Key Points
Median platelet counts increased to 50 × 109/L or more by week 2 in patients with ITP and were maintained for ≥2 years.
Lower platelet counts, more previous therapies, and/or splenectomy resulted in good but somewhat lower responses to eltrombopag.
Abstract
In phase 2/3 trials, eltrombopag treatment of 6 months or less in patients with chronic/persistent immune thrombocytopenia (ITP) increased platelet counts and reduced bleeding. The open-label EXTEND study evaluated long-term safety and efficacy of eltrombopag in adults with ITP who had completed a previous eltrombopag study. For the 302 patients enrolled, median duration of eltrombopag treatment was 2.37 years (2 days-8.76 years). Median platelet counts increased to 50 × 109/L or more by week 2 and were sustained throughout the treatment period. Overall, 259 patients (85.8%) achieved a response (platelet count ≥50 × 109/L at least once in the absence of rescue), and 133 (52%) of 257 patients achieved a continuous response of 25 weeks or longer. Responses in patients with platelet counts lower than 15 × 109/L, more previous therapies, and/or splenectomy were somewhat lower. Thirty-four (34%) of 101 patients receiving concomitant ITP medication discontinued 1 or more medication. In patients with assessments, bleeding symptoms (World Health Organization grades 1-4) decreased from 57% at baseline to 16% at 1 year. Forty-one patients (14%) withdrew because of adverse events. Hepatobiliary adverse events (n = 7), cataracts (n = 4), deep vein thrombosis (n = 3), cerebral infarction (n = 2), headache (n = 2), and myelofibrosis (n = 2) occurred in more than 1 patient; the remaining adverse events occurred only once. Rates of thromboembolic events (6%) and hepatobiliary adverse events (15%) did not increase with treatment duration past 1 year. EXTEND demonstrated that long-term use of eltrombopag was effective in maintaining platelet counts of 50 × 109/L or more and reducing bleeding in most patients with ITP of more than 6 months’ duration. Important adverse events (eg, thrombosis, hepatobiliary, and bone marrow fibrosis) were infrequent. (ClinicalTrials.gov:NCT00351468).
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by reduced platelet counts and increased bleeding risk. Although traditionally a disorder of accelerated antibody-mediated platelet destruction, the pathophysiology of ITP also encompasses impaired platelet production.1-4 ITP of 12 or more months’ duration is currently defined as chronic.5 Bleeding symptoms may range from common, relatively mild events such as petechiae and bruising to much less common but more serious events such as intracranial hemorrhage. The severity of thrombocytopenia imperfectly correlates with bleeding risk; very low platelet counts are permissive but not sufficient to cause bleeding.6
Eltrombopag is a small-molecule, oral, nonpeptide, thrombopoietin-receptor-agonist (TPO-RA) widely approved for treatment of patients with chronic ITP who are older than 1 year, patients with chronic hepatitis C and thrombocytopenia to allow interferon-based therapy, and patients with severe aplastic anemia who have insufficient response to immunosuppressive therapy.7 Eltrombopag increases platelet production by binding to the transmembrane domain of the TPO-R and activating proliferation of megakaryocytes from bone marrow progenitors.8,9 In 6-week and 6-month placebo-controlled studies in patients with previously treated ITP, eltrombopag increased platelet counts to 50 × 109/L or more in approximately 60% to 80% of patients and reduced bleeding.10-14 Although headache and nasopharyngitis were the most frequently reported adverse events (AEs) with use of eltrombopag in ITP, more serious AEs (SAEs) have occurred such as increased liver enzymes, development of cataracts, thrombosis,7 and bone marrow fibrosis, all of which have sometimes led to discontinuation of treatment.12-15
The other widely approved TPO-RA is romiplostim.16
Because of their unique mechanism of action, which has limited anticipation of producing curative effects, TPO-RAs are usually considered for bridging and maintenance therapy, and administered on a daily or weekly basis.5 Because of the unremitting nature of ITP in most patients with chronic disease, treatment with eltrombopag may be prolonged, extending for not just months but years. Hence, the efficacy and safety of eltrombopag beyond 6 months needed to be investigated.
EXTEND (Eltrombopag Extended Dosing) was a phase 3, open-label, long-term extension study of the safety, tolerability, and efficacy of eltrombopag in patients with ITP of at least 6 to 12 months’ duration who had completed a previous eltrombopag study. Interim results from this study showed that treatment with eltrombopag was safe, well-tolerated, and effective in maintaining platelet counts in the desired range for most of the 299 patients treated for up to 3 years.17 This final study report describes up to 8 years of continuous treatment with eltrombopag.17
Patients and methods
Study design
EXTEND (ClinicalTrials.gov identifier: NCT00351468) included adults with ITP who had previously been enrolled in one of the following eltrombopag trials: two 6-week and one 6-month randomized, double-blind, placebo-controlled studies (TRA100773A, TRA100773B, and RAISE)12-14 and an open-label, single-arm study of intermittent dosing (REPEAT).15 All enrolled patients had completed the treatment and follow-up periods in the prior studies. To qualify for the prior studies, patients must have had thrombocytopenia for at least 6 months (chronic ITP was previously defined as thrombocytopenia for 6 or more months), insufficient response to at least 1 previous ITP treatment, and a platelet count lower than 30 × 109/L (20 to 50 × 109/L in REPEAT).12-15 Patients with secondary ITP were excluded. There was no platelet-count criterion for entry into EXTEND. Patients who had experienced an SAE related to eltrombopag in their previous study were excluded. Medications prohibited during the study included nonsteroidal anti-inflammatory drugs, aspirin or aspirin-containing compounds, salicylates, rosuvastatin, pravastatin, anticoagulants, quinine, and herbal supplements.
The eltrombopag dose could be adjusted. The starting dose was 50 mg daily and was titrated to as low as 25 mg daily or less often if platelet counts were too high, and to as high as 75 mg daily if platelet counts were too low at 50 mg.
The study was conducted in 4 stages.17
Stage 1: Dosing was initiated at 50 mg once daily and adjusted to identify a dose that increased platelet counts to 100 × 109/L or more.
Stage 2: Concomitant ITP medications were reduced or eliminated while maintaining platelet counts of 50 × 109/L or more with eltrombopag.
Stage 3: The dose of eltrombopag was adjusted to identify the minimal dose of eltrombopag necessary to maintain platelet counts of 50 × 109/L or more in conjunction with the minimal dose of any concomitant ITP medication.
Stage 4: The safety and efficacy of eltrombopag were monitored at the minimal effective dose (with or without the minimal dose of any concomitant ITP medication) that maintained platelet counts at 50 × 109/L or more.
Patients who received at least 2 years of therapy on study and had transitioned off study because of the commercial availability of eltrombopag were considered to have completed EXTEND, whether or not they continued eltrombopag. Patients who did not achieve platelet counts of 50 × 109/L or more but experienced benefit from eltrombopag treatment (as determined with their physicians) were permitted to remain on treatment in the study. Patients were discontinued if they were significantly nonadherent with the protocol, became pregnant, or had an adverse experience that could lead to unacceptable risk. Study medication was discontinued for any thrombosis unless the investigator, in consultation with the study sponsor, determined that the benefit of continuing eltrombopag outweighed the potential risk. Study medication was also discontinued for specific hepatobiliary laboratory abnormalities (HBLAs)18 or ocular changes of clinical concern.
The study was approved by the ethics committee at every participating institution and was conducted according to the recommendations of Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent to participate in the study.
Endpoints and assessments
The primary endpoints were safety and tolerability parameters, including clinical laboratory tests (hematology, coagulation assays, urine dipstick, and clinical chemistry including liver enzyme assessments), ocular examinations (slit lamp examination, visual acuity test, and general ophthalmoscopic examination), and frequency of all AEs. AEs, including hepatobiliary AEs, were assessed with the Common Terminology Criteria for Adverse Events, version 3.19
Prespecified secondary endpoints included the proportion of patients achieving platelet count thresholds (≥50 × 109/L and ≥30 × 109/L) during treatment, maximum continuous duration of platelet count elevation of 50 × 109/L or more or 30 × 109/L or more during treatment; reduction or sparing of concomitant ITP therapies while maintaining a platelet count of 50 × 109/L or more; proportion of patients achieving stable platelet counts of 50 × 109/L or more while remaining free of concomitant ITP medication during treatment; proportion of patients needing rescue treatment (defined as a composite of new ITP medication, increased dose of a concomitant ITP medication, platelet transfusion, or splenectomy); and incidence and severity of signs and symptoms associated with ITP measured using the World Health Organization (WHO) bleeding scale20 and ITP bleeding score.21
Platelet counts and safety assessments were performed weekly during the first 4 weeks of eltrombopag treatment or after any change in dose of eltrombopag or concomitant ITP medications. In patients receiving a stable dose of eltrombopag for 4 or more weeks, platelet counts and safety assessments were performed every 4 weeks.
Safety events of special interest were cataracts, HBLAs (assessed according to the US Food and Drug Administration guidance for potential drug-induced liver injury18 ), thromboembolic events (TEEs), and bone marrow (BM) fibrosis. Secondary, post-hoc analyses of time-to-event for TEE and HBLA were assessed using Kaplan-Meier analysis. Cataracts were reported as an SAE. After the study started, BM examinations were performed annually or when clinically indicated.22 Analysis was primarily descriptive.
Results
EXTEND began in June 2006 and ended in July 2015. The median time from ITP diagnosis to entry into EXTEND was 58.8 (range, 9-552) months. Before entering EXTEND, 290 (96%) patients had a duration of ITP of more than 12 months. At baseline, 33% of patients were using concomitant ITP medications, 53% had received 3 or more prior ITP treatments, 38% had previously undergone splenectomy, and 70% had a baseline platelet count lower than 30 × 109/L (Table 1). The most common prior ITP medications were corticosteroids (81%), intravenous immunoglobulin (46%), and rituximab (23%).
Baseline demographics and characteristics
| Characteristic . | Patients, n (%) (N = 302)* . |
|---|---|
| Age, y | |
| Mean (SD) | 48.9 (15.6) |
| Median (min, max) | 50.0 (18, 86) |
| Female | 201 (67) |
| Race‡ | |
| White | 240 (79) |
| Asian† | 45 (15) |
| American Indian/Alaskan native | 13 (4) |
| African American | 4 (1) |
| Splenectomized | 115 (38) |
| Concomitant ITP medication use at baseline | 101 (33) |
| Baseline platelet counts, ×109 | |
| <30 | 211 (70) |
| 30-50 | 52 (17) |
| >50 | 39 (13) |
| Number of prior ITP therapies | |
| 1 | 67 (22) |
| 2 | 75 (25) |
| ≥3 | 160 (53) |
| Characteristic . | Patients, n (%) (N = 302)* . |
|---|---|
| Age, y | |
| Mean (SD) | 48.9 (15.6) |
| Median (min, max) | 50.0 (18, 86) |
| Female | 201 (67) |
| Race‡ | |
| White | 240 (79) |
| Asian† | 45 (15) |
| American Indian/Alaskan native | 13 (4) |
| African American | 4 (1) |
| Splenectomized | 115 (38) |
| Concomitant ITP medication use at baseline | 101 (33) |
| Baseline platelet counts, ×109 | |
| <30 | 211 (70) |
| 30-50 | 52 (17) |
| >50 | 39 (13) |
| Number of prior ITP therapies | |
| 1 | 67 (22) |
| 2 | 75 (25) |
| ≥3 | 160 (53) |
A total of 494 patients were enrolled in the previous studies feeding into EXTEND. Of these, 371 completed their first study and 302 were enrolled in EXTEND.
Asians included 4 patients of Central/South Asian heritage and 41 of Japanese, East Asian/Southeast Asian heritage.
Percentages do not add up to 100% because of rounding.
Of 302 patients enrolled, 135 (45%) completed the study and 167 (55%) withdrew; 60% of patients were treated for at least 2 years, and 35% for at least 3 years (supplemental Table 1, available on the Blood Web site). The main reasons for withdrawal were AEs (41 [14%]), patient decision (39 [13%]), lack of efficacy (32 [11%]), and other (39 [13%]; supplemental Table 2). The most frequent on-therapy AEs leading to withdrawal were hepatobiliary AEs (16 events in 7 patients), cataracts (4 events in 4 patients), and deep vein thrombosis (3 events in 3 patients). Cerebral infarction, headache, and myelofibrosis occurred in 2 patients each. The remaining AEs leading to withdrawal occurred only once.
Efficacy
Platelet counts.
Overall, 259 (86%) of 302 patients achieved a platelet count of at least 50 × 109/L, and 276 (91%) of 302 patients achieved a platelet count of at least 30 × 109/L at least once in the absence of rescue therapy. Response rates of at least 1 platelet count higher than 50 × 109/L were slightly lower in patients with lower baseline platelet counts (<30 × 109/L, 81.5% [172/211]; 30-50 × 109/L, 98.1% [51/52]; >50 × 109/L, 92.3% [36/39]), those with splenectomy (80% [92/115] of splenectomized patients vs 89.3% [167/187] of nonsplenectomized patients), and those who had 4 or more prior ITP medications (79.6% [90/113] vs 90.5% [171/189] of those with 1 to 3 prior therapies). Of patients who achieved a platelet count of 50 × 109/L or more in their initial eltrombopag study, 92% to 96% achieved this level of response again in EXTEND.
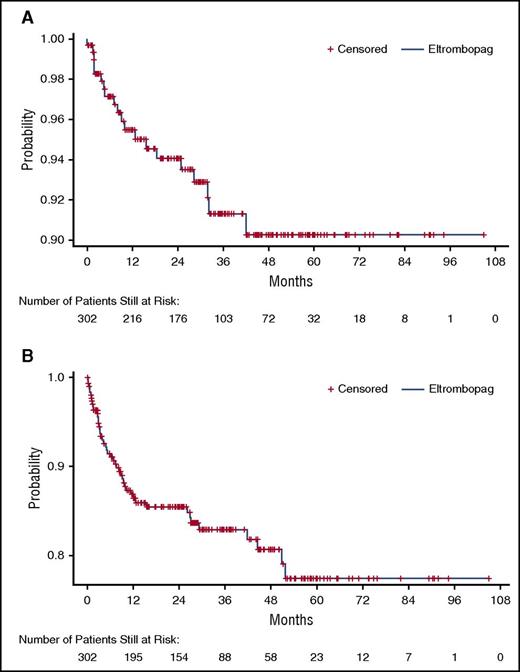
Median platelet counts increased to 50 × 109/L or more by week 2 and remained at least 50 × 109/L throughout 250 weeks of treatment (Figure 1A). More than half of patients achieving a count higher than 50 × 109/L (133/257; 52%) had a continuous platelet count of 50 × 109/L or more or twice baseline for at least 25 weeks in the absence of rescue therapy, and 183 (71%) of 257 patients achieved a continuous platelet count of at least 30 × 109/L in the absence of rescue therapy (Figure 1B) during the same period. A platelet count of 50 × 109/L or more for at least 50% of on-treatment assessments (continuous or intermittent) was achieved by 185/302 patients (61%), with lower rates achieved in patients with splenectomy (59/115; 51%) vs without splenectomy (126/187; 67%), and in those receiving concomitant ITP medications at baseline (55/101; 54%) vs no ITP medications at baseline (130/201; 65%). A platelet count of at least 50 × 109/L for at least 75% of on-treatment assessments was achieved by 126 (42%) of 302 patients, with lower rates achieved in patients with splenectomy (36/115; 31%) vs without splenectomy (90/187; 48%), and in those receiving concomitant ITP medications at baseline (40/101; 40%) vs no ITP medications at baseline (86/201; 43%). Rates of response at various platelet cutoffs are shown in supplemental Table 3.
Platelet count responses throughout EXTEND. (A) Median platelet counts with interquartile ranges (IQR) over time. (B) Weeks of continuous response at platelet thresholds of 30 × 109/L or more, or 50 × 109/L or more and twice baseline in the absence of rescue therapy. The “n” under each figure represents the total number of patients who had been exposed to eltrombopag for the indicated duration unless otherwise indicated. *Platelet count data were collected throughout the study as part of the complete blood count, weekly during the first 4 weeks, and at any dose change (eltrombopag or concomitant ITP medication). If a patient continued on a stable dose during any stage of the study for more than 4 weeks, platelets were assessed every 4 weeks. Because patients were not all assessed on the same schedule, the same patient is not necessarily shown at each assessment. Fewer than 15 patients had platelet counts at each assessment after week 250. †Number of patients with platelet counts of at least 50 × 109/L and at least twice baseline for the indicated number of weeks. ‡Number of patients with platelet counts of 30 × 109/L or more for the indicated number of weeks.
Platelet count responses throughout EXTEND. (A) Median platelet counts with interquartile ranges (IQR) over time. (B) Weeks of continuous response at platelet thresholds of 30 × 109/L or more, or 50 × 109/L or more and twice baseline in the absence of rescue therapy. The “n” under each figure represents the total number of patients who had been exposed to eltrombopag for the indicated duration unless otherwise indicated. *Platelet count data were collected throughout the study as part of the complete blood count, weekly during the first 4 weeks, and at any dose change (eltrombopag or concomitant ITP medication). If a patient continued on a stable dose during any stage of the study for more than 4 weeks, platelets were assessed every 4 weeks. Because patients were not all assessed on the same schedule, the same patient is not necessarily shown at each assessment. Fewer than 15 patients had platelet counts at each assessment after week 250. †Number of patients with platelet counts of at least 50 × 109/L and at least twice baseline for the indicated number of weeks. ‡Number of patients with platelet counts of 30 × 109/L or more for the indicated number of weeks.
Of 101 patients receiving concomitant ITP treatment at baseline, 34 (34%) of 101 permanently stopped 1 or more concomitant ITP medication. In addition, 39% (39/101) reduced or permanently stopped at least 1 ITP medication without ever receiving rescue therapy, and 37 (95%) of the 39 patients had a sustained reduction of at least 24 weeks. The most frequently discontinued or reduced ITP medications were corticosteroids (11% [34/302]), danazol (2% [5/302]), and azathioprine (1% [4/302]). Medications taken at baseline and at the end of treatment are shown in Table 2.
Comparison of medications for treating ITP that were taken at baseline and at the end of treatment in patients who never received a rescue therapy
| Class and drug . | Baseline, n (%) . | End of study, n (%)* . |
|---|---|---|
| Any medication | 101 (33) | 68 (23) |
| Corticosteroids | ||
| Any | 89 (29) | 54 (18) |
| Prednisone | 50 (17) | 29 (10) |
| Prednisolone | 30 (10) | 19 (6) |
| Methylprednisolone | 7 (2) | 5 (2) |
| Corticosteroid NOS | 1 (<1) | 1 (<1) |
| Hydrocortisone | 0 | 1 (<1) |
| Dexamethasone | 1 (<1) | 0 |
| Steroids NOS | 1 (<1) | 1 (<1) |
| Azathioprine | 12 (4) | 6 (2) |
| Danazol | 12 (4) | 3 (<1) |
| Cyclophosphamide | 2 (<1) | 1 (<1) |
| Cyclosporin | ||
| Any | 2 (<1) | 6 (2) |
| Ciclosporin | 1 (<1) | 6 (2) |
| Cilazapril | 1 (<1) | 0 |
| Anti-D (ρ) immunoglobulin | 1 (<1) | 1 (<1) |
| Mycophenolate | ||
| Any | 1 (<1) | 4 (1) |
| Mycophenolic acid | 1 (<1) | 3 (<1) |
| Mycophenolate sodium | 0 | 1 (<1) |
| Vincristine/Vinblastine | ||
| Vincristine | 1 (<1) | 1 (<1) |
| Other | ||
| Any | 2 (<1) | 7 (2) |
| Hydroxychloroquine sulfate | 1 (<1) | 1 (<1) |
| Oxymetholone | 1 (<1) | 0 |
| Rituximab | 2 (<1) | 2 (<1) |
| Eltrombopag† | 0 | 4 (1) |
| Romiplostim | 0 | 2 (<1) |
| Class and drug . | Baseline, n (%) . | End of study, n (%)* . |
|---|---|---|
| Any medication | 101 (33) | 68 (23) |
| Corticosteroids | ||
| Any | 89 (29) | 54 (18) |
| Prednisone | 50 (17) | 29 (10) |
| Prednisolone | 30 (10) | 19 (6) |
| Methylprednisolone | 7 (2) | 5 (2) |
| Corticosteroid NOS | 1 (<1) | 1 (<1) |
| Hydrocortisone | 0 | 1 (<1) |
| Dexamethasone | 1 (<1) | 0 |
| Steroids NOS | 1 (<1) | 1 (<1) |
| Azathioprine | 12 (4) | 6 (2) |
| Danazol | 12 (4) | 3 (<1) |
| Cyclophosphamide | 2 (<1) | 1 (<1) |
| Cyclosporin | ||
| Any | 2 (<1) | 6 (2) |
| Ciclosporin | 1 (<1) | 6 (2) |
| Cilazapril | 1 (<1) | 0 |
| Anti-D (ρ) immunoglobulin | 1 (<1) | 1 (<1) |
| Mycophenolate | ||
| Any | 1 (<1) | 4 (1) |
| Mycophenolic acid | 1 (<1) | 3 (<1) |
| Mycophenolate sodium | 0 | 1 (<1) |
| Vincristine/Vinblastine | ||
| Vincristine | 1 (<1) | 1 (<1) |
| Other | ||
| Any | 2 (<1) | 7 (2) |
| Hydroxychloroquine sulfate | 1 (<1) | 1 (<1) |
| Oxymetholone | 1 (<1) | 0 |
| Rituximab | 2 (<1) | 2 (<1) |
| Eltrombopag† | 0 | 4 (1) |
| Romiplostim | 0 | 2 (<1) |
NOS, not otherwise specified.
Number of patients who received any ITP medication at the 2 points.
Eltrombopag was included if there were an overlap in dates between the investigational product and the poststudy commercial availability.
Of 302 patients on study, 103 (34%) received 1 or more rescue treatments: 82 (27%) started a new ITP medication, 27 (9%) increased their dose of ITP medication from baseline, 21 (7%) received a platelet transfusion, and 3 (<1%) underwent a splenectomy on study.
Bleeding.
Bleeding symptoms occurred in 171 (57%) of 302 patients at baseline and decreased to 16% (13/80 patients) at 1 year; 50 patients (17%) reported WHO grade 2 to 4 bleeding at baseline. During the study, rates of bleeding declined in both splenectomized and nonsplenectomized patients; the great majority were grade 1 or 2 (Figure 2). Nineteen patients reported grade 3 bleeding, and 1 patient reported grade 4 bleeding. The incidence of WHO grade 3 or 4 bleeding was 4.01/100 patient-years. On the ITP bleeding scale, most patients had no bleeding and those with grade 2 other than skin bleeding were evenly divided among oral bleeding, epistaxis, and gynecological bleeding (supplemental Table 4).
Incidence of bleeding during EXTEND (WHO bleeding scale) up to week 117. Bleeding assessments were performed weekly and then monthly or every 2 months for patients with a stable dose of eltrombopag. WHO grade 1, petechiae; grade 2, mild blood loss; grade 3, gross blood loss; grade 4, debilitating blood loss. One patient had WHO grade 4 bleeding, which occurred at week 24. Another patient had grade 4 bleeding during follow-up.
Incidence of bleeding during EXTEND (WHO bleeding scale) up to week 117. Bleeding assessments were performed weekly and then monthly or every 2 months for patients with a stable dose of eltrombopag. WHO grade 1, petechiae; grade 2, mild blood loss; grade 3, gross blood loss; grade 4, debilitating blood loss. One patient had WHO grade 4 bleeding, which occurred at week 24. Another patient had grade 4 bleeding during follow-up.
Dose adjustments
The median duration of eltrombopag exposure was 2.37 years (range, 2 days to 8.76 years). During the study, 285 (94%) of 302 patients required a change in the dose (including frequency) of eltrombopag. Of these, 252 (83%) of 302 patients had at least 1 dose increase (or frequency of dosing) and 218 patients (72%) had at least 1 dose decrease (or frequency of dosing) (supplemental Table 5). A dose interruption occurred in 166 patients (55%), with 77 (25%) interrupting dosing once and 36 (12%) interrupting dosing at least 5 times. Only 15 patients (5%) required no dose modifications and received eltrombopag 50 mg during the entire study period. Among patients with dose adjustments, 48% had a combination of eltrombopag dose increases, decreases, and interruptions based on platelet fluctuations; 18% had both increases and decreases, 23% had increases only, and 11% had only decreases or decreases and dose interruptions (Figure 3). Most of these were minor changes; only a very small number of patients had problematic fluctuations.
At the end of the study, the majority of patients were receiving either eltrombopag 50 mg daily (23%) or 75 mg daily (39%); 108 patients (36%) used an alternate daily dosing regimen (eg, eltrombopag 25 mg every other day or less frequently). In 133 patients with a continuous response for at least 25 weeks, the median dose of eltrombopag was lower (44.4 [range, 1-74.9] mg) than in 168 patients without at least a 25-week continuous response (63.2 [range, 3.5-74.6] mg; supplemental Table 5).
Safety
Adverse events.
The most frequent AEs were headache, nasopharyngitis, and upper respiratory tract infection (Table 3). Most AEs were grade 1 or 2. Grade 3 and 4 AEs occurred in 78 (26%) and 19 (6%) patients, respectively. Most grade 3 or 4 events occurred in 1 patient each, and none occurred in more than 6 patients. Grade 3 events occurring in at least 3 patients included 6 (2%) patients with pain in extremity; 5 (2%) each with pneumonia, fatigue, back pain, alanine aminotransferase (ALT) increase, aspartate aminotransferase (AST) increase, anemia, or hypertension; 4 (1%) with cataracts; and 3 (1%) each with diarrhea, headache, migraine, dyspnea, platelet count decreased, or menorrhagia. Three of the patients included here had both grade 3 ALT increase and grade 3 AST increase. Grade 4 events occurred in 19 patients. Anemia and thrombocytopenia occurred in 3 (1%) and 4 (1%) patients, respectively; all other grade 4 events occurred in 1 patient each.
Adverse events on therapy plus 1 d (N = 302)
| Adverse events . | Patients, n (%) . |
|---|---|
| Any AE | 277 (92) |
| AEs occurring in 10% or more patients | |
| Headache | 86 (28) |
| Nasopharyngitis | 74 (25) |
| Upper respiratory tract infection | 69 (23) |
| Fatigue | 50 (17) |
| Diarrhea | 47 (16) |
| Arthralgia | 45 (15) |
| Back pain | 40 (13) |
| Urinary tract infection | 34 (11) |
| Nausea | 34 (11) |
| Cough | 32 (11) |
| Influenza | 30 (10) |
| Anemia | 29 (10) |
| Any SAE | 96 (32) |
| SAEs occurring in 4 (≥1%) or more patients | |
| Cataracts* | 16 (5) |
| Pneumonia | 8 (3) |
| Anemia | 5 (2) |
| ALT increase | 5 (2) |
| Epistaxis | 4 (1) |
| AST increase | 4 (1) |
| Bilirubin increase | 4 (1) |
| Deep vein thrombosis | 4 (1)† |
| Adverse events . | Patients, n (%) . |
|---|---|
| Any AE | 277 (92) |
| AEs occurring in 10% or more patients | |
| Headache | 86 (28) |
| Nasopharyngitis | 74 (25) |
| Upper respiratory tract infection | 69 (23) |
| Fatigue | 50 (17) |
| Diarrhea | 47 (16) |
| Arthralgia | 45 (15) |
| Back pain | 40 (13) |
| Urinary tract infection | 34 (11) |
| Nausea | 34 (11) |
| Cough | 32 (11) |
| Influenza | 30 (10) |
| Anemia | 29 (10) |
| Any SAE | 96 (32) |
| SAEs occurring in 4 (≥1%) or more patients | |
| Cataracts* | 16 (5) |
| Pneumonia | 8 (3) |
| Anemia | 5 (2) |
| ALT increase | 5 (2) |
| Epistaxis | 4 (1) |
| AST increase | 4 (1) |
| Bilirubin increase | 4 (1) |
| Deep vein thrombosis | 4 (1)† |
The study protocol required that cataracts be reported as SAEs.
DVTs listed here were categorized by the preferred term. One patient had 2 DVTs. Another patient was classified as having thrombosis and then later considered to have had a DVT.
The main on-therapy SAEs occurring in at least 2% of patients were cataracts (16; 5%), pneumonia (8; 3%), anemia (5; 2%), and ALT increase (5; 2%). BM fibrosis was not generally considered an SAE, as no corresponding abnormal changes were seen in peripheral blood smears.
Malignancy.
Ten patients (3%) reported a malignancy that started during the study. Basal cell carcinoma was reported in 3 patients (1%), and intramucosal adenocarcinoma, B-cell unclassifiable lymphoma low grade, breast cancer, Hodgkin’s disease, metastases to the lung, ovarian cancer, squamous cell carcinoma, transitional cell carcinoma, and lymphoma were reported in 1 patient (<1%) each.
Cataracts.
At baseline, 192 (66%) of 291 patients assessed had at least 1 cataract risk factor: corticosteroid use (142; 49%); cigarette use (51; 18%); and/or diabetes mellitus (34; 12%). Cataracts developed in 28 patients (9%) while on study and were considered SAEs in 16 (5%). Characteristics and risk factors are shown in Table 4.
Summary of ocular examination: ocular history and risk factors*
| Visit . | Eltrombopag (N = 28) . |
|---|---|
| At the time of cataract | |
| Age | |
| Median, years | 63 |
| ≥60 y, n | 18 |
| <60 y, n | 10 |
| Baseline | |
| Visually significant cataracts | |
| N | 27 |
| Unilateral, n (%) | 5 (19) |
| Bilateral, n (%) | 7 (26) |
| Cataract risk factors, n (%)† | |
| No risk factors | 6 (22)‡ |
| At least 1 risk factor | 21 (78) |
| Chronic steroid use | 16 (59) |
| Diabetes mellitus | 6 (22) |
| History of intraocular surgery | 4 (15) |
| History of ocular disease requiring medical treatment | 2 (7) |
| Serious eye trauma | 1 (4) |
| Gout | 1 (4) |
| Cigarette use | 4 (15) |
| Other | 3 (11) |
| Visit . | Eltrombopag (N = 28) . |
|---|---|
| At the time of cataract | |
| Age | |
| Median, years | 63 |
| ≥60 y, n | 18 |
| <60 y, n | 10 |
| Baseline | |
| Visually significant cataracts | |
| N | 27 |
| Unilateral, n (%) | 5 (19) |
| Bilateral, n (%) | 7 (26) |
| Cataract risk factors, n (%)† | |
| No risk factors | 6 (22)‡ |
| At least 1 risk factor | 21 (78) |
| Chronic steroid use | 16 (59) |
| Diabetes mellitus | 6 (22) |
| History of intraocular surgery | 4 (15) |
| History of ocular disease requiring medical treatment | 2 (7) |
| Serious eye trauma | 1 (4) |
| Gout | 1 (4) |
| Cigarette use | 4 (15) |
| Other | 3 (11) |
No patient had family history of cataract at a young (<60 y) age, history of radiation treatment to the head or orbit, chronic occupational exposure to ultraviolet light, or alcohol abuse.
One patient was not assessed for risk factors at baseline.
The 6 patients assessed for and without risk factors at baseline were aged 25, 33, 54, 58, 63, and 66 y, respectively, at the time of the event.
Thromboembolic events.
During treatment on EXTEND, 19 patients (6%) experienced 24 TEEs including 8 deep vein thrombosis (6 SAEs), 5 myocardial infarctions (all SAEs), 1 pulmonary embolism and 1 pulmonary infarction (both SAEs), and 4 cerebral infarction (3 SAEs; 1 was a nonserious event of left centrum semiovale infarct [small stroke]; Table 5). This corresponds to an incidence rate of 2.69 TEE/100 patient-years receiving eltrombopag (95% confidence interval, 1.67-4.11). Of these 19 patients, 9 were splenectomized.
On-therapy plus 1 day thromboembolic events (safety population)
| . | Eltrombopag (N = 302) . | |
|---|---|---|
| All AEs . | SAEs . | |
| Patients with thromboembolic AEs, n (%)* | 19 (6) | 16 (5) |
| Thromboembolic events, n (%) | 24 (8) | 18 (6) |
| Venous events | 10 (3) | 9 (3) |
| Arterial events† | 14 (5) | 9 (3) |
| Individual events, n (%) | ||
| Deep vein thrombosis‡ | 8 (3) | 6 (2) |
| Cerebral infarction | 4 (1) | 3 (1) |
| Acute myocardial infarction | 2 (<1) | 2 (<1) |
| Myocardial infarction§ | 3 (1) | 3 (1) |
| Transient ischemic attack† | 3 (1) | 0 |
| Cerebral ischemia | 1 (<1) | 1 (<1) |
| Pulmonary embolism | 1 (<1) | 1 (<1) |
| Pulmonary infarction | 1 (<1) | 1 (<1) |
| Thrombophlebitis superficial | 1 (<1) | 1 (<1) |
| . | Eltrombopag (N = 302) . | |
|---|---|---|
| All AEs . | SAEs . | |
| Patients with thromboembolic AEs, n (%)* | 19 (6) | 16 (5) |
| Thromboembolic events, n (%) | 24 (8) | 18 (6) |
| Venous events | 10 (3) | 9 (3) |
| Arterial events† | 14 (5) | 9 (3) |
| Individual events, n (%) | ||
| Deep vein thrombosis‡ | 8 (3) | 6 (2) |
| Cerebral infarction | 4 (1) | 3 (1) |
| Acute myocardial infarction | 2 (<1) | 2 (<1) |
| Myocardial infarction§ | 3 (1) | 3 (1) |
| Transient ischemic attack† | 3 (1) | 0 |
| Cerebral ischemia | 1 (<1) | 1 (<1) |
| Pulmonary embolism | 1 (<1) | 1 (<1) |
| Pulmonary infarction | 1 (<1) | 1 (<1) |
| Thrombophlebitis superficial | 1 (<1) | 1 (<1) |
Patients could have more than 1 event. Two patients had 2 events, and 2 other patients had 3 events, while on study. Two additional patients had 1 on-study event (a pulmonary embolism and a pulmonary infarction, respectively) and 1 off-study event (a deep vein thrombosis and a pulmonary infarction, respectively).
One patient had both a speech disorder and unsteadiness on the same day, and was later considered to have a transient ischemic attack.
One patient had 2 deep vein thromboses. Another patient was classified as having thrombosis and then later considered to have had a deep vein thrombosis.
One patient had 2 myocardial infarctions.
A post-hoc analysis of time-to-first TEE revealed that most events occurred in the first year and none after year 4 (Figure 4A). Supplemental Figure 1 shows the relationship between platelet counts and timing of TEEs in the individual patients.
Kaplan-Meier time-to-first event curves for TEE and HBLA. (A) TEEs. (B) HBLAs. Patients still at risk do not include patients who already experienced the event in question or patients who withdrew from the study.
Kaplan-Meier time-to-first event curves for TEE and HBLA. (A) TEEs. (B) HBLAs. Patients still at risk do not include patients who already experienced the event in question or patients who withdrew from the study.
Hepatobiliary adverse events and laboratory abnormalities.
A post-hoc Kaplan-Meier analysis showed that the highest incidence of hepatobiliary events occurred within the first year (Figure 4B). Forty-five patients (15%) had hepatobiliary AEs (supplemental Table 6), none of which were grade 4. Grade 3 hepatobiliary AEs included 5 patients (2%) with increased ALT, 5 (2%) with increased AST, 4 (1%) with increased bilirubin, 2 (<1%) with increased transaminase, 1 (<1%) with increased blood alkaline phosphatase, and 1 (<1%) with increased hepatic enzymes. Five patients met criteria for Hy’s law (ALT or AST >3 × upper limit of normal [ULN] with bilirubin >2 × ULN and ALP <2 × ULN); all 5 cases were confounded (see narratives in supplemental Materials). One patient was rechallenged with eltrombopag after 7 days, and 1 patient continued eltrombopag 75 mg. Of the 37 patients with HBLAs (supplemental Table 6), 24 were considered to have hepatobiliary AEs.
BM fibrosis.
Centrally22 and locally reviewed BM biopsies (n = 356) were obtained from 166 patients treated with eltrombopag for up to 7 years and were analyzed using the European Consensus Scale (supplemental Table 7).23 In the combined centrally and locally reviewed samples, the majority of patients remained at a marrow fibrosis (MF) score of 0 (87, 52%) or had a maximal increase to MF-1 (68, 41%). Ten patients (6%) had a maximal increase to MF-2, and 1 (<1%) increased to MF-3. The 356 on-treatment biopsies were all prompted by study protocol; none was prompted by abnormal cell counts, morphology, or clinical symptoms suggestive of BM dysfunction.22
Deaths
Four patients died while receiving therapy plus 1 day. The causes of deaths included a traffic accident; bleeding, sepsis syndrome, and cardiovascular events in a patient with fever on steroids; multiorgan failure resulting from pulmonary sepsis; and adenocarcinoma. Two other patients died more than 50 days after treatment. Causes included hypovolemic shock secondary to gastrointestinal hemorrhage and intracranial hemorrhage. None of the 6 deaths was considered related to eltrombopag treatment.
Discussion
On the EXTEND study, median platelet levels increased to 50 × 109/L or more within 2 weeks. Just more than 40% of all patients and 50% treated for at least 30 weeks achieved a 25-week continuous platelet count of 50 × 109/L or more, and nearly 70% of patients achieved a continuous platelet count of 30 × 109/L or more, for at least 25 weeks. Responses were seen in heavily pretreated patients, splenectomized patients, more previous therapies, and those with lower platelet counts at baseline; however, response rates in these groups were lower than those in nonsplenectomized patients, those receiving 3 or fewer prior therapies, and those with higher platelet counts at baseline, respectively. None of the differences between subgroups was substantial enough to be considered prognostic factors or to recommend using or withholding eltrombopag in specific patient subgroups.
WHO grade 3 to 4 bleeding events were infrequent (approximately 4 events/100 patient-years), and TEEs occurred at approximately half that rate. One third of patients receiving concomitant ITP medications were able to stop 1 or more concomitant medications without ever receiving on-treatment rescue therapy, despite many patients having already reduced these medications in their prior eltrombopag study.
Here, we extend the interim report of the EXTEND study reporting results in patients treated for up to 3 years with eltrombopag, and further explore its long-term safety and efficacy.17 Overall, eltrombopag was well-tolerated and reasonably safe, with 18 patients being exposed for 6 or more years. Looking at AEs of special interest, TEEs occurred in 6% of patients overall, very similar to results reported with romiplostim treatment of similar duration24 and in retrospective (6%-8%)25,26 and prospective (5%) studies of patients with ITP.27 Sarpatwari et al reported a 4.6% incidence of TEEs in patients without ITP vs 6.1% in those with ITP.25 Notably, many TEEs occurred in the first year of treatment, as was found with hepatobiliary events, which occurred at relatively low rates. Nonetheless, patients should be monitored for TEEs and HBLA; when to reduce the frequency of monitoring (eg, after 1-3 years) remains to be determined. Rates of cataracts were also low. Eltrombopag’s contribution to cataract development was difficult to determine given patients’ multiple confounding risk factors (eg, past steroid use, age >60 years, and smoking). At this time, screening and monitoring patients for cataracts before initiating and during eltrombopag treatment is not routine, but might be appropriate for patients with 1 or more risk factors.
BM fibrosis of more than MF-1 was uncommon in EXTEND, with MF-2 and MF-3 assessment at any time during the study period in 10 and 1 patients, respectively. A prospective longitudinal study of BM changes during 2 years of eltrombopag found that 5 (3%) of 162 patients had biopsies graded MF-2 or MF-3 at any time during the study.28 A single-center study reported a similar higher frequency of MF-2 or MF-3 over time with TPO-RAs.29 The current international consensus is not to perform routine marrow surveillance in patients treated with TPO-RAs,30 and to perform them only after loss of platelet response or when abnormal cells are seen in peripheral blood smears; in this study, the latter did not occur.5
The goal of managing chronic ITP is to minimize the bleeding risk by increasing platelet counts to within the hemostatic range,5 with maximum tolerability and the fewest possible treatment-associated AEs. Guidelines and expert opinion recommend clinical intervention in adult patients with platelet counts lower than 30 × 109/L or lower than 50 × 109/L with significant bleeding or risk factors for bleeding or with fatigue.5,31-34 After initial treatment with corticosteroids or intravenous immunoglobulin or anti-D immunoglobulin, many patients require second-line therapy, which in addition to TPO-RAs may also include immunosuppressants, rituximab, or splenectomy.33,35 As extensively discussed previously, there is no clear way to decide which second-line therapy to use in an individual patient.36,37 Randomized studies comparing eltrombopag (or romiplostim) with any other treatments are lacking.
An important premise of using TPO-RAs has been that they combine a high rate of efficacy with good tolerability and low toxicity rates. Intravenous immunoglobulin, steroids, and splenectomy have comparable rates of efficacy (eg, >50% responding).38-41 However, intravenous immunoglobin is limited by difficulty with intravenous administration, the postinfusion headaches associated with its use, and the roller-coaster effects on platelet counts. Rituximab and splenectomy both have the advantages of long-term and potentially curative effects.42,43 Rituximab alone has an approximately 20% to 40% long-term response rate in adults.42-44 Even with 3 cycles of dexamethasone, the cure rates for rituximab are similar, except in women with ITP duration of less than 1 year, whose response rates appear to be higher.45 Splenectomy has a higher curative rate, but has its own set of toxicities and currently has decreasing acceptance by patients.46 Therefore, despite the lack of curative effects and the dietary limitations of eltrombopag, which must be taken 1 hour before or 2 hours after a meal and 4 hours from supplemental calcium (eg, dairy products),7 use of eltrombopag and other TPO-RAs are increasing among patients with chronic ITP.
It is worth noting that the dosing of eltrombopag is not straightforward. As shown by the number of patients who required dose increases, decreases, and interruptions, including those done to reduce concomitant medications (eg, reduced steroids), all but 5% required dose adjustment, and many required increases and decreases in the dose. Therefore, in addition to regular liver tests to monitor HBLA, monitoring of blood counts is required at regular intervals even in patients with apparently stable platelet counts.
The EXTEND study demonstrates that even if eltrombopag is used for years as maintenance treatment, it may be not only consistently efficacious but also tolerable and safe in most patients. The American Society of Hematology convened an expert panel in 2015 to revise the 2011 evidence-based practice guideline for immune thrombocytopenia, with publication of new guidelines anticipated in late 2017. Assessing where TPO-RAs fit within the recommended treatment paradigm will be hampered by the lack of comparative evidence to other treatments and uncertainty as to the existence of curative effects.
Limitations include that patients who completed the study were not followed, so their long-term outcomes are unknown and data on patients who were able to discontinue therapy were not captured.
In conclusion, in this clinical trial setting, eltrombopag increased platelet counts in most patients with ITP in a consistent fashion to adequate levels, was well-tolerated, and appeared safe, with a low frequency of SAEs, TEEs, and BM fibrosis. It was effective in essentially all patient subgroups, although less so in splenectomized and heavily pretreated patients and in those with very low baseline platelet counts. Good responders maintained platelet counts of at least 30 to 50 × 109/L for prolonged periods, leading to lower bleeding rates and reducing the need for concurrent ITP medications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the study investigators and sites. A complete list is provided in the supplemental Materials. Editorial support (assembling tables and figures, collating author comments, copyediting, fact checking, and referencing) and graphic services were provided by Meher Dustoor, and Nancy Price, of AOI Communications, LP, and was funded by Novartis Pharmaceuticals Corporation.
This study (NCT00351468) is/remains sponsored by GlaxoSmithKline; however, as of March 2, 2015, eltrombopag is an asset of Novartis AG.
Authorship
Contribution: All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. R.S.M.W. and A.K. contributed to the acquisition of the data and to the data analysis or interpretation; M.N.S. and A.S. contributed to the acquisition of the data; M.S.O.P. and P.B. contributed to the data analysis or interpretation of the data; J.B.B. contributed to the conception or design of the study, contributed to the acquisition of the data, and performed data analysis or interpretation of the data; and all authors provided critical review of the manuscript and approval of the final version of the manuscript.
Conflict-of-interest disclosure: R.S.M.W. provided consultancy for Bayer, Biogen-Idec, and Novartis; received research funding from Bayer, Biogen-Idec, Bristol-Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Merck Sharp & Dohme, Novartis, Pfizer, and Roche; and is an advisory board member of Biogen-Idec and Novartis. M.N.S. has participated in speaker bureaus, provided consultancy, and received research funding from GlaxoSmithKline. M.S.O.P. is an employee of Novartis. P.B. is currently an employee of Novartis and formerly an employee of GlaxoSmithKline and holds equity in GlaxoSmithKline. J.B.B. has been a consultant for GlaxoSmithKline and Novartis; received research funding from Amgen, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Prophylix Pharma, Protalex, and Rigel Pharmaceuticals; participated in advisory boards for Amgen, Momenta Pharmaceuticals, Novartis, Prophylix Pharma, Protalex, and Rigel Pharmaceuticals; has received a royalty from UpToDate; and has participated in a speakers bureau for Novartis and Physicians Education Resource. The remaining authors declare no competing financial interests.
Correspondence: Raymond S. M. Wong, Department of Medicine & Therapeutics, Prince of Wales Hospital, 30-32 Ngan Shing St, Shatin, NT, Hong Kong; e-mail: raymondwong@cuhk.edu.hk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal