Key Points
BV plus DTIC is an active and well-tolerated combination for patients aged ≥60 years with HL.
Although highly active at the doses evaluated, BV plus bendamustine has unacceptable toxicity in patients aged ≥60 years with HL.
Abstract
Patients aged ≥60 years with treatment-naive Hodgkin lymphoma (HL) have few treatment options and inferior survival due to treatment-related toxicities and comorbidities. This phase 2, nonrandomized, open-label study evaluated brentuximab vedotin (BV) monotherapy (results previously reported), BV plus dacarbazine (DTIC), and BV plus bendamustine. Patients had classical HL and were ineligible for or declined frontline chemotherapy. Twenty-two patients received 1.8 mg/kg BV and 375 mg/m2 DTIC for up to 12 cycles, and 20 more patients received 1.8 mg/kg BV plus 90 or 70 mg/m2 bendamustine for up to 6 cycles (dose reduced due to toxicity). Subsequent BV monotherapy was allowed. Approximately 30 patients were to receive BV plus bendamustine; however, the incidence of serious adverse events (65%) and 2 deaths on study led to discontinuation of bendamustine and cessation of enrollment. Most patients had stage III/IV disease, and approximately half had ≥3 comorbidities or were impaired in ≥1 aspect that significantly interfered with quality of life. For BV plus DTIC, the objective response rate (ORR) was 100% and the complete remission (CR) rate was 62%. To date, the median progression-free survival (PFS) is 17.9 months. For BV plus bendamustine, the ORR was 100% and the CR rate was 88%. Neither the median PFS nor overall survival was reached. For elderly patients with HL, BV plus DTIC may be a frontline option based on tolerability and response duration. Despite activity, BV plus bendamustine is not a tolerable regimen in these patients. This trial was registered at www.clinicaltrials.gov as #NCT01716806.
Introduction
Most patients with Hodgkin lymphoma (HL), even those in advanced stage, are curable using standard chemotherapy regimens. A recent US National Clinical Trials Network study for patients aged <60 years with advanced-stage HL used a response-adapted approach with a backbone of Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD), which resulted in a 2-year progression-free survival (PFS) of 79%.1 More aggressive regimens, such as dose-escalated bleomycin, etoposide, Adriamycin, cyclophosphamide, Oncovin, procarbazine, and prednisone, may result in even higher PFS rates.2
Although HL is typically a disease of young patients (median age, ∼29 years), there is a bimodal age distribution of incidence, with >20% of patients aged ≥ 60 years.3 These older patients with HL are significantly underrepresented in clinical trials and have a markedly inferior prognosis compared with younger patients. For example, only 5% of patients were aged >60 years in a randomized Eastern Cooperative Oncology Group (ECOG) trial comparing ABVD with Stanford V (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone followed by radiation therapy).4 The 5-year overall survival (OS) for those patients was 58% compared with 90% for patients aged ≤60 years. The inferior survival was mostly driven by common comorbidities in older patients with HL and treatment toxicities resulting in deaths. Given these findings, patients aged ≥60 years represent the greatest unmet clinical need in HL, and alternative therapies are needed for these vulnerable patients.5
Brentuximab vedotin (BV; ADCETRIS) is an antibody–drug conjugate linking the microtubule-disrupting agent monomethylauristatin E to an anti-CD30 antibody. BV monotherapy yields an objective response rate (ORR) of 75% in relapsed HL, with a subset of patients having durable remissions at 5 years.6 In a retrospective analysis of BV activity in patients aged ≥60 years with relapsed HL, ORR was 56%.7 Although higher rates of adverse events (AEs) such as anemia, fatigue, and neuropathy were seen in older compared with younger patients, BV was tolerable overall, and a significant proportion of older patients had clinical benefit. Based upon this favorable experience, our phase 2 study evaluated BV monotherapy as frontline therapy in 27 efficacy-evaluable patients aged ≥60 years with HL, resulting in an ORR of 92%, a complete remission (CR) rate of 73%, and median response duration of 9.1 months.8
Additionally, BV is tolerable in combination with several chemotherapy agents studied thus far. When combined with AVD (ABVD minus bleomycin), response rates are high in small single-arm studies.9 Therefore, our study was amended to evaluate efficacy and safety of novel combination regimens in elderly patients. Herein are the results for combining BV with alkylating agents with established single-agent activity in HL, including dacarbazine (DTIC), a necessary component of ABVD,10 and bendamustine, a drug with demonstrated efficacy in relapsed HL.11
Patients and methods
This phase 2, nonrandomized, open-label, multicenter study evaluated the efficacy and safety of BV monotherapy, BV plus DTIC, and BV plus bendamustine as frontline therapy of HL in patients aged ≥60 years. The results for BV monotherapy were previously published.8
This study complied with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practices, and the applicable US Food and Drug Administration regulations. Each institutional review board approved the study before initiation at each site, and written informed consent was obtained from each patient before study participation.
Patient eligibility
Eligible patients were aged ≥60 years, had treatment-naive classical HL (excluding nodular lymphocyte predominant HL), fluorodeoxyglucose positron emission tomography (PET)–avid disease, bidimensional measurable disease of ≥1.5 cm in the greatest transverse diameter, and an ECOG performance status of ≤3 and were ineligible for or declined standard frontline chemotherapies (eg, ABVD or bleomycin, etoposide, Adriamycin, cyclophosphamide, Oncovin, procarbazine, and prednisone). As detailed in supplemental Methods (available on the Blood Web site), all patients had adequate baseline laboratory values for eligibility. For safety purposes, patients treated with BV plus bendamustine were required to meet liver and kidney function parameters based on bendamustine prescribing information (Treanda Prescribing Information; Cephalon, Inc., a wholly owned subsidiary of Teva Pharmaceutical Industries Ltd.; November 2015). Patients were not eligible for enrollment if they had symptomatic neurologic disease compromising instrumental activities of daily living (iADLs) or requiring medications (eg, ≥ grade 2 neuropathy) per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), with the exception of patients with mild cognitive impairment who were still able to perform age-appropriate iADLs, even if on medication. Patients with kidney disease requiring ongoing dialysis were excluded. Other key eligibility criteria are detailed in supplemental Methods.
Study design and treatment
The primary objective was ORR per investigator based on Revised Response Criteria for Malignant Lymphoma.12 Key secondary end points were safety, CR rate, response duration, PFS, B symptom resolution, and selected pharmacokinetic (PK) parameters. OS was an additional end point.
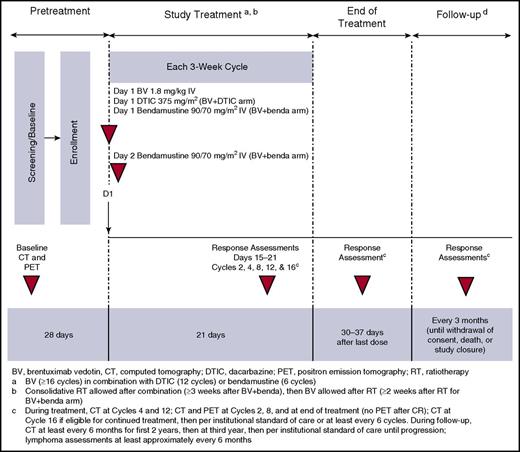
The study design, including treatment, response assessments, and follow-up, is presented in Figure 1. After the BV monotherapy arm completed enrollment, the study was amended to enroll ∼20 patients for BV 1.8 mg/kg plus DTIC 375 mg/m2 treatment on day 1 of each 3-week cycle for cycles 1 through 12, followed by BV 1.8 mg/kg for cycles 13 through 16 or more. Patients with severe renal impairment (estimated CrCl <30 mL/min) were to receive lower doses of BV (1.2 mg/kg) and DTIC (262 mg/m2; ∼30% reduced).
Another cohort of ∼30 patients subsequently opened for BV 1.8 mg/kg treatment on day 1 and bendamustine 90 mg/m2 on days 1 and 2 of each 3-week cycle for up to 6 cycles, followed by BV monotherapy for 10 additional cycles or more. As detailed in the safety results below, the study Safety Monitoring Committee (SMC) recommended a protocol amendment to reduce bendamustine dosing to 70 mg/m2 due to the number of serious adverse events (SAEs) observed. Although no specific safety signal was identified, ongoing toxicities resulted in a sponsor decision to suspend bendamustine treatment and enrollment in this arm.
Patients with unacceptable toxicity to DTIC or bendamustine could continue on BV monotherapy, and patients who completed 16 cycles of treatment and experienced clinical benefit per the investigator could remain on BV monotherapy until disease progression, unacceptable toxicity, or study closure. Dose reductions and delays are detailed in the supplemental Methods.
Study assessments
Diagnosis, medical history, current conditions, treatment and response for other prior malignancies, and concomitant medications were evaluated before enrollment. Each patient completed a baseline geriatric assessment using validated tools evaluating aspects of function, comorbidity, cognition, psychological state, social activity/support, and nutritional status.13-15
Response assessments were performed between days 15 and 21 with computed tomography (CT) scans at cycles 2, 4, 8, and 12 and at end of treatment (EOT) and PET scans at cycles 2 and 8 and at EOT. PET was not required after CR. Combined CT/PET of diagnostic quality was allowed. CT was required at cycle 16 for patients eligible for continued BV treatment. After cycle 16, CT and PET scanning frequency followed institutional standard of care, with CT at least every 6 cycles. Patients who received BV were followed for survival every 3 months until withdrawal of consent, death, or study closure. Patients off treatment without progressive disease (PD) had disease assessments per institutional standard of care or at least every 6 months for the first 2 years, then during the third year, and then per institutional standard of care until progression. Lymphoma assessments (medical history and physical examination) were performed at least every 6 months (Figure 1).
Safety assessments included AEs, SAEs (per 21CFR312.32), physical examinations, and laboratory tests. Severity of AEs and laboratory abnormalities was graded per the NCI CTCAE, version 4.03. As detailed in supplemental Methods, a study SMC monitored patient safety of treatment with BV plus bendamustine at protocol-specified time points and as needed.
PK parameters were estimated based on serum or plasma concentrations of BV antibody–drug conjugate and monomethylauristatin E at the following selected time points: predose, end of infusion, and at 24, 48, 168, and 336 hours after infusion (days 2, 3, 8, and 15) for cycle 1.
Statistical analysis
The ORR was defined as the proportion of patients with CR or partial remission (PR) and the CR rate as the proportion of patients with CR. Duration of response was defined as time from the start of CR or PR to time of tumor progression or death, whichever came first, and PFS as time from start of treatment to first documentation of tumor progression or death, whichever came first. OS was defined as time from start of treatment to date of death. In the absence of confirmation of death, survival was censored at the last date the patient was known to be alive.
Treatment-emergent AEs (TEAEs) were defined as AEs newly occurring or worsening after treatment. AEs were summarized by treatment group using Medical Dictionary for Regulatory Activities (version 15.1) and severity was graded using the NCI CTCAE (version 4.03).
PK parameters of area under the concentration–time curve, maximum concentration, and time of maximum concentration were estimated by noncompartmental analysis using Phoenix WinNonlin 6.3 (Pharsight, Mountain View, CA) and summarized for patients who received BV at cycle 1.
Results
Patients
Twenty-two patients received BV plus DTIC, then 20 additional patients received BV plus bendamustine. All had a diagnosis of classical HL. Enrollment occurred between February 2014 and September 2015 at 23 sites in the United States. Enrollment in the BV plus bendamustine arm was stopped in October 2015 due to acute toxicities detailed in the safety results. All patients were off treatment by February 2016, and 19 (86%) treated with BV plus DTIC and 13 (65%) treated with BV plus bendamustine remain in follow-up at the time of this analysis. Approximately half of patients discontinued treatment with either regimen due to AEs (55% BV plus DTIC; 60% BV plus bendamustine). Three patients (14%) on BV plus DTIC and 1 patient (5%) on BV plus bendamustine discontinued treatment due to PD. One patient (5%) in each arm discontinued the study due to withdrawal consent (BV plus DTIC, noncardiac chest pain; BV plus bendamustine, reason unknown) and 1 additional BV plus DTIC patient (5%) was lost to follow-up. One patient (5%) in the BV plus DTIC arm and 6 (30%) in the BV plus bendamustine arm died during follow up (2 received 1 dose of BV plus bendamustine, died less than 30 days after treatment, and had no postbaseline response assessment).
Patient demographics and baseline disease characteristics are presented in Table 1. Median age was 69 years for the BV plus DTIC arm (range, 62 to 88 years) and 75 years for the BV plus bendamustine arm (range, 63 to 86 years). Most patients in both arms had stage III/IV disease, and 9 patients (41%) in the BV plus DTIC arm and 8 patients (40%) in the BV plus bendamustine arm had extranodal disease. More patients in the BV plus bendamustine arm had mild to moderate renal impairment at baseline than those in the BV plus DTIC arm (70% and 41%, respectively). Two patients (9%) in the BV plus DTIC arm had severe renal impairment, and none in the BV plus bendamustine arm had severe renal impairment per the eligibility criteria.
Patient demographics and baseline characteristics
| . | BV+DTIC (n = 22) . | BV+bendamustine (n = 20) . |
|---|---|---|
| Median age, y (min, max) | 69 (62, 88) | 75 (63, 86) |
| Male | 16 (73) | 10 (50) |
| Race | ||
| Black or African American | 3 (14) | 0 |
| White | 19 (86) | 20 (100) |
| ECOG performance status | ||
| 0/1 | 15 (68) | 16 (80) |
| 2/3 | 7 (32) | 4 (20) |
| Patients ineligible for conventional chemotherapy | 19 (86) | 17 (85) |
| Histologic subtype of Hodgkin HL | ||
| Nodular sclerosis | 9 (41) | 10 (50) |
| Mixed cellularity | 9 (41) | 4 (20) |
| Lymphocyte-rich classical HL | 0 | 1 (5) |
| Lymphocyte-depleted classical HL | 1 (5) | 1 (5) |
| Classical HL not otherwise specified | 3 (14) | 4 (20) |
| Disease stage at diagnosis | ||
| I/II | 6 (27) | 5 (25) |
| III/IV | 16 (72) | 15 (75) |
| Median percent CD30 expression (min, max) | 95 (4, 100) | 95 (40, 100) |
| Baseline B symptoms | ||
| Fever | 2 (9) | 2 (10) |
| Night sweats | 5 (23) | 6 (30) |
| Weight loss >10% | 3 (14) | 5 (25) |
| Bulky disease | 2 (9) | 1 (5) |
| Extranodal involvement | 9 (41) | 8 (40) |
| Baseline renal function | ||
| Unimpaired (CrCl >80 mL/min) | 11 (50) | 6 (30) |
| Mild (CrCl >50 to ≤80 mL/min) | 6 (27) | 10 (50) |
| Moderate (CrCl ≥30 to ≤50 mL/min) | 3 (14) | 4 (20) |
| Severe (CrCl <30 mL/min) | 2 (9) | 0 |
| Median percent cardiac ejection fraction* (min, max) | 60 (25, 72) | 60 (45, 70) |
| . | BV+DTIC (n = 22) . | BV+bendamustine (n = 20) . |
|---|---|---|
| Median age, y (min, max) | 69 (62, 88) | 75 (63, 86) |
| Male | 16 (73) | 10 (50) |
| Race | ||
| Black or African American | 3 (14) | 0 |
| White | 19 (86) | 20 (100) |
| ECOG performance status | ||
| 0/1 | 15 (68) | 16 (80) |
| 2/3 | 7 (32) | 4 (20) |
| Patients ineligible for conventional chemotherapy | 19 (86) | 17 (85) |
| Histologic subtype of Hodgkin HL | ||
| Nodular sclerosis | 9 (41) | 10 (50) |
| Mixed cellularity | 9 (41) | 4 (20) |
| Lymphocyte-rich classical HL | 0 | 1 (5) |
| Lymphocyte-depleted classical HL | 1 (5) | 1 (5) |
| Classical HL not otherwise specified | 3 (14) | 4 (20) |
| Disease stage at diagnosis | ||
| I/II | 6 (27) | 5 (25) |
| III/IV | 16 (72) | 15 (75) |
| Median percent CD30 expression (min, max) | 95 (4, 100) | 95 (40, 100) |
| Baseline B symptoms | ||
| Fever | 2 (9) | 2 (10) |
| Night sweats | 5 (23) | 6 (30) |
| Weight loss >10% | 3 (14) | 5 (25) |
| Bulky disease | 2 (9) | 1 (5) |
| Extranodal involvement | 9 (41) | 8 (40) |
| Baseline renal function | ||
| Unimpaired (CrCl >80 mL/min) | 11 (50) | 6 (30) |
| Mild (CrCl >50 to ≤80 mL/min) | 6 (27) | 10 (50) |
| Moderate (CrCl ≥30 to ≤50 mL/min) | 3 (14) | 4 (20) |
| Severe (CrCl <30 mL/min) | 2 (9) | 0 |
| Median percent cardiac ejection fraction* (min, max) | 60 (25, 72) | 60 (45, 70) |
Data are presented as n (%) of patients unless otherwise specified.
CrCl, creatinine clearance.
By echocardiogram (17 patients in the BV plus DTIC arm and 19 patients in BV plus bendamustine arm).
Results from the baseline geriatric assessments are presented in Table 2. Approximately half of patients had at least 3 comorbidities or were impaired in at least 1 aspect that significantly interfered with quality of life in both arms. The majority of patients in both arms reported being “limited a lot” for at least 1 physical activity (73% BV plus DTIC; 70% BV plus bendamustine).16 Of these patients, a proportion had preexisting peripheral neuropathy (PN) (25% BV plus DTIC; 36% BV plus bendamustine). Six BV plus DTIC patients (29%) and 5 patients (26%) BV plus bendamustine patients reported a fall within 6 months before enrolling. Two patients (9%) in the BV plus DTIC arm and 4 patients (20%) in the BV plus bendamustine arm reported being completely dependent on others for at least 1 of 7 iADLs evaluated in the geriatric assessment tool.17 A greater number of patients in the BV plus bendamustine arm had “timed up and go” >13.5 s compared with the BV plus DTIC arm (70% and 41%, respectively).
Comorbidities and impaired functional status
| . | BV+DTIC (n = 22) . | BV+bendamustine (n = 20) n . |
|---|---|---|
| Physical health (OARS) | ||
| ≥3 comorbidities OR ≥1 that significantly interfered with quality of life*,† | 11 (50) | 9 (45) |
| Physical function (MOS) | ||
| Limited a lot for ≥1 of 10 activities* | 16 (73) | 14 (70) |
| Preexisting PN | 4 (25) | 5 (36) |
| Patients with ≥1 fall within prior 6 mo‡ | 6 (29) | 5 (26) |
| Preexisting PN | 1 (17) | 2 (40) |
| Instrumental activities of daily living (OARS) | ||
| Completely dependent for ≥1 of 7 iADLs* | 2 (9) | 4 (20) |
| Timed “up and go” | ||
| >13.5 s to complete action§ | 9 (41) | 14 (70) |
| Unintentional weight loss of ≥10% within the past 6 mo | 5 (23) | 7 (35) |
| BOMC test score of >6 | 7 (32) | 4 (20) |
| . | BV+DTIC (n = 22) . | BV+bendamustine (n = 20) n . |
|---|---|---|
| Physical health (OARS) | ||
| ≥3 comorbidities OR ≥1 that significantly interfered with quality of life*,† | 11 (50) | 9 (45) |
| Physical function (MOS) | ||
| Limited a lot for ≥1 of 10 activities* | 16 (73) | 14 (70) |
| Preexisting PN | 4 (25) | 5 (36) |
| Patients with ≥1 fall within prior 6 mo‡ | 6 (29) | 5 (26) |
| Preexisting PN | 1 (17) | 2 (40) |
| Instrumental activities of daily living (OARS) | ||
| Completely dependent for ≥1 of 7 iADLs* | 2 (9) | 4 (20) |
| Timed “up and go” | ||
| >13.5 s to complete action§ | 9 (41) | 14 (70) |
| Unintentional weight loss of ≥10% within the past 6 mo | 5 (23) | 7 (35) |
| BOMC test score of >6 | 7 (32) | 4 (20) |
Data are presented as n (%) of patients.
MOS, Medical Outcomes Study; OARS, Older American Resources and Services.
Patient reported.
Thirteen specific comorbidities plus eyesight and hearing (only if fair to totally blind/totally deaf).
Patients with results: n = 21 (BV plus DTIC) and n = 19 (BV plus bendamustine).
Stand up from chair, walk 3 m, turn, walk back, and sit down.
Efficacy
Treated patients who had both a baseline and postbaseline disease assessment or had PD after receiving BV were included in the efficacy analysis. One patient treated with BV plus DTIC and 3 treated with BV plus bendamustine did not have postbaseline response assessments. Best response, PFS, subsequent therapies, and OS are presented by patient in supplemental Table 1 for BV plus DTIC and supplemental Table 2 for BV plus bendamustine.
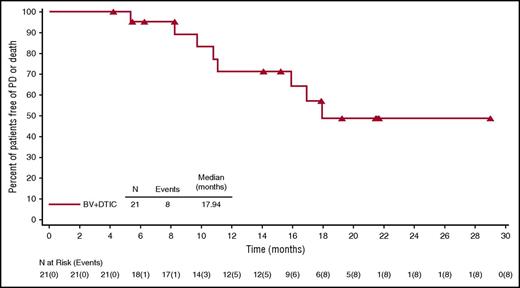
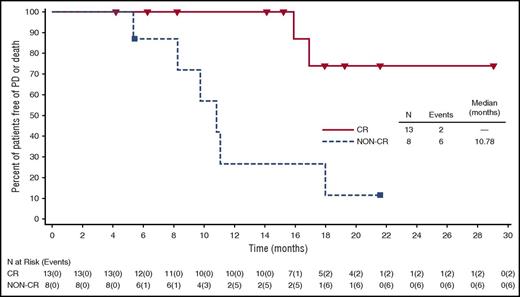
For efficacy-evaluable patients treated with BV plus DTIC (n = 21), the ORR was 100%, and the CR rate was 62% (Table 3). One patient received consolidative radiotherapy (allowed per protocol). Of the 6 patients who had B symptoms at baseline, 4 (67%) experienced resolution during treatment. At the time of this analysis, the median observation time from first dose was 21.6 months (range, 14.8 to 29.0 months), and median PFS was 17.9 months (range, ≥4.2 to ≥29 months) (Figure 2). Eight efficacy-evaluable patients (38%) had events of PD or death (7 PD, 1 death). For patients with CR, median PFS had not been reached (range, ≥4.2 to ≥29 months), whereas patients who had not achieved a CR with BV plus DTIC (all PR) had a median PFS of 10.8 months (range, 5.3 to ≥21.5 months) (Figure 3). Median OS also had not been reached for all patients treated with BV plus DTIC (range, ≥14.8 to ≥29 months) (supplemental Figure 1). After discontinuing treatment, 13 patients (62%) had no subsequent therapy. All subsequent therapies for the other 8 efficacy-evaluable patients are presented by patient in supplemental Table 1, including 1 BV plus DTIC retreatment, 1 subsequent BV monotherapy, 3 checkpoint inhibitor treatments (2 nivolumab and 1 pembrolizumab), and other single-agent or combination chemotherapies.
Summary of best clinical response
| . | BV+DTIC (n = 21) . | BV+bendamustine (n = 17) . |
|---|---|---|
| ORR* | 21 (100) | 17 (100) |
| 95% CI† | 83.9, 100 | 80.5, 100 |
| Best clinical response | ||
| CR | 13 (62) | 15 (88) |
| PR | 8 (38) | 2 (12) |
| 95% CI† for CR rate | 38.4, 81.9 | 63.6, 98.5 |
| . | BV+DTIC (n = 21) . | BV+bendamustine (n = 17) . |
|---|---|---|
| ORR* | 21 (100) | 17 (100) |
| 95% CI† | 83.9, 100 | 80.5, 100 |
| Best clinical response | ||
| CR | 13 (62) | 15 (88) |
| PR | 8 (38) | 2 (12) |
| 95% CI† for CR rate | 38.4, 81.9 | 63.6, 98.5 |
Data are presented as n (%) of patients unless otherwise indicated.
CI, confidence interval.
CRs and PRs per Cheson et al.12 Responses are mutually exclusive.
Two-sided 95% exact confidence interval computed using the Clopper Pearson method.
PFS of treatment-naive, elderly patients with HL treated with BV plus DTIC. PFS was analyzed using Kaplan-Meier methodology. Censored patients are indicated on the graph. Twenty-one efficacy-evaluable patients were included in the analysis, and 1 patient was excluded because of a lack of postbaseline response assessments.
PFS of treatment-naive, elderly patients with HL treated with BV plus DTIC. PFS was analyzed using Kaplan-Meier methodology. Censored patients are indicated on the graph. Twenty-one efficacy-evaluable patients were included in the analysis, and 1 patient was excluded because of a lack of postbaseline response assessments.
PFS of CR vs non-CR in elderly patients with HL treated with BV plus DTIC. Censored patients are indicated on the graph. Analysis used Kaplan-Meier methodology and was based on 21 efficacy-evaluable patients; all 8 patients with non-CR had a response of PR.
PFS of CR vs non-CR in elderly patients with HL treated with BV plus DTIC. Censored patients are indicated on the graph. Analysis used Kaplan-Meier methodology and was based on 21 efficacy-evaluable patients; all 8 patients with non-CR had a response of PR.
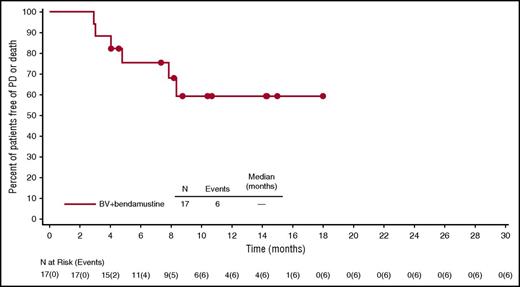
For efficacy-evaluable patients treated with BV plus bendamustine (n = 17), the ORR was also 100%, with an 88% CR rate (Table 3). Two patients received consolidative radiotherapy. Four of 7 patients (57%) with B symptoms at baseline had resolution. The median observation time from first dose was 10.8 months (range, 2.9 to 18.2 months). Neither the median PFS (range, 2.9 to ≥18 months) (Figure 4) nor the median OS (range, 2.9 to ≥18.2 months) (supplemental Figure 2) was reached at the time of this analysis. Six efficacy-evaluable patients (35%) had events of PD or death (2 PD, 4 deaths). After discontinuing treatment, 14 patients (82%) have had no subsequent therapy. All subsequent therapies for the other 3 patients are presented by patient in supplemental Table 2.
PFS of treatment-naive, elderly patients with HL treated with BV plus bendamustine. PFS was analyzed using Kaplan-Meier methodology. Censored patients are indicated on the graph. Seventeen efficacy-evaluable patients were included in the analysis, and 3 patients were excluded because of a lack of postbaseline response assessments.
PFS of treatment-naive, elderly patients with HL treated with BV plus bendamustine. PFS was analyzed using Kaplan-Meier methodology. Censored patients are indicated on the graph. Seventeen efficacy-evaluable patients were included in the analysis, and 3 patients were excluded because of a lack of postbaseline response assessments.
Safety
Patients who received at least 1 dose of any study drug were included in the safety analysis. A summary of AEs is presented in Table 4; TEAEs occurring in ≥25% of patients are shown by grade in Table 5, and grade ≥3 laboratory abnormalities are listed in supplemental Table 3.
Summary of AEs
| . | BV+DTIC (n = 22) . | BV+bendamustine (n = 20) . |
|---|---|---|
| Any TEAE* | 22 (100) | 20 (100) |
| Treatment-related AEs | 22 (100) | 19 (95) |
| Grade ≥3 AEs | 10 (45) | 18 (90) |
| SAEs | 4 (18) | 13 (65) |
| AEs leading to treatment discontinuation | 12 (55) | 12 (60) |
| Deaths within 30 d of last dose | 0 | 2 (10)† |
| . | BV+DTIC (n = 22) . | BV+bendamustine (n = 20) . |
|---|---|---|
| Any TEAE* | 22 (100) | 20 (100) |
| Treatment-related AEs | 22 (100) | 19 (95) |
| Grade ≥3 AEs | 10 (45) | 18 (90) |
| SAEs | 4 (18) | 13 (65) |
| AEs leading to treatment discontinuation | 12 (55) | 12 (60) |
| Deaths within 30 d of last dose | 0 | 2 (10)† |
Data are presented as n (%) of patients.
TEAEs are presented and defined as newly occurring (not present at baseline) or worsening after first dose of investigational product.
Unrelated to study treatment.
TEAEs by maximum severity occurring in at least 25% of patients in any treatment arm
| Preferred term . | BV+DTIC (n = 22) . | BV+bendamustine (n = 20) . | ||||||
|---|---|---|---|---|---|---|---|---|
| G1/2 . | G3 . | G4 . | Total . | G1/2 . | G3 . | G4 . | Total . | |
| Diarrhea | 6 (27) | — | — | 6 (27) | 17 (85) | — | — | 17 (85) |
| Nausea | 9 (41) | — | — | 9 (41) | 13 (65) | — | — | 13 (65) |
| Fatigue | 9 (41) | — | — | 9 (41) | 7 (35) | 3 (15) | — | 10 (50) |
| Decreased appetite | 5 (23) | — | — | 5 (23) | 8 (40) | 1 (5) | — | 9 (45) |
| PSN | 11 (50) | 6 (27) | — | 17 (77) | 5 (25) | 3 (15) | — | 8 (40) |
| Pyrexia | 2 (9) | — | — | 2 (9) | 8 (40) | — | — | 8 (40) |
| Constipation | 10 (45) | — | — | 10 (45) | 7 (35) | — | — | 7 (35) |
| Dehydration | 1 (5) | — | — | 1 (5) | 6 (30) | 1 (5) | — | 7 (35) |
| Urinary tract infection | 1 (5) | 1 (5) | — | 2 (9) | 4 (20) | 2 (10) | 1 (5) | 7 (35) |
| Cough | 4 (18) | — | — | 4 (18) | 5 (25) | 1 (5) | — | 6 (30) |
| Edema peripheral | 7 (32) | — | — | 7 (32) | 6 (30) | — | — | 6 (30) |
| Hypokalemia | — | — | — | — | 4 (20) | 1 (5) | 1 (5) | 6 (30) |
| Hypotension | — | 1 (5) | — | 1 (5) | 4 (20) | 2 (10) | — | 6 (30) |
| Abdominal pain | 3 (14) | — | — | 3 (14) | 5 (25) | — | — | 5 (25) |
| Alopecia | 4 (18) | — | — | 4 (18) | 5 (25) | — | — | 5 (25) |
| Dyspnea | 5 (23) | — | — | 5 (23) | 4 (20) | 1 (5) | — | 5 (25) |
| Fall | 5 (23) | — | — | 5 (23) | 5 (25) | — | — | 5 (25) |
| Neutropenia | 1 (5) | 1 (5) | 1 (5) | 3 (14) | 3 (15) | 1 (5) | 1 (5) | 5 (25) |
| Weight decreased | 4 (18) | 1 (5) | — | 5 (23) | 4 (20) | 1 (5) | — | 5 (25) |
| Preferred term . | BV+DTIC (n = 22) . | BV+bendamustine (n = 20) . | ||||||
|---|---|---|---|---|---|---|---|---|
| G1/2 . | G3 . | G4 . | Total . | G1/2 . | G3 . | G4 . | Total . | |
| Diarrhea | 6 (27) | — | — | 6 (27) | 17 (85) | — | — | 17 (85) |
| Nausea | 9 (41) | — | — | 9 (41) | 13 (65) | — | — | 13 (65) |
| Fatigue | 9 (41) | — | — | 9 (41) | 7 (35) | 3 (15) | — | 10 (50) |
| Decreased appetite | 5 (23) | — | — | 5 (23) | 8 (40) | 1 (5) | — | 9 (45) |
| PSN | 11 (50) | 6 (27) | — | 17 (77) | 5 (25) | 3 (15) | — | 8 (40) |
| Pyrexia | 2 (9) | — | — | 2 (9) | 8 (40) | — | — | 8 (40) |
| Constipation | 10 (45) | — | — | 10 (45) | 7 (35) | — | — | 7 (35) |
| Dehydration | 1 (5) | — | — | 1 (5) | 6 (30) | 1 (5) | — | 7 (35) |
| Urinary tract infection | 1 (5) | 1 (5) | — | 2 (9) | 4 (20) | 2 (10) | 1 (5) | 7 (35) |
| Cough | 4 (18) | — | — | 4 (18) | 5 (25) | 1 (5) | — | 6 (30) |
| Edema peripheral | 7 (32) | — | — | 7 (32) | 6 (30) | — | — | 6 (30) |
| Hypokalemia | — | — | — | — | 4 (20) | 1 (5) | 1 (5) | 6 (30) |
| Hypotension | — | 1 (5) | — | 1 (5) | 4 (20) | 2 (10) | — | 6 (30) |
| Abdominal pain | 3 (14) | — | — | 3 (14) | 5 (25) | — | — | 5 (25) |
| Alopecia | 4 (18) | — | — | 4 (18) | 5 (25) | — | — | 5 (25) |
| Dyspnea | 5 (23) | — | — | 5 (23) | 4 (20) | 1 (5) | — | 5 (25) |
| Fall | 5 (23) | — | — | 5 (23) | 5 (25) | — | — | 5 (25) |
| Neutropenia | 1 (5) | 1 (5) | 1 (5) | 3 (14) | 3 (15) | 1 (5) | 1 (5) | 5 (25) |
| Weight decreased | 4 (18) | 1 (5) | — | 5 (23) | 4 (20) | 1 (5) | — | 5 (25) |
Data are presented as n (%) of patients. TEAEs are presented and defined as newly occurring (not present at baseline) or worsening after first dose of investigational product and in descending order of patient incidence in the BV plus bendamustine arm.
G, grade.
Patients in the BV plus DTIC arm (n = 22) received a median of 12.5 cycles of BV (range, 2 to 27 cycles) and a median of 12 cycles of DTIC (range, 1 to 12 cycles). Twelve patients completed 12 cycles of DTIC, and 4 patients completed 16 cycles of BV. All patients who received BV plus DTIC experienced at least 1 treatment-related TEAE, 45% experienced grade ≥3 AEs, and 18% had SAEs (Table 4). Peripheral sensory neuropathy (PSN) was the most common TEAE overall (77%) and the most frequently occurring grade ≥3 TEAE (27%, no grade 4) (Table 5). Overall, 13 of 19 patients who had treatment-emergent PN had resolution or improvement of events, and 5 patients had resolution of all events. No association of time to resolution or improvement relative to the risk factors of diabetes and hypothyroidism was observed. PSN was also the principal AE that led to dose modifications (32%) and treatment discontinuations (36%). All other AEs leading to treatment discontinuation of BV plus DTIC occurred in 1 patient each (5% each). One patient had grade 3 neutropenia, and another patient experienced both a grade 3 and 4 TEAE of neutropenia; low neutrophils was the highest occurring grade ≥3 laboratory abnormality in the BV plus DTIC arm (supplemental Table 3).
Patients in the BV plus bendamustine arm (n = 20) received a median of 5 cycles of BV (range, 1 to 16 cycles) and a median of 3.5 cycles of bendamustine (range, 1 to 6 cycles). Seven patients completed 6 cycles of bendamustine, and 1 patient completed 16 cycles of BV. Nearly all patients who received BV plus bendamustine experienced a treatment-related TEAE (95%); 90% had a grade ≥3 AE, and 65% had SAEs (Table 4). Diarrhea was the most common TEAE in these patients (Table 5). PSN was the primary treatment-related TEAE (15%) for this combination and was the top AE that led to dose modifications and treatment discontinuations (both 25%). Six of 10 patients who had treatment-emergent PN had resolution or improvement of events, and 3 patients had resolution of all events. No association of time to resolution or improvement relative to the risk factors of diabetes and hypothyroidism was observed. All other AEs leading to treatment discontinuation occurred in 1 patient each (5% each). Six patients (30%) had grade 4 TEAEs, including acute respiratory failure, pneumomediastinum, and pneumonitis (n = 1); septic shock and urinary tract infection (n = 1); and acute myocardial infarction, hypokalemia, interstitial lung disease, and neutropenia (n = 1 each). SAEs occurring in >1 patient included asthenia, urinary tract infection, febrile neutropenia, and pneumonia. A low lymphocyte count was the highest occurring laboratory abnormality in the BV plus bendamustine arm (supplemental Table 3).
For the BV plus bendamustine arm, an SMC reviewed safety data after the first 5 patients completed 1 treatment cycle and again after 5 patients completed 2 cycles. After 5 patients completed the second treatment cycle, the SMC observed that 4 of 7 patients treated with 1.8 mg/kg BV plus 90 mg/m2 bendamustine had SAEs resulting in hospitalization, including 2 patients with grade 3 asthenia (1 related, 1 unrelated), 1 patient with unrelated grade 3 febrile neutropenia, and 1 patient with a related delayed hypersensitivity reaction. To improve safety and tolerability, the dose of bendamustine was reduced to 70 mg/m2. Further acute toxicities were observed, including SAEs in 12 of 20 patients treated with BV plus bendamustine and 2 deaths within the 30-day safety observation period (1 disease related and 1 unknown cause of death; both after 1 dose of treatment). Additionally, although the study was not designed to compare treatment arms, a higher incidence of specific TEAEs (diarrhea, asthenia/fatigue, hypokalemia, dehydration, weight decrease, hypotension, neutropenia, candidiasis, and pneumonia) and TEAEs grade ≥3 were observed in patients treated with BV plus bendamustine compared with patients treated with BV plus DTIC (Table 5) or BV monotherapy.8 Although no specific safety signal was identified and neither of the deaths was considered related to treatment, the sponsor determined that the combination of BV plus bendamustine at the dose levels evaluated did not represent a suitable regimen for these patients. Based on the SMC recommendation, the sponsor suspended treatment with bendamustine and stopped enrollment for this treatment regimen. Five patients who were receiving BV plus bendamustine demonstrated tolerability and clinical benefit and continued on BV monotherapy.
The median age of the BV plus bendamustine arm was 75 years, and the median age of the BV plus DTIC arm was 69 years. A count of the 13 patients who experienced SAEs on BV plus bendamustine showed that 12 were ≥70 years, while 2 of the 4 patients with SAEs on the BV plus DTIC arm were ≥70 years.
Pharmacokinetics and pharmacodynamics
PK parameters, including area under the concentration–time curve, maximum concentration, and time of maximum concentration, were determined for BV when combined with DTIC or bendamustine (supplemental Table 4). Results were consistent with those from the monotherapy arm and from historic data for BV.8,18
Discussion
In this nonrandomized, open-label, phase 2 study, both BV plus DTIC and BV plus bendamustine demonstrated significant activity (100% ORR) in elderly patients with newly diagnosed HL. The study enrolled patients who were not candidates for or declined ABVD, with approximately half who had 3 or more comorbidities or at least 1 comorbidity that significantly interfered with quality of life and a majority who demonstrated functional impairment by geriatric assessment tools. Despite the advanced age (median age, 69 years [BV plus DTIC] and 75 years [BV plus bendamustine]) and significant comorbidity burden of these patients, the majority achieved a CR (62% BV plus DTIC; 88% BV plus bendamustine), and median PFS was ∼18 months for BV plus DTIC (but not reached for BV plus bendamustine).
BV plus DTIC was well tolerated overall, with a similar toxicity profile to BV monotherapy in this population.8 Conversely, BV plus bendamustine treatment was stopped prematurely due to an unacceptably high rate of SAEs and deaths. Other studies of BV plus bendamustine have been conducted in younger, transplant-eligible patients with relapsed HL. A preliminary report confirmed high activity in this younger population.19 Nonetheless, despite encouraging activity and tolerability in fit patients, the combination of BV plus bendamustine is too toxic for elderly patients at the ages, dose levels, and frequency studied. Exposure is consistent with results from the monotherapy arm and from historic data for BV. Our toxicity experience emphasizes the importance of dedicated trials evaluating novel regimens in elderly patients.
The outcomes of BV plus DTIC treatment appear to suggest a trend of improved durability compared with BV monotherapy, especially among CR patients (Figure 3).8 Although follow-up is ongoing and patients in our study have been followed for slightly less than 2 years, the results with BV plus DTIC also appear to be favorable thus far relative to the limited literature of chemotherapy combinations for elderly patients with HL.10,20 For example, the German Hodgkin Study Group developed the treatment regimen of prednisone, vinblastine, doxorubicin and gemcitabine for elderly patients with early unfavorable and advanced-stage HL, resulting in a 3-year PFS of 58%.21 Similarly, a subset of patients with advanced stage disease had a 3-year PFS of 58% in the Study of Hodgkin in the Elderly/Lymphoma Database (SHIELD) program.22 The majority of these patients were treated on a prospective phase 2 study of the chemotherapy combination of vinblastine, cyclophosphamide, procarbazine, etoposide, mitoxantrone, bleomycin, and prednisolone. Compared with these chemotherapy experiences, our patients were older and had more advanced HL.
Baseline comprehensive geriatric assessments were conducted in our study, which is, to the best of our knowledge, the largest experience of geriatric assessments in HL published to date. Similar to solid tumors,23 geriatric assessments have robustly predicted OS and tolerance to chemotherapy in non-HL.24,25 A recent consensus panel emphasized the importance of comprehensive geriatric assessments evaluating functional status, comorbidity, cognition, mental health status, fatigue, social status and support, nutrition, and presence of geriatric syndrome for patients with cancer.26 Our results from geriatric assessments emphasize the significant frailty, comorbidities, and geriatric syndromes in an elderly cohort of patients with HL and have implications on future trial design for these patients, as well as enhancing the ability to compare results across studies. Additionally, our results explain, in part, the repeated observation that elderly patients with HL tolerate conventional chemotherapy poorly and have an increased risk of death from complications of treatment, such as bleomycin toxicity.4,27
The results of this study should serve as a benchmark for future studies of novel induction regimens for elderly patients with HL. It is particularly important to refine induction treatment strategies, because poor outcomes have been universally observed in these vulnerable patients.28 In addition to poor treatment tolerance resulting in increased morbidity and mortality and inadequate delivery of chemotherapy, poor outcomes in these patients may also be from biologically higher risk disease.29 Mixed cellularity subtype (41% BV plus DTIC; 20% BV plus bendamustine), which has an inferior prognosis, is enriched in older patients, and most older patients present with clinical high-risk features.4
Recently, checkpoint blockade with pembrolizumab or nivolumab has demonstrated significant efficacy in relapsed/refractory HL in several studies enrolling younger patients.30-32 This approach is based upon the observation that PD-L1/PD-L2 alterations are a defining feature of classical HL.33 Amplification and alterations of chromosome 9p24.1 (PD-L1/PD-L2) increase the abundance of the PD-1 ligands and are more common in patients with advanced-stage HL and associated with shorter PFS. A preliminary report demonstrating tolerability and activity of the combination of BV plus nivolumab in relapsed HL has been presented.34 Based upon this report and the antitumor activity observed with BV combinations, we have recently opened another arm of our study evaluating the combination of BV plus nivolumab as frontline treatment of elderly HL, including biomarker studies to evaluate correlation between PD-L1/2 status and outcomes (www.clinicaltrials.gov #NCT01716806).
In conclusion, high activity was demonstrated with BV in combination with DTIC or bendamustine in elderly, frail patients with newly diagnosed HL; however, BV plus bendamustine was poorly tolerated by these patients at the dose levels and frequency studied. BV plus DTIC was not only highly active, with 13 of 21 patients achieving CR and an overall median PFS of 17.9 months, but also well tolerated. Although our patients were older and had more advanced HL, results demonstrated favorable tolerability and activity compared with historical chemotherapy regimens in elderly patients. Confirmation of our findings in larger trials is needed; nevertheless, given the poor outcomes observed with standard chemotherapy, these results suggest BV plus DTIC may be a treatment option for elderly patients with newly diagnosed HL.
Presented in abstract and poster form at the 10th International Symposium on Hodgkin Lymphoma, Cologne, Germany, 22-25 October 2016.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Peter Haughney, an employee of Seattle Genetics, Inc., for PK analysis, and Tiffany Griffin, a contract employee of Seattle Genetics, Inc., for medical writing assistance. Supriya G. Mohile (University of Rochester Medical Center, Rochester, NY) acted as a consultant and provided expertise on conducting the geriatric assessment and evaluation of the resulting data.
Authorship
Contribution: J.W.F., A.F.-T., R.C., and C.A.Y. contributed to the concept and design of the study; J.W.F., A.F.-T., R.E.B., V.J.M.C., D.P.D., P.J.F., G.O., R.C., and C.A.Y. contributed to data acquisition; J.W.F. and C.A.Y. wrote the manuscript and along with Y.W. analyzed and interpreted the data; and all authors critically reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: Seattle Genetics, Inc., provided research funding to the institutions of J.W.F., A.F.-T., R.E.B., V.J.M.C., D.P.D., P.J.F, G.O., R.C., and C.A.Y. R.C. and C.A.Y. have acted as consultants for Seattle Genetics, Inc. A.F.-T. has participated in a Seattle Genetics, Inc. speakers’ bureau. A.F. and Y.W. are employees of and have equity ownership in Seattle Genetics, Inc.
Correspondence: Jonathan W. Friedberg, James P. Wilmot Cancer Center, University of Rochester Medical Center, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: jonathan_friedberg@urmc.rochester.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal