Taken together, these results demonstrate that ZFP521/ZNF521 facilitates MLL-AF9–mediated leukemogenesis in mice and regulates the growth of human leukemia cell lines possessing MLL-AF9 translocations.
In conclusion, our results identify ZNF521/ZFP521 as a critical regulator of HSC biology and a facilitator of MLL-AF9–driven leukemic disease in mice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ulrich H. von Andrian and Aude Thiriot and A. Zguro for their valuable assistance.
This work was supported by the National Institutes of Health (grants R01HL107630, U01HL107440, U01HL099997, and U19HL129903 from the National Heart, Lung and Blood Institute, and grant 1UC4DK104218 from the National Institute of Diabetes and Digestive and Kidney Diseases) (D.J.R.), Google, Inc. (D.J.R.), the Leona M. and Harry B. Helmsley Charitable Trust (D.J.R.), the New York Stem Cell Foundation (D.J.R.), the American Federation for Aging (D.J.R.), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; grant R01AR 057769) (R.B.).
Authorship
Contribution: B.S.G. performed the WT and Zfp521−/− cell transplantation experiments and ex vivo experiments with assistance from B.H. and F.E.M.; B.S.G. and A.P.R. performed ex vivo HSC experiments, MLL-AF9–transduced cell transplantation, and in vitro experiments; A.P.R. performed experiments involving human cell lines; I.B. analyzed the microarray data; R.B., D.B., and D.T.S. provided intellectual guidance; R.B. provided Zfp521−/− mice and some Zfp521 reagents; B.S.G., A.P.R., and D.J.R. designed experiments and interpreted data; and B.S.G., A.P.R., and D.J.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Derrick J. Rossi, 200 Longwood Ave, Warren Alpert Building, Room WAB-149e, Boston Children’s Hospital, Boston, MA 02115; e-mail: derrick.rossi@childrens.harvard.edu.
References
Author notes
B.S.G. and A.P.R. contributed equally to this study.

![Figure 2. ZFP521 regulates self-renewal ex vivo and facilitates MLL-AF9–mediated AML leukemia in mice. (A) Serial replating assays of HSCs transduced with lentiviruses encoding ZsGreen (control), ZFP521, or ZFP521(ΔNuRD). (B) Representative histograms (left) and quantitation (right) of DNA content per cell cycle from 3 independent experiments. The percentage of cells in S/G2/M phase of the cell cycle is presented as mean ± SEM. Unpaired t test: ***P < .001. (C) Wright-Giemsa staining (G, granulocyte; M, macrophage; PL, progenitor-like cell) of cultured HSC-derived cells ectopically expressing ZsGreen (control), ZFP521, or ZFP521(ΔNuRD). Scale bar represents 50 µm. (D) Heatmap showing relative expression (red [high expression] to blue [low expression]) of 26 conserved HSC-enriched genes, including the 6 indicated transcription factors within patient samples from 4 AML cytogenetic subtypes. See supplemental Table 4 for a complete gene list. (E-F) Kaplan-Meier survival curves following (E) primary and (F) secondary transplantation of MLL-AF9–transduced cells on a WT or Zfp521-deficient (KO) background, with the number (n) of mouse recipients indicated per group. Log-rank (Mantel-Cox) testing was conducted for statistical analyses; *P < .05, **P < .01. (G) Serial plating of bone marrow–derived MLL-AF9–transduced cells. Number of colonies at each passage is presented as mean ± SEM of 3 independent experiments. Unpaired t test: *P < .05, **P < .01. (H) Relative cell growth of MLL-AF9–positive (NOMO-1 and THP-1) or non–MLL-AF9 AML cells (OCI-AML3, HL-60, and U937) following doxycycline (+DOX)–induced shRNA-mediated ZNF521 knockdown (shZNF521) or scramble shRNA (shCONTROL) compared with the uninduced (−DOX) condition. Relative cell growth (+DOX/−DOX) values are presented as mean ± SEM of 3 independent experiments. Unpaired t test: *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/5/10.1182_blood-2016-09-738591/4/m_blood738591f2.jpeg?Expires=1771331778&Signature=e0MghPZXa5scCawRiUuknpdzP~ecpABH81kfHtHV9G-8ysGpEFPaFAtmkDzL3hbYy1ZlCFXw-4nkjz6-dIVHoOoDAy19byaLLCHJnFkFx~SuFMuUuaOmHItptcQIT3QQEuFIBQ33hosLJLh~kntFftnDyqImwGES2C75Msg2dw9m92nn7Mag1zYkp-jfSbsSioqlGaP3ii~VWlqQKNtNhywOBWxkjvPzQYCvYNVyJJCbZXPD6Y8ija8vCLB210EBKfyBh5v~sU~HtJp7RdhmDB6xtsmlzQYtMa909xA~lWGaLWM0z5E3-ofRmxgw4kdR-z4X9kQgqod0bSKnqPEidw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal