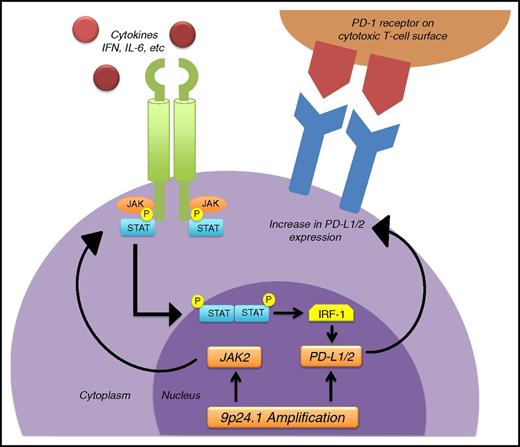
In this issue of Blood, Zinzani et al1 report that the immune check point inhibitor pembrolizumab is active against relapsed/refractory primary mediastinal large B-cell lymphoma (PMLBCL). This unique clinicopathologic entity accounts for up to 10% of all diffuse large B-cell lymphomas (DLBCLs) and is most commonly seen in females between 20 and 40 years of age. Gene expression profiling has shown that the genes expressed in PMLBCL are essentially those of Hodgkin lymphoma (HL).2 Some of these genes are related to the signaling pathways of NF-κB and JAK-STAT (see figure). Of interest is that chromosome 9p24.1 alterations (see figure), which have been associated with programmed death ligand (PD-L1)/L2 upregulation in classical HL, have also been observed in PMLBCL.3 In view of these similarities in gene expression between PMLBCL and HL, it should not come as a surprise that overexpression of the PD-L1 gene, typically seen in HL, is also observed in PMLBCL.
Schematic showing the mechanism of upregulation of PD-L1/2, responsible for the efficacy of the anti–PD-1 antibody pembrolizumab in primary mediastinal large B-cell and in Hodgkin lymphoma. Chromosome 9p24.1 amplification occurs commonly in these 2 disorders, leading to increased production of PD-L1/2, which in turn leads to overexpression of PD-L1/2 on the surface of the malignant cells. Another contributor to overexpression of PD-L1/2 is the JAK-STAT pathway, which when activated by different cytokines, such as interferon-γ (IFN-γ), or by overproduction of JAK2, can induce the transcription of genes that include the interferon regulatory factor 1 (IRF-1), which binds to the PD-L1/2 promoter and induces transcription and surface expression of PD-L1/2. When PD-L1/2 on the lymphoma cell surface binds with PD-1 receptor on the cytotoxic T cells, it suppresses the capacity of the immune system to attack the tumor by inactivating cytotoxic T cells. Pembrolizumab, an anti–PD-1 antibody, blocks the inactivation of the cytotoxic T cells and induces remissions mediated by the immune system. IL-6, interleukin 6.
Schematic showing the mechanism of upregulation of PD-L1/2, responsible for the efficacy of the anti–PD-1 antibody pembrolizumab in primary mediastinal large B-cell and in Hodgkin lymphoma. Chromosome 9p24.1 amplification occurs commonly in these 2 disorders, leading to increased production of PD-L1/2, which in turn leads to overexpression of PD-L1/2 on the surface of the malignant cells. Another contributor to overexpression of PD-L1/2 is the JAK-STAT pathway, which when activated by different cytokines, such as interferon-γ (IFN-γ), or by overproduction of JAK2, can induce the transcription of genes that include the interferon regulatory factor 1 (IRF-1), which binds to the PD-L1/2 promoter and induces transcription and surface expression of PD-L1/2. When PD-L1/2 on the lymphoma cell surface binds with PD-1 receptor on the cytotoxic T cells, it suppresses the capacity of the immune system to attack the tumor by inactivating cytotoxic T cells. Pembrolizumab, an anti–PD-1 antibody, blocks the inactivation of the cytotoxic T cells and induces remissions mediated by the immune system. IL-6, interleukin 6.
In the KEYNOTE-013 trial reported by Zinzani et al, pembrolizumab was administered to a subset of 17 patients with PMLBCL, of which 7 (41%) responded and an additional 6 (35%) of patients had some tumor shrinkage.
The study was a phase 1 trial. It is well-known that patients entered into that type of study usually have far advanced disease and are very refractory to treatment. Response rates are usually poor. As expected, the population entered consisted of multiply relapsed patients with 3 prior lines of therapy, which translates into fourth-line therapy. As compared with previous salvage studies in aggressive lymphomas, in which the average number of prior lines of therapy is 1 or 2, clearly this population was more heavily pretreated. In that context, the response rate of 41%, which at first glance might not appear extremely encouraging, must be considered in a different light. Despite the advanced nature of the patient population, it was encouraging that at a median follow-up of 11.3 months, median duration of response has not been reached. Only 5 of 17 patients have progressed and only 4 have died. Two patients reached the maximum 2-year treatment duration and remain in remission; furthermore, only 1 of the responders has relapsed.
Another crucial point to consider is that the duration of response in all cases was longer than their response to first-line therapy, a highly unusual finding. In addition, there were 3 cases whose response to first-line therapy was only stable disease, yet their response to pembrolizumab was complete response in 1 case and partial response in the other 2.
Currently, the preferred front-line treatment of PMLBCL is an intensive approach reported by Dunleavy et al using chemotherapy with cyclophosphamide, doxorubicin, etoposide, prednisone, rituximab, and vincristine sulfate (DA-R-EPOCH) without radiation therapy.4 With that approach, the failure-free survival is 93%, leaving little room for salvage therapy with pembrolizumab. Although real-world results perhaps might not be as impressive as those reported by Dunleavy et al, the future use of pembrolizumab, instead of rescue therapy, might consist of its use as front-line therapy combined with the classical R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, prednisone, vincristine sulfate), which is much less toxic than DA-R-EPOCH. By doing so, the toxicity of DA-R-EPOCH might be obviated. Most patients with PMLBCL are females of child-bearing potential, which makes the goal of preserving fertility an important objective. Pembrolizumab might be the first step in developing a chemotherapy-free combination with other biological agents that have little or no gonadal toxicity, certainly a desirable goal.
We are being faced with increasingly new knowledge about the biology of lymphomatous disorders, which is already having an impact on our treatment choices. We must stand ready to integrate this new knowledge and apply it to our therapeutic strategy for these tumors.
As clinicians, we have grown accustomed to perceiving lymphoid disorders in a histological context, but it is time we start developing novel approaches to categorizing them that will be more clinically meaningful and relevant. The first steps have been taken already. The 2016 revision of the World Health Organization classification of lymphoid neoplasms takes into consideration the cell of origin, germinal center B-cell vs activated B-cell type, which clearly has the potential of affecting treatment selection5 because both lenalidomide and ibrutinib appear to be active in activated B-cell type DLBCL.
The time is now ripe to devise a clinically relevant classification that would take into consideration biological features that have treatment implications. For instance, PDL-1–expressing lymphomas could be grouped together. Aside from HL and PMLBCL, this category would also include mediastinal gray zone lymphomas6 and Richter transformation of chronic lymphocytic leukemia.7 Potentially, other lymphomas could be included. There is frequent overexpression of PD-L1 in Epstein-Barr virus as well as other virus-related lymphomas,8 which include Burkitt lymphoma, HIV-associated DLBCL, HIV-associated primary central nervous system lymphoma, Epstein-Barr virus+ posttransplant lymphoproliferative disorder, plasmablastic lymphomas, extranodal natural killer/T-cell lymphoma, angioimmunoblastic T-cell lymphoma, human herpesvirus 8-associated primary effusion lymphoma, and multicentric Castleman disease.
Some of these entities are associated with suboptimal treatment outcomes. For example, mediastinal gray zone lymphoma, despite its similarity with HL and PMLBCL, is associated with a relatively adverse clinical outcome, worse than for HL and PMLBCL. There is currently no standard therapy. Incorporating pembrolizumab into the front-line management of this disorder highlights an opportunity for improving the management of this challenging lymphoma. Such a clinical classification could also include a category of CD30-expressing lymphomas such as anaplastic large-cell lymphomas, HL, mycosis fungoides, and PMLBCL, in which treatment with brentuximab could have an effect.
Discovery of new biologically based therapeutic agents will unquestionably continue at a fast pace. As clinicians, we will constantly be faced with new opportunities to exploit them. The challenges will be to modulate our mindset so we can make treatment decisions that are based on biological rather than on histological language and to discover new ways of integrating these agents into our salvage and front-line treatments.
Conflict-of-interest disclosure: The authors report Merck research grant support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal