Key Points
Organ progression at second-line therapy predicated inferior survival.
Patients relapsing from >VGPR had a longer time to develop organ progression.
Abstract
Among patients with immunoglobulin light chain (AL) amyloidosis, there is little consensus on when reinstitution of chemotherapy should occur. We conducted a retrospective study to evaluate the patterns of relapse or progression (R/P) and the timing of reinitiating therapy among 235 patients initially treated with autologous stem cell transplant (ASCT) at Mayo Clinic. The median time from ASCT to second-line therapy was 24.3 months. At the time of restarting therapy, median difference of free light chain (dFLC) was 9.9 mg/dL (42% of diagnosis value), 32% had a dFLC <5 mg/dL, and 63% met criteria for organ R/P. The indications for retreatment were (1) clinical suspicion of R/P, 10%; 92) hematologic R/P only, 23%; (3) organ R/P only, 32%; (4) both hematologic and organ R/P, 31%; and (5) suboptimal response to ASCT and second-line therapy as consolidation, 4%. Patients with organ progression at the time of second-line therapy had inferior survival. Although a dFLC of >5 mg/dL at the time of reinstituting therapy was associated with risk, patients relapsing from very good partial response (VGPR) or better had a longer time to develop organ progression after hematologic R/P (24.2 vs 3.2 months, P = .007). These data suggest that the best candidates for clinical trials testing novel plasma cell–directed chemotherapy beyond first line may be those patients who are either relapsing from VGPR or better (dFLC at diagnosis was >5 mg/dL) or having inadequate response to prior therapy. This strategy should allow for hematologic response assessment while avoiding the risk of deleterious organ progression. Implementation of more stringent progression criteria may also be warranted.
Introduction
Immunoglobulin light chain (AL) amyloidosis is a systemic disease characterized by the deposition of misfolded AL proteins leading to progressive organ dysfunction.1 Since its introduction for the treatment of AL amyloidosis in the 1990s,2,3 autologous stem cell transplant (ASCT) has been proved as an effective treatment to achieve hematologic and organ responses, as well as to improve survival outcome.4,5 Studies have demonstrated that 25% to 43% of AL patients receiving ASCT survive a decade or more.6,7 Among AL amyloidosis patients who progress, the median time to relapse/progression (R/P) after ASCT is 26 months, but the 5-year overall survival (OS) from posttransplant R/P approaches 40%.8 Given the complexity and heterogeneity of AL patients, there is little consensus on when reinstitution of chemotherapy should occur,9 which makes rational clinical trial design difficult. Patients with AL amyloidosis who have been treated with high-dose chemotherapy followed by ASCT are a relatively low-risk and homogenous population making them an ideal group to study practice patterns and the implications thereof in terms of the timing of resuming therapy.
Patients and methods
After approval by the Mayo Clinic Institutional Review Board, clinical and laboratory data of those patients who granted approval to access their medical records for research were reviewed to evaluate the patterns of R/P and the timing of reinitiation of therapy. The inclusion criteria of this retrospective review were biopsy-proven AL amyloidosis, ASCT as planned up-front treatment either with or without pretransplant induction chemotherapy, and documented reinstitution of a second-line therapy post-ASCT. The patients who died within 3 months of ASCT, who died anytime beyond 3 months post-ASCT without starting a second-line therapy, or who were alive and did not require therapy at the last follow-up were excluded from this study. Two-hundred thirty-five patients with AL amyloid patients who underwent ASCT at Mayo Clinic between 1 January 1996 and 31 December 2014 met the selection criteria. All of these patients received a second-line therapy post-ASCT between 9 July 1997 and 29 June 2016. The disposition of patients for the analyses is shown in supplemental Figure 1 (available on the Blood Web site).
The definitions of organ involvement and the criteria used to classify hematologic and organ R/P were based on the consensus opinion from the 10th International Symposium on Amyloidosis10 and the updated consensus response criteria.11 We used the newest criteria for renal response (≥30% decrease in proteinuria or drop of proteinuria below 0.5 g/24 hours in the absence of a dropping estimated glomerular filtration rate), which is associated with longer renal survival.12 Renal R/P was defined as an increase in proteinuria by >50% from nadir that was at least 1 g/24 hours or 25% worsening of serum creatinine or creatinine clearance,10 cardiac response was defined as >30% and >300 ng/L decrease if baseline N-terminal pro-B-type natriuretic peptide (NT pro-BNP) ≥650 ng/L, and R/P was defined as an increase in NT pro-BNP by >30% from nadir and minimum of 300 or increase in troponin T by ≥33%11 ; liver R/P was defined as 50% increase of alkaline phosphatase from nadir value.10 Hematologic relapse from complete response (CR) and hematologic progression were according to the current consensus criteria.13 The former is defined as 2 consecutive detectable monoclonal protein or abnormal FLC ratio, and the latter as either 50% increase of difference of free light chain (dFLC) to >10 mg/dL, 50% increase in serum M protein to >0.5 g/dL, or 50% increase in urine M protein to >200 mg/dL from partial response (PR). In this study, we defined subtle hematologic R/P as a dFLC increase of 25% that was also an increment of at least 5 mg/dL.
Statistical analyses were performed using JMP software (SAS, Cary, NC). All value comparisons used median and interquartile range (IQR). Two-sided χ2 test or Fisher’s exact test was used to test for differences between categorical variables. Wilcoxon rank sum test was used to compare continuous variables. OS was calculated from both the date of ASCT and the date of initiation of second-line therapy to the date of death attributable to any cause and from the time of reinstitution of therapy post-ASCT till death. Patients were censored at the last follow-up date if alive at the time of last follow-up. The survival outcomes were estimated using the Kaplan-Meier method, and survival curves were compared by the log-rank test. Risk ratios were estimated based on the univariate and a stepwise multivariable Cox proportional hazard models. Variables tested in risk modeling included age, gender, year of ASCT, AL amyloid staging, indications for second-line therapy post-ASCT, number of organs involved, post-ASCT hematologic and organ response. Mayo amyloid staging systems were excluded from the stepwise multivariable analysis because nearly one-third of patients had missing values, and there was a time period effect associated with the absence of this variable. In addition, the variable dividing patients by whether they instituted second-line therapy before or after 2009 was also excluded because of a strong interaction with the use of novel agents at second-line therapy. Statistical significance was inferred at P value <.05 for all comparisons.
Results
Patient characteristics
Among the 235 patients in this series, the median age at diagnosis was 58 years (IQR: 51-64.0), and 62% were male. Sixty-eight percent of the patients had renal involvement, and 48.5% had cardiac involvement. The median time from diagnosis to ASCT was 2.2 months (IQR: 1.7-3.3), and the median time from ASCT to initiating a second-line therapy was 24.3 months (IQR: 10-49.6). Post-ASCT, 59% of this cohort of patients achieved a very good partial response (VGPR) or better, and the organ response rate was 44%. A higher organ response rate was seen in the VGPR or better patients compared with those who did not achieve post-ASCT VGPR (59% vs 25%, P < .0001).
Based on their R/P status at the time of second-line therapy institution, patients were divided into 5 groups (Table 1). Group 1 patients (10%) were retreated at early disease recurrence as determined by providers but did not yet meet either hematologic or organ R/P criteria. Group 2 patients (23%) had evidence of hematologic R/P without signs of organ R/P. Group 3 (32%) had evidence of organ R/P without hematologic R/P. Group 4 (31%) had both hematologic and organ R/P. Group 5 patients (4%) had had suboptimal response to ASCT and were thus given second-line therapy as consolidation with the intent of achieving a deeper response. It is notable that the year of ASCT differed by group, with patients in group 4 representing the earliest calendar years, and group 1 the more recent calendar years. As expected, group 5 patients had the lowest rates of PR and VGPR post-ASCT and the shortest interval between ASCT and second-line therapy. The patients with both hematologic and organ R/P had the highest median value of dFLC when restarting treatment. The patients with organ-only R/P had the lowest dFLC at diagnosis and at second-line therapy initiation. The organ distribution of the organ-only R/P patients was 80% with renal involvement, 47% with cardiac involvement with 33% with both renal and cardiac involvement.
Patient characteristics by indication for instituting second-line therapy
| Patient characteristics . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | Group 5 . | P . |
|---|---|---|---|---|---|---|
| No documented R/P (N = 24) . | Hematologic . | Organ R/P only . | Both hematologic and organ R/P (N = 72) . | Suboptimal response (N = 10) . | ||
| R/P only (N = 53) . | (N = 76) . | |||||
| Age, median (IQR) | 57 (50-70) | 60 (53-64) | 57 (53-62) | 58 (49-65) | 54 (50-61) | .43 |
| Male sex, n (%) | 15 (62) | 30 (57) | 54 (71) | 43 (60) | 5 (50) | .39 |
| Year of ASCT, median (IQR) | 2008 (2004-2013) | 2006 (2003-2010) | 2007 (2004-2010) | 2005 (2003-2007) | 2009 (2007-2011) | .007 |
| Diagnosis median dFLC, mg/dL (IQR) | 19.5 (5.4, 67.4) | 38.0 (13.1, 90.3) | 9.4 (5.1, 26.4) | 24.6 (11.9, 82.2) | 24.8 (13.6, 46.5) | <.001 |
| Diagnosis dFLC <5 mg/dL, % | 20 | 11 | 24 | 7 | 10 | .08 |
| AL stage >2, n (%)* | ||||||
| 2004 stage† | 0 (0) | 8 (20) | 8 (14) | 8 (17) | 2 (22) | .10 |
| 2012 stage‡ | 1 (5) | 11 (28) | 15 (26) | 12 (26) | 3 (33) | .18 |
| Organs >2, n (%) | 2 (8) | 5 (9) | 13 (17) | 11 (15) | 0 (0) | .26 |
| Post-ASCT CR, n (%) | 3 (12) | 18 (33) | 15 (27) | 19 (35) | 0 (0) | .06 |
| Post-ASCT ≥VGPR, n (%) | 10 (50) | 35 (67) | 47 (66) | 37 (54) | 0 (0) | <.001 |
| Post-ASCT ≥PR, n (%) | 21 (88) | 48 (91) | 60 (79) | 62 (86) | 4 (40) | .009 |
| ASCT organ response, n (%) | 7 (29) | 29 (55) | 29 (38) | 35 (49) | 3 (30) | .13 |
| Months from ASCT to second-line (median, IQR) | 13.3 (6.8-24.4) | 31.3 (17.4-60.2) | 16.0 (9.3-38.2) | 29.3 (15.2-51.7) | 3.7 (3.2-9.8) | <.001 |
| Novel agent in second-line therapy, n (%) | 16 (67) | 44 (83) | 49 (65) | 51 (73) | 9 (90) | .12 |
| Second-line therapy post-2009, n (%) | 15 (63) | 31 (60) | 39 (51) | 31 (43) | 6 (60) | .30 |
| Median dFLC at second-line, mg/dL (IQR) | 5.7 (2.7-17.7) | 11.6 (6.7-21.8) | 3.2 (2.0-7.3) | 15.4 (9.1-40.7) | 12.8 (8.4-21.3) | <.001 |
| dFLC at second-line ≥5 mg/dL, n (%) | 13 (62) | 44 (85) | 25 (36) | 63 (89) | 8 (80) | <.001 |
| “Subtle” hematologic R/P or more, n (%) | 10 (42) | 53 (100) | 17 (22) | 72 (100) | 1 (10) | <.001 |
| Patient characteristics . | Group 1 . | Group 2 . | Group 3 . | Group 4 . | Group 5 . | P . |
|---|---|---|---|---|---|---|
| No documented R/P (N = 24) . | Hematologic . | Organ R/P only . | Both hematologic and organ R/P (N = 72) . | Suboptimal response (N = 10) . | ||
| R/P only (N = 53) . | (N = 76) . | |||||
| Age, median (IQR) | 57 (50-70) | 60 (53-64) | 57 (53-62) | 58 (49-65) | 54 (50-61) | .43 |
| Male sex, n (%) | 15 (62) | 30 (57) | 54 (71) | 43 (60) | 5 (50) | .39 |
| Year of ASCT, median (IQR) | 2008 (2004-2013) | 2006 (2003-2010) | 2007 (2004-2010) | 2005 (2003-2007) | 2009 (2007-2011) | .007 |
| Diagnosis median dFLC, mg/dL (IQR) | 19.5 (5.4, 67.4) | 38.0 (13.1, 90.3) | 9.4 (5.1, 26.4) | 24.6 (11.9, 82.2) | 24.8 (13.6, 46.5) | <.001 |
| Diagnosis dFLC <5 mg/dL, % | 20 | 11 | 24 | 7 | 10 | .08 |
| AL stage >2, n (%)* | ||||||
| 2004 stage† | 0 (0) | 8 (20) | 8 (14) | 8 (17) | 2 (22) | .10 |
| 2012 stage‡ | 1 (5) | 11 (28) | 15 (26) | 12 (26) | 3 (33) | .18 |
| Organs >2, n (%) | 2 (8) | 5 (9) | 13 (17) | 11 (15) | 0 (0) | .26 |
| Post-ASCT CR, n (%) | 3 (12) | 18 (33) | 15 (27) | 19 (35) | 0 (0) | .06 |
| Post-ASCT ≥VGPR, n (%) | 10 (50) | 35 (67) | 47 (66) | 37 (54) | 0 (0) | <.001 |
| Post-ASCT ≥PR, n (%) | 21 (88) | 48 (91) | 60 (79) | 62 (86) | 4 (40) | .009 |
| ASCT organ response, n (%) | 7 (29) | 29 (55) | 29 (38) | 35 (49) | 3 (30) | .13 |
| Months from ASCT to second-line (median, IQR) | 13.3 (6.8-24.4) | 31.3 (17.4-60.2) | 16.0 (9.3-38.2) | 29.3 (15.2-51.7) | 3.7 (3.2-9.8) | <.001 |
| Novel agent in second-line therapy, n (%) | 16 (67) | 44 (83) | 49 (65) | 51 (73) | 9 (90) | .12 |
| Second-line therapy post-2009, n (%) | 15 (63) | 31 (60) | 39 (51) | 31 (43) | 6 (60) | .30 |
| Median dFLC at second-line, mg/dL (IQR) | 5.7 (2.7-17.7) | 11.6 (6.7-21.8) | 3.2 (2.0-7.3) | 15.4 (9.1-40.7) | 12.8 (8.4-21.3) | <.001 |
| dFLC at second-line ≥5 mg/dL, n (%) | 13 (62) | 44 (85) | 25 (36) | 63 (89) | 8 (80) | <.001 |
| “Subtle” hematologic R/P or more, n (%) | 10 (42) | 53 (100) | 17 (22) | 72 (100) | 1 (10) | <.001 |
Unless otherwise stated, characteristics are at diagnosis.
R/P, relapse or progression.
Data available for only 172 patients.
2004 stage is defined by troponin T and NT pro-BNP threshold (0.035 ng/mL and 332 pg/mL). Stage I, both below threshold; stage II, 1 above threshold; stage II, both above threshold.19
2012 stage is defined by troponin T, NT pro-BNP, and dFLC threshold (0.05 ng/mL, 1800 pg/mL, and 18 mg/dL, respectively). Stage I, all below threshold; stage II, 3 below threshold; stage II, 2 below threshold; stage IV, all above threshold.20
Covariates for timing of initiation of second-line therapy
We explored interactions between post-ASCT response and the timing of starting second-line therapy (Table 2). There was a striking interaction between both degree of post-ASCT hematologic response (either VGPR or PR) and time to initiation of therapy regardless of grouping. The better the post-ASCT response, the longer was the time to initiate therapy. This same pattern held true for post-ASCT organ response and time to initiation of therapy.
Post-ASCT response and time to initiation of second-line therapy
| Post-ASCT response . | Time in months from ASCT to initiation of second-line therapy (number of patients) . | P . | ||||
|---|---|---|---|---|---|---|
| Group 1 . | Group 2 . | Group 3 . | Group 4 . | Group 5 . | ||
| ≥VGPR | ||||||
| Yes | 18.2 (10) | 36.6 (34) | 25.5 (47) | 47.2 (37) | <.05 | |
| No | 8.4 (10) | 17.7 (17) | 9.4 (24) | 15.3 (32) | 3.7 (9) | .01 |
| P | .11 | .004 | <.001 | <.001 | ||
| ≥PR | ||||||
| Yes | 17.0 (21) | 32.3 (47) | 23.6 (60) | 33.8 (62) | 12.2 (4) | .02 |
| No | 3.9 (3) | 24.5 (5) | 8.2 (16) | 10.9 (10) | 3.6 (6) | .01 |
| P | <.001 | .04 | <.001 | .01 | .08 | |
| Organ response | ||||||
| Yes | 24.3 (7) | 36.7 (29) | 35.4 (29) | 48.6 (35) | 19.3 (3) | .38 |
| No | 9.4 (17) | 26.8 (23) | 12.3 (47) | 22.3 (37) | 3.7 (7) | <.001 |
| P | .008 | .02 | <.001 | <.001 | .03 | |
| Post-ASCT response . | Time in months from ASCT to initiation of second-line therapy (number of patients) . | P . | ||||
|---|---|---|---|---|---|---|
| Group 1 . | Group 2 . | Group 3 . | Group 4 . | Group 5 . | ||
| ≥VGPR | ||||||
| Yes | 18.2 (10) | 36.6 (34) | 25.5 (47) | 47.2 (37) | <.05 | |
| No | 8.4 (10) | 17.7 (17) | 9.4 (24) | 15.3 (32) | 3.7 (9) | .01 |
| P | .11 | .004 | <.001 | <.001 | ||
| ≥PR | ||||||
| Yes | 17.0 (21) | 32.3 (47) | 23.6 (60) | 33.8 (62) | 12.2 (4) | .02 |
| No | 3.9 (3) | 24.5 (5) | 8.2 (16) | 10.9 (10) | 3.6 (6) | .01 |
| P | <.001 | .04 | <.001 | .01 | .08 | |
| Organ response | ||||||
| Yes | 24.3 (7) | 36.7 (29) | 35.4 (29) | 48.6 (35) | 19.3 (3) | .38 |
| No | 9.4 (17) | 26.8 (23) | 12.3 (47) | 22.3 (37) | 3.7 (7) | <.001 |
| P | .008 | .02 | <.001 | <.001 | .03 | |
At time of second-line therapy: group 1, early signs of recurrent disease that did not yet met either hematologic or organ R/P criteria; group 2 evidence of hematologic R/P without signs of organ R/P; group 3, evidence of organ R/P without hematologic R/P; group 4, both hematologic and organ R/P; and group 5, suboptimal response to ASCT and given second line as consolidation.
Because this study covered nearly 20 years of practice, the effects of when treatment was restarted (pre- and post-2009) were evaluated. There was a higher usage of immune modulatory drugs and proteasome inhibitors in the more recent era (92% vs 52%, P < .0001), and there was a significant difference in the dFLC value at reinitiation of therapy (P = .004): prior to 2009, dFLC was 11.6 mg/dL (IQR: 5.3-24.8), which was 50% of the dFLC at diagnosis; and after 2009, dFLC was 6.8 mg/dL (IQR: 2.6-16.2), which was 34.6% of the diagnosis value. In those who started second-line therapy pre-2009, 70% patients had evidence of organ progression compared with 57% in the post-2009 group (P = .05).
At the time of starting second-line therapy, 63% of all patients met criteria for organ progression. The absolute median value of NT pro-BNP was 1585 pg/mL at retreatment of those having cardiac involvement, which was 103% of the diagnosis value and 160% over nadir. Twenty-four hour urinary protein in patients with renal involvement was 4616 mg, 83% of the diagnosis value and 181% of the nadir value (Table 3). Fifty-three percent (n = 125) met criteria for hematologic R/P according to standard criteria,10,11,13 and 65% (n = 153) patients had “subtle” hematologic R/P or more. At second line, median dFLC was 9.9 mg/dL (3.5, 21), which was 42% of the dFLC level at diagnosis and 262% over nadir, and 32% started second-line therapy with a dFLC <5 mg/dL.
Labs at diagnosis, post-ASCT nadir, and initiation of second-line therapy
| Laboratory markers . | At diagnosis . | Posttransplant nadir . | At second-line therapy initiation . | ||
|---|---|---|---|---|---|
| Restarting therapy . | % of diagnosis . | % over nadir . | |||
| All patients (n = 235) | |||||
| dFLC, mg/dL | 17.7 (7.9-64.6) (n = 203) | 1.9 (0.6-7.0) (n = 223) | 9.9 (3.5-21.1) (n = 224) | 42 (22-75) (n = 200) | 262 (132-1007) (n = 219) |
| NT pro-BNP, pg/mL | 435 (138-1675) (n = 175) | 248 (84-836) (n = 199) | 545 (167-2047) (n = 178) | 111 (52-258) (n = 151) | 166 (106-291) (n = 171) |
| Troponin T, ng/mL | 0.01 (<0.01-0.02) (n = 194) | 0.01 (<0.01-0.01) (n = 215) | 0.01 (<0.01-0.03) (n = 191) | 100 (100-100) (n = 173) | 100 (100-140) (n = 187) |
| Creatinine, mg/dL | 1 (0.9-1.2) (n = 235) | 0.7 (0.6-0.9) (n = 233) | 1.1 (0.9-1.4) (n = 227) | 111 (100-130) (n = 227) | 150 (129-172) (n = 256) |
| Proteinuria, mg/d | 2736 (262-7172) (n = 235) | 665 (62-3171) (n = 233) | 2300 (148-6179) (n = 225) | 87 (47-156) (n = 225) | 183 (119-314) (n = 225) |
| Alkaline phosphatase, U/L | 96 (71-153) (n = 234) | 69 (55-91) (n = 232) | 88 (66-128) (n = 222) | 98 (69-125) (n = 221) | 127 (107-159) (n = 219) |
| BMPC (%) | 10 (6-17) (n = 235) | 3 (1-5) (n = 224) | 5 (3-10) (n = 97) | 53 (28-99) (n = 97) | 120 (100-313) (n = 91) |
| Patients with cardiac involvement (n = 114) | |||||
| NT pro-BNP, pg/mL | 1544 (887-3214) (n = 95) | 735 (305-1976) (n = 99) | 1585 (510-3965) (n = 92) | 103 (37-215) (n = 84) | 160 (103-287) (n = 88) |
| Troponin T, ng/mL | 0.02 (0.01-0.04) (n = 103) | 0.01 (0.01-0.02) (n = 108) | 0.02 (0.01-0.05) (n = 96) | 100 (74-163) (n = 92) | 100 (100-200) (n = 93) |
| Patients with renal involvement (n = 161) | |||||
| Creatinine, mg/dL | 1 (0.9-1.2) (n = 161) | 0.8 (0.6-1) (n = 160) | 1.2 (0.9-1.6) (n = 157) | 114 (100-138) (n = 157) | 150 (129-177) (n = 156) |
| Proteinuria, mg/d | 5430 (2554-8721) (n = 161) | 1951 (484-4779) (n = 160) | 4616 (2017-7438) (n = 156) | 83 (43-144) (n = 156) | 181 (120-292) (n = 156) |
| Patients with liver involvement (n = 46) | |||||
| Alkaline phosphatase, U/L | 311 (206-659) (n = 46) | 118 (78-200) (n = 45) | 160 (92-463) (n = 43) | 48 (27-82) (n = 43) | 130 (101-194) (n = 42) |
| Laboratory markers . | At diagnosis . | Posttransplant nadir . | At second-line therapy initiation . | ||
|---|---|---|---|---|---|
| Restarting therapy . | % of diagnosis . | % over nadir . | |||
| All patients (n = 235) | |||||
| dFLC, mg/dL | 17.7 (7.9-64.6) (n = 203) | 1.9 (0.6-7.0) (n = 223) | 9.9 (3.5-21.1) (n = 224) | 42 (22-75) (n = 200) | 262 (132-1007) (n = 219) |
| NT pro-BNP, pg/mL | 435 (138-1675) (n = 175) | 248 (84-836) (n = 199) | 545 (167-2047) (n = 178) | 111 (52-258) (n = 151) | 166 (106-291) (n = 171) |
| Troponin T, ng/mL | 0.01 (<0.01-0.02) (n = 194) | 0.01 (<0.01-0.01) (n = 215) | 0.01 (<0.01-0.03) (n = 191) | 100 (100-100) (n = 173) | 100 (100-140) (n = 187) |
| Creatinine, mg/dL | 1 (0.9-1.2) (n = 235) | 0.7 (0.6-0.9) (n = 233) | 1.1 (0.9-1.4) (n = 227) | 111 (100-130) (n = 227) | 150 (129-172) (n = 256) |
| Proteinuria, mg/d | 2736 (262-7172) (n = 235) | 665 (62-3171) (n = 233) | 2300 (148-6179) (n = 225) | 87 (47-156) (n = 225) | 183 (119-314) (n = 225) |
| Alkaline phosphatase, U/L | 96 (71-153) (n = 234) | 69 (55-91) (n = 232) | 88 (66-128) (n = 222) | 98 (69-125) (n = 221) | 127 (107-159) (n = 219) |
| BMPC (%) | 10 (6-17) (n = 235) | 3 (1-5) (n = 224) | 5 (3-10) (n = 97) | 53 (28-99) (n = 97) | 120 (100-313) (n = 91) |
| Patients with cardiac involvement (n = 114) | |||||
| NT pro-BNP, pg/mL | 1544 (887-3214) (n = 95) | 735 (305-1976) (n = 99) | 1585 (510-3965) (n = 92) | 103 (37-215) (n = 84) | 160 (103-287) (n = 88) |
| Troponin T, ng/mL | 0.02 (0.01-0.04) (n = 103) | 0.01 (0.01-0.02) (n = 108) | 0.02 (0.01-0.05) (n = 96) | 100 (74-163) (n = 92) | 100 (100-200) (n = 93) |
| Patients with renal involvement (n = 161) | |||||
| Creatinine, mg/dL | 1 (0.9-1.2) (n = 161) | 0.8 (0.6-1) (n = 160) | 1.2 (0.9-1.6) (n = 157) | 114 (100-138) (n = 157) | 150 (129-177) (n = 156) |
| Proteinuria, mg/d | 5430 (2554-8721) (n = 161) | 1951 (484-4779) (n = 160) | 4616 (2017-7438) (n = 156) | 83 (43-144) (n = 156) | 181 (120-292) (n = 156) |
| Patients with liver involvement (n = 46) | |||||
| Alkaline phosphatase, U/L | 311 (206-659) (n = 46) | 118 (78-200) (n = 45) | 160 (92-463) (n = 43) | 48 (27-82) (n = 43) | 130 (101-194) (n = 42) |
All values are reported as median (IQR).
BMPC, bone marrow plasma cells.
Figure 1 illustrates the relationships between VGPR status post-ASCT, dFLC, and R/P patterns at second-line treatment. Those patients who did not achieve VGPR post-ASCT were more likely to have absolute values of dFLC that were higher at baseline, at nadir post-ASCT, and at the initiation of second-line therapy (Figure 1A-B). For these non-VGPR patients, at initiation of second-line therapy, patients’ dFLC relative to diagnosis dFLC ranged between 39% and 72%, with the lowest percentages for groups 1 and 2, intermediate for group 3, and the highest percentages for groups 4 and 5 (Figure 1C). In contrast, the patients who achieved VGPR after ASCT had the lowest absolute values of dFLC at baseline, at nadir, and prior to initiating second-line therapy (Figure 1B); therapy was typically started in these groups when the dFLC was <30% of the diagnosis value (Figure 1D) and 180% to 2100% of nadir.
Relationship between free light chain, response status, and indication for initiating second-line therapy. (A) For patients achieving VGPR or better: median absolute values of free light chain at diagnosis, nadir, and initiation of second-line therapy. (B) For patients not achieving VGPR: median absolute values of free light chain at diagnosis, nadir, and initiation of second-line therapy. (C) For patients achieving VGPR or better: median percent chain of free light chain between initiation of therapy and nadir and initiation of second-line therapy. (D) For patients not achieving VGPR: median percent chain of free light chain between initiation of therapy and nadir and initiation of second-line therapy.
Relationship between free light chain, response status, and indication for initiating second-line therapy. (A) For patients achieving VGPR or better: median absolute values of free light chain at diagnosis, nadir, and initiation of second-line therapy. (B) For patients not achieving VGPR: median absolute values of free light chain at diagnosis, nadir, and initiation of second-line therapy. (C) For patients achieving VGPR or better: median percent chain of free light chain between initiation of therapy and nadir and initiation of second-line therapy. (D) For patients not achieving VGPR: median percent chain of free light chain between initiation of therapy and nadir and initiation of second-line therapy.
In an effort to determine the timing between the earliest signs of hematologic R/P and organ R/P, we analyzed the subset of 29 patients who did not initiate therapy until there was documented organ R/P, but in whom there was documentation of “subtle” hematologic R/P at least 1 month prior to the organ progression. Overall, the median time from “subtle” hematologic R/P to organ R/P was 13.7 months with 25% organ R/P at 5 months. Moreover, those patients who achieved at least a hematologic VGPR posttransplant had longer times to develop organ progression after the first appearance of either subtle (24.1 vs 5.9 months, P = .02) or conventional (24.2 vs 3.2 months, P = .007) hematologic R/P. Timing between hematologic R/P and organ R/P of all group 3 and 4 patients is shown in supplemental Figure 2.
OS outcomes
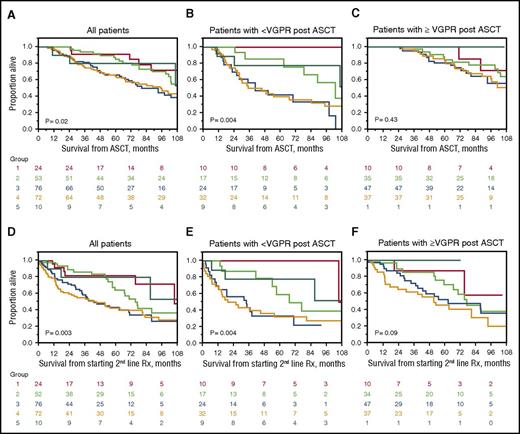
At the time of analysis, 115 patients had died. The median follow-up for surviving patients was 92.4 months (95% confidence interval [CI], 72.8-102.8) from ASCT, and 55.3 months (95% CI, 42.7-62.4) from the time of starting a second-line therapy. Median survival from ASCT (Figure 2A-C) was 102.4 months (95% CI, 92.9-120.8) and from initiation of second-line therapy was 66.8 months (95% CI, 53.0-80.5) (Figure 2D-E). As shown in Figure 2A, survival from diagnosis was worse in those patients with organ relapse at time of starting second-line therapy. The same pattern was seen when survival was calculated from instituting second-line therapy (Figure 2D).
Survival from diagnosis based on indication for starting second-line therapy. Group 1 (red), early signs of recurrent disease not yet meeting hematologic or organ R/P criteria; group 2 (green), hematologic R/P without signs of organ R/P; group 3 (blue), organ R/P without hematologic R/P; group 4 (orange), both hematologic and organ R/P; and group 5 (turquoise), suboptimal response to ASCT. (A) All patients, OS from ASCT. Five-year OS was 91%, 85%, 66%, 64%, and 80% for groups 1, 2,3, 4, and 5, respectively; individual cross-group comparisons were significant for groups 3 and 1 (P = .03), 3 and 2 (P = .008), 4 and 1 (P = .04), and 4 and 2 (P = .03). (B) Patients not achieving a VGPR or better to ASCT, OS from ASCT. Five-year OS was 100%, 86%, 42%, 40%, and 78% for groups 1 to 5, respectively; individual cross-group comparisons were significant for groups 3 and 1 (P = .0006), 3 and 2 (P = .008), 3 and 5 (P = .02), and 4 and 1 (P = .006). (C) Patients achieving a VGPR or better to ASCT, OS from ASCT. Five-year OS was 100%, 85%, 81%, and 80% for groups 1 to 4, respectively; there were no individual differences between groups. (D) All patients, OS from starting second-line therapy. Five-year OS was 82%, 64%, 43%, 42%, and 80% for groups 1 to 5, respectively; individual cross-group comparisons were significant for groups 3 and 1 (P = .009), 3 and 2 (P = .02), 4 and 1 (P = .005), and 4 and 2 (P = .01). (E) Patients not achieving a VGPR or better ASCT, OS from starting second-line therapy. Five-year OS was 100%, 59%, 33%, 32%, and 78% for groups 1 to 5, respectively; individual cross-group comparisons were significant for groups 2 and 1 (P = .06), 3 and 1 (P = .001), 3 and 2 (P = .03), and 3 and 5 (P = .05). (F) Patients achieving a VGPR or better to ASCT, OS from starting second-line therapy. Five-year OS was 88%, 71%, 53%, and 46% for groups 1 to 4, respectively; individual cross-group comparisons were significant between groups 4 and 2 (P = .04).
Survival from diagnosis based on indication for starting second-line therapy. Group 1 (red), early signs of recurrent disease not yet meeting hematologic or organ R/P criteria; group 2 (green), hematologic R/P without signs of organ R/P; group 3 (blue), organ R/P without hematologic R/P; group 4 (orange), both hematologic and organ R/P; and group 5 (turquoise), suboptimal response to ASCT. (A) All patients, OS from ASCT. Five-year OS was 91%, 85%, 66%, 64%, and 80% for groups 1, 2,3, 4, and 5, respectively; individual cross-group comparisons were significant for groups 3 and 1 (P = .03), 3 and 2 (P = .008), 4 and 1 (P = .04), and 4 and 2 (P = .03). (B) Patients not achieving a VGPR or better to ASCT, OS from ASCT. Five-year OS was 100%, 86%, 42%, 40%, and 78% for groups 1 to 5, respectively; individual cross-group comparisons were significant for groups 3 and 1 (P = .0006), 3 and 2 (P = .008), 3 and 5 (P = .02), and 4 and 1 (P = .006). (C) Patients achieving a VGPR or better to ASCT, OS from ASCT. Five-year OS was 100%, 85%, 81%, and 80% for groups 1 to 4, respectively; there were no individual differences between groups. (D) All patients, OS from starting second-line therapy. Five-year OS was 82%, 64%, 43%, 42%, and 80% for groups 1 to 5, respectively; individual cross-group comparisons were significant for groups 3 and 1 (P = .009), 3 and 2 (P = .02), 4 and 1 (P = .005), and 4 and 2 (P = .01). (E) Patients not achieving a VGPR or better ASCT, OS from starting second-line therapy. Five-year OS was 100%, 59%, 33%, 32%, and 78% for groups 1 to 5, respectively; individual cross-group comparisons were significant for groups 2 and 1 (P = .06), 3 and 1 (P = .001), 3 and 2 (P = .03), and 3 and 5 (P = .05). (F) Patients achieving a VGPR or better to ASCT, OS from starting second-line therapy. Five-year OS was 88%, 71%, 53%, and 46% for groups 1 to 4, respectively; individual cross-group comparisons were significant between groups 4 and 2 (P = .04).
Parsing patients by response, the 5-year OS from ASCT for the 92 patients who had achieved less than a hematologic VGPR post-ASCT followed a similar pattern (Figure 2B), but among the 130 patients who had achieved a VGPR or better post-ASCT, there was no significant OS difference among the groups (Figure 2C). Identical patterns were observed when OS was calculated from time of initiation of second-line therapy (Figures 2E-F), once again with the biggest differences seen those not achieving a VGPR to ASCT.
Univariate analysis identified the following adverse risk factors for OS from ASCT: relapse status upon initiating second-line therapy; advanced age at diagnosis; advanced stage by Mayo 2004 and 2012 staging systems; involvement of >2 organs at diagnosis; lack of VGPR after ASCT; lack of CR post-ASCT; absence of novel agents in second-line regimen; second-line therapy initiated before 2009; and dFLC ≥5 mg/dL at time of restarting therapy. The multivariable analysis calculating OS from ASCT (Table 4; multivariate 1) demonstrated that relapse status at second-line therapy initiation (specifically organ R/P), post-ASCT CR, dFLC ≥5 m/dL at second-line therapy, the use of novel agents as salvage, and >2 organs involved at baseline were associated with a higher risk of death.
Multivariate models predicting death given the direction of the response rates (RR)
| Prognostic factors . | Multivariate 1 (OS from ASCT) . | Multivariate 2 (OS from second-line therapy) . | Multivariate 3 (OS from second-line therapy) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) . | . | P . | RR (95% CI) . | . | P . | RR (95% CI) . | . | P . | |
| Age at second-line therapy | Not significant | 1.03 (1.00, 1.05) | .03 | 1.03 (1.01, 1.06) | .02 | ||||
| Post-ASCT CR | <.001 | 0.51 (0.28, 0.88) | .01 | Not included | |||||
| dFLC ≥5 mg/dL at second-line therapy initiation | 2.81 (1.56, 5.16) | <.001 | Not included | 2.83 (1.59, 5.13) | <.001 | ||||
| Novel agents at second line | 0.54 (0.36, 0.81) | .003 | 0.57 (0.39, 0.83) | .004 | 0.60 (0.41, 0.91) | .02 | |||
| Organ >2 at diagnosis | 2.33 (1.34, 3.86) | .004 | Not included | Not included | |||||
| Relapse status at second-line therapy initiation | <.001 | <.001 | <.001 | ||||||
| Group 2 (hematologic only) | 1.0 (Reference) | – | 1.0 (Reference) | – | 1.0 (Reference) | – | |||
| Group 1 (no R/P) | 0.47 (0.1, 1.27) | .14 | 0.54 (0.20, 1.26) | .16 | 0.49 (0.14, 1.30) | .16 | |||
| Group 3 (organ only) | 2.99 (1.56, 5.89) | .001 | 1.69 (0.99, 2.97) | .05 | 3.24 (1.70, 6.24) | <.001 | |||
| Group 4 (hematologic and organ) | 1.55 (0.92, 2.70) | .1 | 1.92 (1.14, 3.35) | .01 | 1.93 (1.15, 3.39) | <.001 | |||
| Group 5 (consolidation) | 0.89 (0.21, 2.63) | .85 | 0.65 (0.15, 1.92) | .47 | 0.78 (0.18, 2.30) | .69 | |||
| Prognostic factors . | Multivariate 1 (OS from ASCT) . | Multivariate 2 (OS from second-line therapy) . | Multivariate 3 (OS from second-line therapy) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) . | . | P . | RR (95% CI) . | . | P . | RR (95% CI) . | . | P . | |
| Age at second-line therapy | Not significant | 1.03 (1.00, 1.05) | .03 | 1.03 (1.01, 1.06) | .02 | ||||
| Post-ASCT CR | <.001 | 0.51 (0.28, 0.88) | .01 | Not included | |||||
| dFLC ≥5 mg/dL at second-line therapy initiation | 2.81 (1.56, 5.16) | <.001 | Not included | 2.83 (1.59, 5.13) | <.001 | ||||
| Novel agents at second line | 0.54 (0.36, 0.81) | .003 | 0.57 (0.39, 0.83) | .004 | 0.60 (0.41, 0.91) | .02 | |||
| Organ >2 at diagnosis | 2.33 (1.34, 3.86) | .004 | Not included | Not included | |||||
| Relapse status at second-line therapy initiation | <.001 | <.001 | <.001 | ||||||
| Group 2 (hematologic only) | 1.0 (Reference) | – | 1.0 (Reference) | – | 1.0 (Reference) | – | |||
| Group 1 (no R/P) | 0.47 (0.1, 1.27) | .14 | 0.54 (0.20, 1.26) | .16 | 0.49 (0.14, 1.30) | .16 | |||
| Group 3 (organ only) | 2.99 (1.56, 5.89) | .001 | 1.69 (0.99, 2.97) | .05 | 3.24 (1.70, 6.24) | <.001 | |||
| Group 4 (hematologic and organ) | 1.55 (0.92, 2.70) | .1 | 1.92 (1.14, 3.35) | .01 | 1.93 (1.15, 3.39) | <.001 | |||
| Group 5 (consolidation) | 0.89 (0.21, 2.63) | .85 | 0.65 (0.15, 1.92) | .47 | 0.78 (0.18, 2.30) | .69 | |||
“Not significant” implies significance on univariate, but driven from model on stepwise multivariate build. “Not included” refers to variables that were not included because not significant on univariate.
When the univariate and multivariate analyses were performed calculating survival from the time of initiating second-line therapy, the findings were similar, but the presence of >2 organs involved by AL amyloidosis was no longer a risk factor, whereas age was. Two models were constructed, one including dFLC at initiation of therapy and the other including CR post-ASCT, because there were strong interactions between these 2 variables. In both models, the presence of organ involvement at the time of starting second-line therapy was a significant risk factor for death.
Discussion
This study investigates the patterns of therapy initiation after R/P following up-front ASCT in patients with AL amyloidosis at our institution. Prior work would suggest that there are not accepted conventions for deciding when to resume therapy among the amyloid community,9 which makes clinical trial design for patients with R/P disease challenging.
There were several striking observations made in the present study. First, 63% of our patients had signs of organ R/P at the time of second-line therapy, with a little more than half of these without documented hematologic R/P using standard progression criteria. Second, only 54% met consensus criteria for hematologic R/P, more than half of whom also had documented evidence of organ progression. Third, the most prominent differences in OS based on R/P status at second-line therapy were seen in patients who did not achieve at least a VGPR post-ASCT; very little difference was seen in those patients who did achieve at least a VGPR. Fourth, patients with “subtle” (or even consensus criteria) hematologic R/P from VGPR had a median of 2 years before evidence of organ R/P, in contrast to those patients who achieved less than a VGPR post-ASCT and who had 3 to 6 months between organ R/P and either consensus criteria or “subtle” hematologic R/P. Fifth, organ progression at the time of instituting second-line therapy was a significant risk factor for death, independent of post-ASCT response, dFLC >5 mg/dL (ie, measureable disease as defined by trial eligibility criteria), age, and the use of novel agents.
Although there are several studies that address outcomes and treatment following relapse,8,14 only 3 studies have addressed practice patterns, all of which have only been presented in abstract form. One is a survey that demonstrates the lack of consensus on when is the “right time” to reinstitute therapy9,15 ; another shares anecdotal cases of the perils of waiting for dFLC ≥5 mg/dL for trial eligibility15 ; and the last enumerates the R/P status at institution of second-line therapy.16 The patterns of disease status at the time of starting second-line therapy described by the Pavia group are comparable to ours: median dFLC of 41% of baseline value (ours, 42%); 48% of patients had dFLC below 5 mg/dL (ours, 32%); and 76% had organ progression (ours 63%).16
The present study is unique in that it carefully delineates outcomes based on R/P status prior to institution of second-line therapy. Although it has limitations, it offers many lessons. Its 3 greatest limitations are that (1) it spans nearly 20 years resulting in different treatment patterns and access to drugs. The long time span particularly influences the goal of frontline therapy resulting from the new criteria of treatment response, updated laboratory diagnostic techniques, and availability of second-line therapy. (2) The data are retrospectively captured without a standardized testing interval. (3) Some of the subgroups used to draw conclusions are small.
It was not until the middle of the first decade of the 21st century that drugs other than alkylators and corticosteroids were available to treat AL amyloidosis. This lack of availability of options along with the fragility of many of these patients resulted in a de facto conservatism in therapeutic strategies. With the advent of the immunoglobulin free light chain assay as a measurement tool of precursor protein around the same time and the acceptance of a definition of VGPR in 2012,11 goals of therapy have morphed to a more aggressive approach.17 Attaining VGPR or better is widely accepted as the goal of therapy in current practice.13 A significant proportion of patients in this study were treated before the new criteria of hematologic response were proposed, and frontline therapy was discontinued even with lack of PR or VGPR following ASCT. Patients who were noted to have deterioration in their organ function may not have had actual amyloid progression, but rather the organ deterioration might have been the natural progressive deterioration that occurs after injury. Despite the limitations of this study, conclusions can be drawn and recommendations can be made based on the findings of this study. First, patients who are showing signs of hematologic R/P from at least a VGPR may be able to tolerate a gradual rise in dFLC, assuming they did not have a dFLC of 5 mg/dL at diagnosis. Waiting for a dFLC of 5 mg/dL (as long as it remains under 30% of the diagnostic level) appears safe in patients who had previously achieved VGPR or better. Second, consolidation post-ASCT appears to be a viable strategy and can be recommended for those not achieving a VGPR, and this population may also be a good population in whom novel anti–plasma cell therapies can be tested in clinical trials. Third, a less restrictive definition of R/P could be considered, which could potentially detect 10% to 20% of patients sooner. It is likely that the organ progression that is seen without hematologic progression is as much a function of the deleterious effect of existing circulating amyloidogenic light chains as it is a function of the current tools we have to measure disease status being suboptimal. Nearly a quarter of the organ R/P patients had a baseline dFLC <5 mg/dL at diagnosis, and nearly two-thirds of these same patients had a dFLC <5 mg/dL at a time when they had documented organ R/P. A more sensitive tool than the serum free light chain assay is required in order to improve our ability to detect (and measure) early hematologic R/P before there is organ R/P. It may well be that mass spectrometry of blood may serve that function in the future.18
In summary, these data would suggest that the best candidates to direct to clinical trials testing novel plasma cell–directed chemotherapy beyond first line may be those who either are relapsing from VGPR or better or are having inadequate response to prior therapy. This approach should allow for hematologic response assessment while avoiding the risk of deleterious organ progression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Predolin Foundation, the JABBS Foundation, and the Robert A. Kyle Hematology Malignancies Fund.
Authorship
Contribution: Y.L.H. and A.D. were responsible for study conception and design; all authors were responsible for provision of study materials of patients; Y.L.H. and A.D. performed data analysis and interpretation; and all authors wrote the manuscript and had final approval of the manuscript.
Conflict-of-interest disclosure: The following authors received research support and/or honoraria: A.D., from Celgene, Takada, Pfizer, Jannsen, and Alnylam; M.A.G., from Celgene, Takeda, Onyx, Novartis, and Smith Kline; S.K.K., from Celgene, Takeda, Onyx, AbbVie, Janssen, Sanofi, Skyline, and Noxxon; and D.D., from Takeda, Karyopharm Therapeutics, and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Angela Dispenzieri, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: dispenzieri.angela@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal