Key Points
STIM1 and STIM2 cooperatively regulate neutrophil SOCE.
The interaction of oxidative stress and calcium signaling pathways imparts a specific role for STIM2 in neutrophil cytokine synthesis.
Abstract
Neutrophils are key effector cells of the innate immune system. Calcium-dependent signaling pathways initiated by store-operated calcium entry (SOCE) are known to regulate neutrophil activation; however, the precise mechanism of this process remains unclear. STIM1 and STIM2 are calcium-sensing molecules that link calcium depletion of the endoplasmic reticulum with opening of plasma membrane calcium channels. Although a role for STIM1 in neutrophil SOCE and activation has been established, the function of STIM2 is unknown. Here we use mice with conditional ablation of Stim1 and/or Stim2 to investigate the role of STIM2 in neutrophil activation. We demonstrate that loss of STIM2 results in decreased SOCE, particularly at lower doses of agonists. Reactive oxygen species (ROS) production, degranulation, and phagocytosis are normal in the absence of STIM2, suggesting STIM1 is the dominant calcium sensor required for classical short-term neutrophil responses. However, neutrophil cytokine production required STIM2, but not STIM1, at least in part as a result of redox regulation of cytokine gene expression. In vivo loss of STIM2 results in lower cytokine levels and protection from mortality in a mouse model of systemic inflammatory response syndrome. These data, combined with previous studies focusing on STIM1, define distinct but cooperative functions for STIM1 and STIM2 in modulating neutrophil bactericidal and cytokine responses.
Introduction
Neutrophils combat invading microorganisms through phagocytosis and release of antiseptic agents; however, activated neutrophils can also injure bystander tissues, and thus must be tightly regulated.1 The neutrophil response is finely tuned via signals transmitted by cell surface receptors. Calcium has been described as a modulator of neutrophil activation for more than 50 years2,3 ; however, the mechanisms by which calcium signals are initiated and transmitted into downstream responses are not clear. In immune cells, the primary mechanism of calcium entry into the cytosol is through the process of store-operated calcium entry (SOCE). Signaling complexes assemble in response to receptor ligation and activate phospholipases that cleave phosphatidylinositol 4,5 bisphosphate into diacylglycerol and inositol triphosphate (IP3). IP3 stimulates release of calcium from intracellular stores via the endoplasmic reticulum (ER) IP3 receptor, resulting in a transient increase in cytosolic calcium. Calcium sensors STIM1 and STIM2 are transmembrane proteins located in the ER membrane that detect the fall in ER calcium. They undergo conformational change to bind and open plasma membrane calcium-release-activated calcium channels comprised of ORAI channel proteins, allowing calcium influx from the extracellular space.4-8
STIM1 and STIM2 are structurally similar molecules consisting of an N-terminal EF hand that binds calcium in the ER lumen, a sterile α motif that mediates STIM oligomerization, and C-terminal cytosolic regions that regulate calcium-release activated calcium channel activation.9 However, there are several biochemical differences between STIM1 and STIM2 that affect function. STIM2 has a lower affinity for calcium, resulting in calcium dissociation and activation, with smaller decreases in ER calcium concentration. This feature has been proposed to render STIM2 more sensitive to weak stimuli compared with STIM1.10-12 In addition, slower activation kinetics alters the gating characteristics of STIM2, such that channel opening by STIM2 is less potent than STIM1.10,13,14 STIM2 has also been proposed to regulate basal calcium levels.15 STIM1 and STIM2 are broadly expressed in human and mouse tissues, and studies indicate STIM1 plays a dominant role in SOCE in most cells.16 In immune cells, ample evidence supports that SOCE is primarily mediated by STIM1.17-21 Children with STIM1 mutations manifest a SCID-like immunodeficiency, highlighting the importance of STIM1 in the immune system.22 STIM2 appears to play a lesser role in most immune cells, with typically mild defects in SOCE in cells lacking STIM2.18,19
In neutrophils, calcium signaling underlies several functional processes, including migration, degranulation, reactive oxygen species (ROS) production, and phagocytosis; however, little is known about the role of STIM1 or STIM2 in these cells. In mice, SOCE is diminished in STIM1-deficient neutrophils, resulting in impaired phagocytosis, degranulation, and ROS production.23,24 These defects translate into defective bacterial clearance in vivo, but also protection from neutrophil-mediated tissue damage in ischemia-reperfusion injury and inflammatory psoriatic lesions.23,25 The role of STIM1 in human neutrophils is less clear. Early studies using siRNA knockdown in cell lines suggested STIM1 is important for SOCE.2,26 However, a recent study using primary cells from patients with mutations in STIM1 found only mildly impaired SOCE.27 Together, the residual SOCE and functional responses in both mouse and human neutrophils suggest STIM2 might contribute to SOCE and calcium-mediated neutrophil activation.
Classically, neutrophils are described as “professional phagocytes,” whose primary role is killing invading microorganisms. However, current research demonstrates that neutrophils synthesize a variety of cytokines that contribute to modulation of the immune response.1,28 Several receptor families mediate cytokine production in neutrophils, including Toll-like receptors (TLRs), C-type lectin receptors, Fc-receptors, and integrins. Although neutrophils have been shown to activate MAP kinases and NF-κB downstream of these receptors,29-32 the signaling mechanisms regulating cytokine production in neutrophils are largely unknown, or are indirectly inferred from studies in other lineages. The role of calcium in activation of transcription factors in neutrophils is unknown. Although TLRs do not induce SOCE, C-type lectin receptors, including the fungal receptor Dectin-1, and Fc-receptors, have signaling pathways analogous to the T- and B-cell receptors and induce SOCE, suggesting cytokine secretion through these pathways might be calcium-dependent.
Using mice with conditional deletion of Stim1 and/or Stim2, we report that STIM1 and STIM2 cooperatively regulate neutrophil SOCE. ROS production, degranulation, and phagocytosis are minimally impaired in the absence of STIM2, suggesting STIM1 is the dominant calcium sensor required for neutrophil bactericidal functions. In contrast, STIM2-deficient, but not STIM1-deficient, neutrophils display a marked defect in cytokine production. In vivo, mice with neutrophil-specific deletion of Stim2, but not Stim1, are protected in a model of systemic inflammatory response syndrome, suggesting a role for STIM2-dependent cytokine production in neutrophil-driven inflammatory disease. These results further define the cooperative regulation of SOCE by STIM1 and STIM2 and demonstrate a unique role for STIM2 in neutrophil cytokine production and inflammation.
Materials and methods
Mice
Stim1f/f and Stim2f/f mice were a gift from Monika Vig (Washington University in St. Louis) and have been previously described.18 Single- and double-mutant (Stim1/2f/f) mice were bred to mice bearing transgenes with Cre recombinase under control of either the Vav or human S100A8 (MRP8) promoters (Jackson Labs). All mice were backcrossed more than 8 generations on the C57BL6 background. For controls, we used predominantly littermate (flox/flox) mice. Neutrophils from these mice functioned equivalently to Vav-Cre and Mrp8-Cre mice. Mice were maintained in a specific pathogen-free barrier facility at University of California, San Francisco and used in accordance with approved Institutional Animal Care and Use Committee protocols.
Measurement of intracellular calcium levels
Bone marrow was isolated and red blood cells lysed with a hypotonic saline solution. A single-cell suspension (107 cells/mL) was resuspended in Hanks balanced salt solution (HBSS)/H and loaded with 3 μM Indo-1 AM and 2.5 mM probenecid (Molecular Probes) in the dark at room temperature for 40 minutes. Cells were washed and resuspended in cold HBSS/H (2 × 107/mL), and neutrophils were labeled with Ly-6G-PE (IA8; BD Pharmingen). Before analysis, cells were aliquoted into fluorescence-activated cell sorter (FACS) tubes containing HBSS/H with 0.5 mM MgCl2, warmed to 37°C for 1.5 minutes, and then run on the flow cytometer (LSRFortessa; BD) for 30 seconds to establish a baseline reading. Stimuli were added and the samples analyzed continuously in the presence of the indicated calcium concentrations. For FcγR cross-linking, cells were also incubated with 4 μg/mL anti-CD16/32 (2.4G2) at 4°C for 20 minutes. Data were analyzed using FlowJo software (TreeStar).
Intracellular cytokine staining
Bone marrow cells were resuspended in RPMI supplemented with 10% fetal bovine serum and Brefeldin A (eBioscience, 1:500) and stimulated for 6 hours. Cells were washed once with cold phosphate-buffered saline (PBS) and resuspended in FACS buffer (PBS with 2% fetal bovine serum and Brefeldin A). Cells were surface-stained with Fixable live/dead Aqua (Life Technologies), Ly6G, and CD11b. Cells were then fixed, permeabilized (eBioscience), and stained for tumor necrosis factor α (TNFα), interleukin 10 (IL-10), and interferon γ (IFN-γ). Samples were washed and analyzed by flow cytometry (LSRFortessa; BD).
Zymosan-induced peritonitis
Age- and sex-matched mice (8-12 weeks old) were injected intraperitoneally with 500 mg/kg zymosan (Sigma) in sterile PBS. For survival experiments, mice were checked every 6 hours, and moribund mice were euthanized. For cellular infiltrate and cytokine measurements, mice were sacrificed 6 hours after injection. The peritoneum was flushed with 5 mL PBS. Total cell count was measured using a Nucleocounter NC-200 (Chemometec, Allerad, Denmark). Neutrophils were identified by staining with Ly6G and CD11b and analyzed by flow cytometry. Peritoneal supernatants were collected and TNFα and IL6 concentrations measured by enzyme-linked immunosorbent assay (ELISA) (eBioscience, San Diego, CA).
Additional methods are described in supplemental Data, available on the Blood Web site.
Results
SOCE is not required for neutrophil development
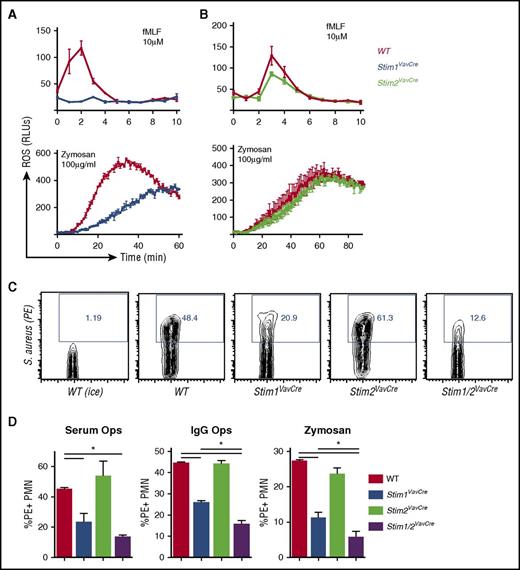
STIM1-deficient murine neutrophils display diminished SOCE and modestly impaired degranulation, phagocytosis, and ROS production.23,24 However, residual function suggests other proteins also contribute to SOCE in these cells. To explore whether STIM2 contributes to neutrophil SOCE, we first examined expression levels of Stim2 in wild-type (WT) cells. Stim2 expression reported in the Immgen database is significantly higher in bone marrow granulocytes compared with other immune cells. We analyzed Stim1 and Stim2 expression in bone marrow neutrophils by quantitative reverse transcription polymerase chain reaction (RT-qPCR) (primers previously verified to be 100% efficient ±5%), confirming Stim2 mRNA expression is up to 10-fold higher than Stim1 in resting neutrophils (Figure 1A). Both Stim1 and Stim2 mRNA decreased in stimulated neutrophils, suggesting downregulation of SOCE machinery may contribute to terminating neutrophil activation (Figure 1B). To investigate the role for STIM2 in neutrophil function, we took a genetic approach and generated mice with hematopoietic deletion of STIM molecules (Stim1VavCre, Stim2VavCre, Stim1/2VavCre). Neutrophils from these mice showed complete loss of the targeted STIM protein by western blot, and deletion of Stim1 or Stim2 did not alter levels of the remaining family member (Figure 1C). Previous work has demonstrated that neutrophil development does not require STIM1.23,25 Similarly, Stim1VavCre and Stim2VavCre neutrophils were present in normal number and percentages in bone marrow and peripheral blood (Figure 1D and not shown), with equivalent expression of surface markers (CD11b, CD16/32, and Ly6G) (Figure 1E-F). Furthermore, Stim1/2VavCre mice, in which SOCE is completely disrupted, exhibit normal neutrophil development, confirming granulopoiesis does not require SOCE (Figure 1D-F).
SOCE is not required for neutrophil development. (A-B) Expression of Stim1 and Stim2 determined by qPCR on cDNA from WT neutrophils either resting (A) or stimulated with zymosan (100 μg/mL) for 4 hours (B). (C) STIM protein levels in neutrophil lysates from Stim1f/f, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice blotted for STIM1, STIM2, or actin control. (Right) Quantitation of band intensities from 2 to 3 blots. (D-F) Analysis of neutrophil number and phenotype in bone marrow isolated from Stim1f/f, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice. Bar graphs represent mean ± standard error of the mean (SEM) compiled from 2 to 3 independent experiments. (D) Percentage of Ly6Ghi, CD11b+ neutrophils in bone marrow (n = 9-11). (E) Representative FACS plots from D. (F) Surface levels of CD16/32 and Ly6G (markers of mature neutrophils).
SOCE is not required for neutrophil development. (A-B) Expression of Stim1 and Stim2 determined by qPCR on cDNA from WT neutrophils either resting (A) or stimulated with zymosan (100 μg/mL) for 4 hours (B). (C) STIM protein levels in neutrophil lysates from Stim1f/f, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice blotted for STIM1, STIM2, or actin control. (Right) Quantitation of band intensities from 2 to 3 blots. (D-F) Analysis of neutrophil number and phenotype in bone marrow isolated from Stim1f/f, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice. Bar graphs represent mean ± standard error of the mean (SEM) compiled from 2 to 3 independent experiments. (D) Percentage of Ly6Ghi, CD11b+ neutrophils in bone marrow (n = 9-11). (E) Representative FACS plots from D. (F) Surface levels of CD16/32 and Ly6G (markers of mature neutrophils).
SOCE is modestly impaired in STIM2-deficient neutrophils
SOCE is initiated downstream of multiple receptor families in neutrophils including formyl peptide and chemokine receptors, Fcγ-receptors, and Dectin-1. To explore the role of STIM2 in neutrophil SOCE, we measured calcium influx in response to receptor agonists and pharmacological store depletion with ionomycin and thapsigargin. Cell stimulation in the absence of extracellular calcium demonstrated equivalent ER store release in WT, Stim1VavCre, and Stim2VavCre neutrophils (Figure 2A,C; supplemental Figure 1B,E). Readdition of calcium demonstrated 30% to 60% reduction in SOCE in the absence of STIM1.23 In comparison, Stim2VavCre neutrophils displayed rapid entry of calcium, but a modest reduction in sustained calcium entry that was most prominent at lower doses of agonists (Figure 2A,D; supplemental Figure 1B-F). The defect in STIM2-deficient cells was even more apparent in cells stimulated in media containing physiological concentrations of calcium (Figure 2E-F; supplemental Figure 1H). These results indicate that both STIM1 and STIM2 regulate SOCE in neutrophils; however, the effect of STIM2 deletion is less pronounced than STIM1. Deletion of both Stim1 and Stim2 completely abrogated calcium entry in response to all receptor agonists (Figure 2B-D; supplemental Figure 1C), confirming SOCE is completely dependent on STIM1 and STIM2 in neutrophils.
SOCE is selectively impaired at low doses of agonists in STIM2-deficient neutrophils. Analysis of SOCE in neutrophils from Stim1f/f, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice measured by flow cytometry. (A) Bone marrow cells loaded with Indo-1 and labeled for Ly6G were stimulated with the indicated doses of fMLF, ionomycin, or thapsigargin. Cells were stimulated first in calcium-free media to analyze ER Ca2+ store release, followed by readdition of extracellular calcium. (B) Analysis of SOCE in neutrophils from WT and Stim1/2VavCre mice stimulated with the indicated agonist. (C) Quantification of ER store release (area under the curve of cells stimulated in 0 mM Ca2+) and (D) SOCE (area under the curve of segment after addition of 1 mM Ca2+); mean ± SEM from 3 to 4 independent experiments. *P < .05. (E) Calcium flux in cells stimulated with the indicated agonists in media containing physiological calcium (1 mM). To synchronize zymosan particle uptake and calcium flux, cells were incubated with zymosan on ice in media containing 1 mM Ca2+ and then placed in a 37°C water bath during flow cytometry to induce stimulation. (F) Quantification of E, area under the curve of indicated time segment represented as mean ± SEM from 3 to 4 independent experiments.
SOCE is selectively impaired at low doses of agonists in STIM2-deficient neutrophils. Analysis of SOCE in neutrophils from Stim1f/f, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice measured by flow cytometry. (A) Bone marrow cells loaded with Indo-1 and labeled for Ly6G were stimulated with the indicated doses of fMLF, ionomycin, or thapsigargin. Cells were stimulated first in calcium-free media to analyze ER Ca2+ store release, followed by readdition of extracellular calcium. (B) Analysis of SOCE in neutrophils from WT and Stim1/2VavCre mice stimulated with the indicated agonist. (C) Quantification of ER store release (area under the curve of cells stimulated in 0 mM Ca2+) and (D) SOCE (area under the curve of segment after addition of 1 mM Ca2+); mean ± SEM from 3 to 4 independent experiments. *P < .05. (E) Calcium flux in cells stimulated with the indicated agonists in media containing physiological calcium (1 mM). To synchronize zymosan particle uptake and calcium flux, cells were incubated with zymosan on ice in media containing 1 mM Ca2+ and then placed in a 37°C water bath during flow cytometry to induce stimulation. (F) Quantification of E, area under the curve of indicated time segment represented as mean ± SEM from 3 to 4 independent experiments.
STIM2 is minimally required for neutrophil bactericidal responses
To determine the functional consequence of STIM2 deficiency, we first tested classical neutrophil bactericidal functions including ROS production, phagocytosis, and degranulation. Consistent with previous results, STIM1 was required for neutrophil ROS production induced through multiple agonists (Figure 3A). In contrast, ROS production in STIM2-deficient neutrophils was mildly decreased in response to fMLF, and normal after zymosan stimulation (Figure 3B). ROS production by STIM2-deficient cells in response to other stimuli including LTB4 and fibrinogen (integrin ligand) was equivalent to WT cells at all doses tested (not shown). Phagocytosis of labeled heat-killed Staphylococcus aureus or zymosan particles was decreased 50% in Stim1VavCre neutrophils (Figure 3C-D), similar to previous studies.23,24 In contrast, STIM2-deficient neutrophils showed normal uptake of both serum- and immunoglobulin G-opsonized particles (Figure 3C-D). Phagocytosis of serum-opsonized S aureus by Stim1/2VavCre neutrophils was equivalent to Stim1VavCre neutrophils; however, uptake of immunoglobulin G-coated particles and zymosan was further diminished in neutrophils lacking both STIM1 and 2 (Figure 3D). These data suggest that although phagocytosis is primarily mediated by STIM1, internalization of particles through Fc receptors and Dectin-1 may cooperatively use STIM1 and STIM2.
STIM2 is minimally required for neutrophil bactericidal responses. (A-C) Analysis of ROS production in WT, Stim1VavCre (A), and Stim2VavCre neutrophils (B) stimulated with formyl-methionine-leucine-phenylalanine (fMLF) or zymosan. (C-D) Analysis of phagocytosis by WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre neutrophils. Neutrophils were incubated for 30 minutes with pHrodo Red-labeled S aureus opsonized with serum or anti-S aureus immunoglobulin G or pHrodo Red zymosan particles. Internalized particles were quantified by percentage of phycoerythrin-stained cells, compared with samples on ice (D). A representative FACS plot is shown in (C). Mean ± SEM compiled from 2 to 4 independent experiments. *P < .05.
STIM2 is minimally required for neutrophil bactericidal responses. (A-C) Analysis of ROS production in WT, Stim1VavCre (A), and Stim2VavCre neutrophils (B) stimulated with formyl-methionine-leucine-phenylalanine (fMLF) or zymosan. (C-D) Analysis of phagocytosis by WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre neutrophils. Neutrophils were incubated for 30 minutes with pHrodo Red-labeled S aureus opsonized with serum or anti-S aureus immunoglobulin G or pHrodo Red zymosan particles. Internalized particles were quantified by percentage of phycoerythrin-stained cells, compared with samples on ice (D). A representative FACS plot is shown in (C). Mean ± SEM compiled from 2 to 4 independent experiments. *P < .05.
Previous studies have suggested that migration and degranulation are calcium-dependent. Although degranulation of mast cells, NK cells, and cytotoxic T cells requires STIM1, STIM1-deficient neutrophils have only a mild degranulation impairment.20,23,33 Similarly, STIM1-deficient neutrophils migrate normally in transwell assays and in vivo.23,34 Therefore, we posited that STIM2 might play a role in neutrophil degranulation and/or migration. However, loss of STIM2 did not affect lactoferrin release or CD11b upregulation (secretory granules), and Stim1/2VavCre neutrophils showed no additional defect compared with Stim1VavCre neutrophils (supplemental Figure 2A-C). Neutrophil migration in response to chemoattractants fMLF or macrophage inflammatory protein-2 was intact in STIM2 and STIM1/2-deficient neutrophils (supplemental Figure 2D). In addition, migration in vivo into air pouches treated with TNFα as an inflammatory stimulus revealed no significant differences in any STIM-deficient animals (supplemental Figure 2E). Together, these data indicate STIM2 is not required for neutrophil degranulation, and calcium-dependent cell migration likely occurs though SOCE-independent pathways.
Neutrophil cytokine synthesis requires STIM2
Neutrophils are a significant source of chemokines and cytokines that can direct the nascent immune response.28 Zymosan induces cytokine production in neutrophils through ligation of both TLR2 and Dectin-1.35 Although TLR2 signaling is calcium-independent, Dectin-1 initiates a signaling cascade that activates PLCγ2 and SOCE.36,37 WT neutrophils stimulated with zymosan produced an abundant amount of TNFα that was inhibited by calcium chelation with 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) (supplemental Figure 3A), suggesting cytokine production requires calcium signaling in neutrophils. Surprisingly, despite the requirement for STIM1 for other responses, production of TNFα, IL-10, and IFN-γ by STIM1-deficient neutrophils was nearly equivalent to WT (Figure 4A). In contrast, cytokine levels were significantly diminished at low and high doses of zymosan in Stim2VavCre and Stim1/2VavCre neutrophils, suggesting cytokine production specifically requires STIM2 (supplemental Figure 3B and Figure 4A). Stimulation with Pam3CSK4 induced TNFα, but not IL-10 or IFN-γ, production, and as predicted, neither STIM1 nor STIM2 was required for TLR2-mediated TNFα production (Figure 4B). These results suggest STIM2, but not STIM1, modulates zymosan-induced cytokine production via calcium signals initiated by Dectin-1 ligation. We also tested cytokine production in cells stimulated with insoluble immune complexes, which signal through Fcγ receptors. Immune complexes induced very little IL-10 or IFN-γ (not shown); however, TNFα was decreased in STIM2 and STIM1/2-deficient neutrophils (Figure 4C), suggesting STIM2-mediated SOCE is required for cytokine production downstream of multiple receptors. Baseline differences or altered survival were not responsible for these changes, as basal cytokine levels and neutrophil viability after stimulation were equivalent among WT and STIM-deficient neutrophils (supplemental Figure 1C-D).
Neutrophil cytokine synthesis requires STIM2. (A-C) Analysis of cytokine production in whole bone marrow cells (1 × 106) from WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice incubated with (A) zymosan (100 μg/ml), (B) PAM3CSK (1 μg/mL), or (C) insoluble immune complexes (10 μg/mL) in RPMI ([Ca2+] 0.7 mM) for 5 hours. Neutrophils were identified by double staining for Ly-6G and CD11b, and cytokine production was measured by intracellular staining for TNFα, IL-10, and IFN-γ. (A) FACS plots (left) are representative of 3 independent experiments, and bar graphs (right) represent mean ± SEM compiled from multiple experiments. (D) Analysis of cytokine transcripts by RT-qPCR. Neutrophils were purified from WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice by negative selection yielding more than 95% pure neutrophils. Cells were stimulated with zymosan (100 μg/mL) for 4 hours, and RNA transcript levels of the indicated cytokines measured by RT-qPCR. Mean ± SEM compiled from 3 independent experiments. #P < .05.
Neutrophil cytokine synthesis requires STIM2. (A-C) Analysis of cytokine production in whole bone marrow cells (1 × 106) from WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice incubated with (A) zymosan (100 μg/ml), (B) PAM3CSK (1 μg/mL), or (C) insoluble immune complexes (10 μg/mL) in RPMI ([Ca2+] 0.7 mM) for 5 hours. Neutrophils were identified by double staining for Ly-6G and CD11b, and cytokine production was measured by intracellular staining for TNFα, IL-10, and IFN-γ. (A) FACS plots (left) are representative of 3 independent experiments, and bar graphs (right) represent mean ± SEM compiled from multiple experiments. (D) Analysis of cytokine transcripts by RT-qPCR. Neutrophils were purified from WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice by negative selection yielding more than 95% pure neutrophils. Cells were stimulated with zymosan (100 μg/mL) for 4 hours, and RNA transcript levels of the indicated cytokines measured by RT-qPCR. Mean ± SEM compiled from 3 independent experiments. #P < .05.
To investigate whether changes in cytokine levels occurred at the transcriptional level, we measured cytokine expression by RT-qPCR. Expression of multiple transcripts including Tnfα, Il10, Il6, and Cxcl1 was equivalent in STIM1-deficient cells, but significantly diminished in Stim2VavCre and Stim1/2VavCre neutrophils compared with WT (Figure 4D). In contrast, transcription of Tnfα induced by LPS was equivalent in WT and STIM-deficient neutrophils again, indicating that TLR-mediated cytokine synthesis is independent of SOCE (supplemental Figure 3E). Together, these results demonstrate that STIM2, but not STIM1, regulates the transcription of multiple inflammatory cytokines.
STIM2-mediated SOCE regulates activation of NF-κB
To explore the mechanism by which STIM2 regulates cytokine synthesis, we examined activation of transcription factor pathways in zymosan-stimulated neutrophils. NF-κB resides in the cytoplasm of resting neutrophils and translocates to the nucleus with activation (Figure 5A). Calcium chelation with BAPTA blocked NF-κB translocation, demonstrating that calcium is important for NF-κB activation in neutrophils. Similarly, IκB kinase (IKK) α/β, an upstream modulator of NF-κB, required calcium for phosphorylation (Figure 5B). Neutrophils primarily express 2 isoforms of NFAT: NFAT2 (NFATc1) and NFAT4 (NFATc3). Although both NFAT2 and NFAT4 were present in both cytoplasmic and nuclear fractions, we found no evidence for a change in distribution after stimulation (not shown), suggesting NFAT may not play a role in transcriptional responses in zymosan-activated neutrophils. Calmodulin-dependent kinase II (CAMKII) is proposed to regulate activation of multiple transcription factors,38,39 and in WT neutrophils, we observed calcium-dependent phosphorylation of CAMKII (Figure 5B). In contrast, phosphorylation of MAP kinases ERK1/2 and p38, which are activated downstream of diacylglycerol, was not calcium dependent (Figure 5B and not shown). In STIM-deficient cells, nuclear translocation of NF-κB and phosphorylation of IKKα/β was diminished in Stim2VavCre and Stim1/2VavCre, but not Stim1VavCre, neutrophils (Figure 5C). CAMKII activation was similarly decreased in Stim2VavCre and Stim1/2VavCre cells, whereas phosphorylation of ERK1/2 and p38 was equivalent to WT in all STIM-deficient cells (Figure 5C-D). We conclude that STIM2, but not STIM1, regulates calcium-dependent activation of NF-κB and CAMKII to support neutrophil cytokine synthesis.
STIM2-mediated SOCE regulates activation of NF-κB. (A-B) Analysis of calcium-dependent activation of NF-κB and upstream signaling pathways in WT neutrophils. Neutrophils were stimulated with zymosan (100 μg/mL) in the presence or absence of BAPTA-AM to chelate available calcium. Nuclear and cytoplasmic fractions were immunoblotted for the indicated proteins. (C) Analysis of NF-κB activation and upstream signaling pathways in WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre neutrophils stimulated with zymosan (100 μg/mL). Nuclear and cytoplasmic fractions were blotted for the indicated proteins. n.s., nonspecific band. Blots are representative of more than 3 independent experiments. (Right) Mean band intensities (normalized to WT) ± SEM compiled from 3 to 4 independent experiments. (D) Analysis of phospho-CAMKII in cytoplasmic fractions of WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre neutrophils stimulated with zymosan (100 μg/mL). (Right) Mean band intensities (normalized to WT) ± SEM compiled from 3 to 4 independent experiments. Cyto., cytoplasmic.
STIM2-mediated SOCE regulates activation of NF-κB. (A-B) Analysis of calcium-dependent activation of NF-κB and upstream signaling pathways in WT neutrophils. Neutrophils were stimulated with zymosan (100 μg/mL) in the presence or absence of BAPTA-AM to chelate available calcium. Nuclear and cytoplasmic fractions were immunoblotted for the indicated proteins. (C) Analysis of NF-κB activation and upstream signaling pathways in WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre neutrophils stimulated with zymosan (100 μg/mL). Nuclear and cytoplasmic fractions were blotted for the indicated proteins. n.s., nonspecific band. Blots are representative of more than 3 independent experiments. (Right) Mean band intensities (normalized to WT) ± SEM compiled from 3 to 4 independent experiments. (D) Analysis of phospho-CAMKII in cytoplasmic fractions of WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre neutrophils stimulated with zymosan (100 μg/mL). (Right) Mean band intensities (normalized to WT) ± SEM compiled from 3 to 4 independent experiments. Cyto., cytoplasmic.
ROS-mediated inhibition of cytokine production imparts a selective role for STIM2 in neutrophils
In other immune cells, STIM2 deficiency also impairs cytokine production; however, STIM1-deficient cells display much greater deficits, concordant with the defect in SOCE.18-20 To address why cytokine synthesis in STIM1-deficient neutrophils is nearly normal despite decreased calcium influx, we focused on a major difference between neutrophils and other immune cells: the robust production of ROS. Oxidative stress modulates signaling pathways, and evidence suggests ROS may negatively regulate cytokine production.40,41 Because STIM1, but not STIM2, is required for ROS production, we postulated that cytokine production in Stim1VavCre neutrophils is augmented by a lack of inhibitory oxidative signaling. To test this notion, we stimulated WT neutrophils in the presence of the oxidase inhibitor diphenyleneiodonium (DPI). Interestingly, inhibiting ROS augmented TNFα production in WT neutrophils (Figure 6A). Phosphorylation of IKKα/β and nuclear translocation of NF-κB were also increased in DPI-treated cells (Figure 6B), supporting that ROS negatively regulates cytokine production in neutrophils. We next tested whether inhibiting ROS could rescue cytokine production in STIM2-deficient cells. Indeed, DPI increased TNFα production in zymosan-treated Stim2VavCre, but not Stim1VavCre, neutrophils (Figure 6C-D). Conversely, to rescue ROS, but not calcium production, in STIM1-deficient neutrophils, we stimulated cells with PMA/ionomycin. PMA restores ROS production in Stim1VavCre neutrophils23 (supplemental Figure 4A-B); however, SOCE initiated by the ER ionophore ionomycin requires STIM1 (Figure 2A). Indeed, stimulation with PMA/ionomycin unmasked a defect in cytokine production Stim1VavCre neutrophils (Figure 6C,E). Together, these results suggest neutrophil cytokine production is modulated by both calcium and ROS-dependent signals, and in STIM1-deficient cells, the lack of ROS-mediated inhibition compensates for decreased calcium-dependent cytokine production.
ROS-mediated inhibition of cytokine production imparts a selective role for STIM2 in neutrophils. (A) Analysis of TNFα production in WT neutrophils treated with the NADPH oxidase inhibitor DPI. Bone marrow cells (1 × 106) from WT mice were incubated with zymosan (100 μg/mL) for 5 hours with or without DPI (10 μM), and TNFα levels were measured by intracellular staining. (B) Analysis of NF-κB activation in WT neutrophils treated with DPI. Isolated neutrophils were stimulated with zymosan (100 μg/mL) in the presence or absence of DPI (10 μM), and nuclear and cytoplasmic fractions were blotted for the indicated proteins. (C) Bone marrow cells (1 × 106) from WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice were incubated with zymosan (100 μg/mL) for 5 hours with or without the NADPH oxidase inhibitor DPI (10 μM) or PMA/Ionomycin, and TNFα levels were measured by intracellular staining. (C) Representative FACS plots. (D-E) Mean ± SEM compiled from 2 to 3 independent experiments. *P < .05.
ROS-mediated inhibition of cytokine production imparts a selective role for STIM2 in neutrophils. (A) Analysis of TNFα production in WT neutrophils treated with the NADPH oxidase inhibitor DPI. Bone marrow cells (1 × 106) from WT mice were incubated with zymosan (100 μg/mL) for 5 hours with or without DPI (10 μM), and TNFα levels were measured by intracellular staining. (B) Analysis of NF-κB activation in WT neutrophils treated with DPI. Isolated neutrophils were stimulated with zymosan (100 μg/mL) in the presence or absence of DPI (10 μM), and nuclear and cytoplasmic fractions were blotted for the indicated proteins. (C) Bone marrow cells (1 × 106) from WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice were incubated with zymosan (100 μg/mL) for 5 hours with or without the NADPH oxidase inhibitor DPI (10 μM) or PMA/Ionomycin, and TNFα levels were measured by intracellular staining. (C) Representative FACS plots. (D-E) Mean ± SEM compiled from 2 to 3 independent experiments. *P < .05.
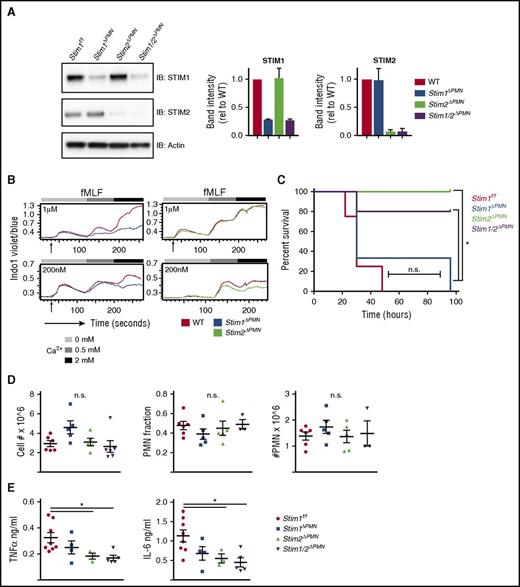
Neutrophil-specific deletion of STIM2 protects against cytokine-mediated systemic inflammation
Neutrophil-derived cytokines contribute to pathology in sterile inflammation.42 To test whether STIM2 deficiency alters a cytokine-mediated disease process in vivo, we used a model of intraperitoneal injection of zymosan, which produces an overwhelming cytokine response that progresses to multiorgan failure and is at least in part dependent on neutrophil activation.43 We generated mice with neutrophil-specific deletion in STIM molecules, using MRP8-Cre (StimΔPMN).44 StimΔPMN neutrophils exhibited 85% to 100% deletion of both STIM1 and STIM2 (Figure 7A). SOCE in StimΔPMN and StimVavCre neutrophils was similar, functionally confirming effective deletion (Figure 7B). Strikingly, when challenged with a lethal dose of zymosan, Stim2ΔPMN and Stim1/2ΔPMN mice were protected, whereas mortality in Stim1ΔPMN mice was similar to that in controls (Figure 7C). Total cellular influx and neutrophil migration into the peritoneum was equivalent (Figure 7D); however, TNFα and IL-6 levels in the peritoneum were significantly less in both Stim2ΔPMN and Stim1/2ΔPMN mice (Figure 7E). Serum cytokine levels trended lower in Stim2ΔPMN and Stim1/2ΔPMN mice, but did not reach statistical significance (not shown). Together, these results demonstrate that STIM2 deficiency in neutrophils protects against mortality in a model of systemic inflammatory response syndrome, likely as a result of decreased inflammatory cytokine release.
Neutrophil-specific deletion of STIM2 protects against cytokine-mediated systemic inflammation. (A) Analysis of STIM1 and STIM2 protein expression in mice with neutrophil-specific deletion. Neutrophils were isolated from WT, Stim1ΔPMN, Stim2ΔPMN, Stim1/2ΔPMN mice, and levels of STIM1 and STIM2 were determined by western blot. (Right) Quantitation of mean ± SEM from 2 to 3 experiments. (B) Functional analysis of calcium flux in bone marrow neutrophils from WT, Stim1ΔPMN, and Stim2ΔPMN mice. Cells were loaded with Indo-1 and calcium flux determined by flow cytometry. (C-E) WT, Stim1ΔPMN, and Stim2ΔPMN and Stim1/2ΔPMN mice were injected with zymosan 500 mg/kg intraperitoneally and followed for survival (C) or sacrificed at 6 hours and the peritoneal lavage obtained for cell counts, identification of neutrophils by flow cytometry (D), and measurement of TNFα and IL-6 by ELISA (E). Survival data represent 7 to 9 mice per group. Cell counts and cytokine levels are representative of 2 independent experiments and expressed as mean ± SEM. *P < .05.
Neutrophil-specific deletion of STIM2 protects against cytokine-mediated systemic inflammation. (A) Analysis of STIM1 and STIM2 protein expression in mice with neutrophil-specific deletion. Neutrophils were isolated from WT, Stim1ΔPMN, Stim2ΔPMN, Stim1/2ΔPMN mice, and levels of STIM1 and STIM2 were determined by western blot. (Right) Quantitation of mean ± SEM from 2 to 3 experiments. (B) Functional analysis of calcium flux in bone marrow neutrophils from WT, Stim1ΔPMN, and Stim2ΔPMN mice. Cells were loaded with Indo-1 and calcium flux determined by flow cytometry. (C-E) WT, Stim1ΔPMN, and Stim2ΔPMN and Stim1/2ΔPMN mice were injected with zymosan 500 mg/kg intraperitoneally and followed for survival (C) or sacrificed at 6 hours and the peritoneal lavage obtained for cell counts, identification of neutrophils by flow cytometry (D), and measurement of TNFα and IL-6 by ELISA (E). Survival data represent 7 to 9 mice per group. Cell counts and cytokine levels are representative of 2 independent experiments and expressed as mean ± SEM. *P < .05.
Discussion
SOCE is clearly critical for immune cell function. Although a role for STIM1 in neutrophil responses has been established, the role of STIM2 in neutrophil SOCE and activation is unknown. Here we report that absence of STIM2 results in a modest reduction in SOCE. Although neutrophil bactericidal functions including ROS production, degranulation and phagocytosis were relatively independent of STIM2, cytokine production required STIM2, but not STIM1, at least in part because of redox regulation of cytokine gene expression. In vivo loss of neutrophil STIM2 results in lower cytokine levels and protection from mortality in a mouse model of systemic inflammatory response syndrome. These data, combined with previous studies focusing on STIM1, define distinct but cooperative functions for STIM1 and STIM2 that finely tune neutrophil responses over a range of signal strengths.
Our finding that STIM2 efficiency results in a mild decrease in SOCE is in line with previous literature describing the role of STIM2 in B cells and T cells.18,19 We measured calcium influx in neutrophils using a flow cytometry-based method that allows for minimal manipulation of bone marrow neutrophils before stimulation. A potential caveat is that the kinetics of calcium influx appear somewhat delayed compared with microscopy or fluorimeter-based methods. Our results are consistent with those obtained in other cell types; however, this must be considered when comparing these findings with those using other methodologies. It is interesting that we see a more prominent effect of STIM2 deficiency under conditions of physiological calcium. Although SOCE is often described and assayed in 2 distinct phases (ER-store release followed by opening of plasma membrane channels), in actuality, both processes occur simultaneously. STIM activation is maximal after full store depletion; however, calcium dissociation kinetics stipulate that STIM activation initiates once calcium levels in the ER fall below the activation threshold. Studies in cell lines have demonstrated that STIM2 is more sensitive to ER calcium depletion, and thus the threshold for STIM2 activation is lower.12,15,45,46 Therefore, we postulate that the defect in STIM2-deficient cells is more apparent when calcium is present throughout stimulation, as these conditions reflect periods of both partial and full ER store-depletion. To truly understand this phenomenon requires further study with simultaneous measurement of ER and cytosolic calcium levels. The increased sensitivity of STIM2 coupled with weak calcium channel opening might facilitate the ability of neutrophils to respond over a wide range of stimuli, a feature that is likely physiologically relevant for neutrophils, which are exquisitely sensitive to calcium-dependent activation.
Although current research demonstrates that neutrophils synthesize a variety of cytokines, few studies have explored the mechanisms regulating transcription factors in neutrophils.29,47 We demonstrate here that STIM2 is specifically required for neutrophil cytokine synthesis via calcium-dependent activation of NF-κB. NFAT and NF-κB are important transcription factors in immune cell function, and evidence supports a role for their calcium-dependent regulation.48-50 NFAT is the classical calcium-dependent transcription factor, and impaired NFAT activation underlies the altered cytokine production in STIM1-deficient lymphocytes.18,19 In neutrophils, we did not see increased NFAT activation with zymosan stimulation, suggesting inflammatory cytokine responses rely largely on NF-κB, although NFAT may still modulate basal transcriptional processes. Calcium-dependent activation of NF-κB has been described, although the mechanism is less clear. STIM1-dependent SOCE was recently shown to regulate NF-κB activation in T cells via calcium-dependent activation of PKCα.49 Calcineurin has also been shown to promote formation of the Carma1-Bcl10-Malt1 complex, which facilitates NF-κB activation.50 In macrophages, calcium-dependent activation of CAMKII has been linked to NF-κB activation via phosphorylation of TAK1, which subsequently activates IKKα/β.38 In neutrophils, we show that both IKKα/β and CAMKII phosphorylation require calcium and STIM2. However, regulation of NF-κB activation is complex and likely involves multiple pathways. More studies are necessary to clearly delineate how these calcium-dependent pathways converge to regulate NF-κB activation in neutrophils.
Our finding that cytokine production in STIM1-deficient cells is minimally decreased, despite significantly altered SOCE, was unexpected. Given that ROS inhibition rescues cytokine production in STIM2-deficient cells while restoring ROS production in STIM1-deficient cells reveals a defect in cytokine production, we propose that this discrepancy is explained at least in part by ROS-mediated inhibitory signaling. The concept of ROS as a negative regulator of cytokine production is certainly not new. Patients with chronic granulomatous disease have a hyper-inflammatory phenotype that is not completely understood, and chronic granulomatous disease neutrophils demonstrate enhanced IL-8 secretion in vitro.41 Furthermore, mouse models of chronic granulomatous disease display increased cytokine levels in a model of lung injury.40 The mechanism of this regulation is not clear; however, ROS has been shown to directly modify transcription factors and MAP kinases, and also induce a DNA damage response, all of which can modify cytokine transcription.51,52 Further studies are required to clearly dissect the molecular mechanism underlying this finding. The observation that STIM1/2-deficiency neutrophils impairs ROS release and cytokine production appears to conflict with this logic. However, it is likely that the more profound loss of SOCE in STIM1/2-deficient cells outweighs loss of ROS-mediated inhibition.
Biologically, the role for STIM2 in neutrophil cytokine production is significant, as loss of STIM2 is specifically protective against cytokine-dependent shock and mortality. We chose this peritonitis model because it is a model of sterile inflammation that induces a robust cytokine response. Testing the contribution of STIM1 vs STIM2 in bacterial infection models is more complex, given the interplay among bacterial killing, pathogen burden, and cytokine response. However, these studies will be important steps toward defining how to best tailor neutrophil activation therapeutically by targeted inhibition of STIM1 vs STIM2.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Clare Abram for critical reading of the manuscript.
This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grants AI65495 and AI68150 [C.A.L.] and K08AI119134 [R.A.C.]) R.A.C. is a fellow of the Pediatric Scientist Development Program, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12-HD000850).
Authorship
Contribution: R.A.C., J.C., and D.G. performed experiments; Y.H. performed mouse experiments; C.A.L. provided laboratory oversight; and all authors participated in composing and editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for R.A.C. is Department of Pediatrics, Washington University, St. Louis, MO.
Correspondence: Clifford A. Lowell, Department of Laboratory Medicine, University of California, San Francisco, 513 Parnassus Ave, HSW1201A, CA 94143-0451; e-mail: clifford.lowell@ucsf.edu.



![Figure 4. Neutrophil cytokine synthesis requires STIM2. (A-C) Analysis of cytokine production in whole bone marrow cells (1 × 106) from WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice incubated with (A) zymosan (100 μg/ml), (B) PAM3CSK (1 μg/mL), or (C) insoluble immune complexes (10 μg/mL) in RPMI ([Ca2+] 0.7 mM) for 5 hours. Neutrophils were identified by double staining for Ly-6G and CD11b, and cytokine production was measured by intracellular staining for TNFα, IL-10, and IFN-γ. (A) FACS plots (left) are representative of 3 independent experiments, and bar graphs (right) represent mean ± SEM compiled from multiple experiments. (D) Analysis of cytokine transcripts by RT-qPCR. Neutrophils were purified from WT, Stim1VavCre, Stim2VavCre, and Stim1/2VavCre mice by negative selection yielding more than 95% pure neutrophils. Cells were stimulated with zymosan (100 μg/mL) for 4 hours, and RNA transcript levels of the indicated cytokines measured by RT-qPCR. Mean ± SEM compiled from 3 independent experiments. #P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/13/10.1182_blood-2016-11-751230/4/m_blood751230f4.jpeg?Expires=1769087584&Signature=gl62bKyk~pGp9ZfCR7fb9H~MwDDE4VKxwQ0mSMj8ykAAfzxBJ4LgFPKTYBQzxSJXmTk0ZO2pCFo38-Ojb4PcIF-lEfYiBgoN~Xp3L9QYuXu8-vIBMBjgM8yziZQNs9JGRibB9o~gF1cRB7IR96t-tKRm2wRJ2lWaGPkqu3PbSpXVPrMACpA2EPD~QiZu7XmzPfD12Qr~7agxEeS19m9d3IrpJnDvOP7PcA-6M8LLGg2D0A3q0clCqdICfsCBoFNdyWpCwV6Hyl0QF9zYThjbzR1nV6v8aHEvdbdzEbV-9OA3bBL6IoqkcMtIwb2I~uKBD59NvuZzjggPwmNjsHVOnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal