In this issue of Blood, Clemens et al identify specific functions of the calcium-sensing molecules STIM1 and STIM2 in calcium-dependent signaling pathways in neutrophils. STIM1 is essential for the regulation of store-operated calcium entry (SOCE) and bactericidal cellular functions like phagocytosis and degranulation, whereas STIM2 is essential for neutrophil cytokine production. Protective effects of STIM2 deficiency in a mouse model of systemic inflammation make STIM2 interesting for therapeutic approaches to different inflammatory diseases.1
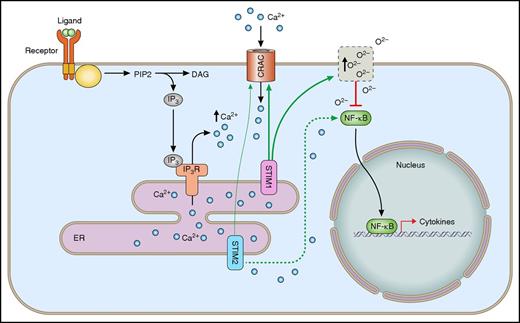
Differential roles of STIM1 and STIM2 in calcium-dependent signaling pathways in neutrophils. Following the engagement of surface receptors, the second messenger IP3 is produced. Following binding of IP3 to its receptors, Ca2+ is released into the cytoplasm, leading to ER-store depletion. STIM1 and STIM2 sense changes in ER-Ca2+ concentration and induce SOCE via the interaction with calcium-release activated channels (CRAC) in the plasma membrane. Additionally, STIM1 induces production of ROS, which in turn inhibit the activation of the transcription factor NF-κB and cytokine synthesis. In contrast, STIM2 activates the calcium-dependent translocation of NF-κB, leading to increased cytokine synthesis. IP3R, IP3 receptor.
Differential roles of STIM1 and STIM2 in calcium-dependent signaling pathways in neutrophils. Following the engagement of surface receptors, the second messenger IP3 is produced. Following binding of IP3 to its receptors, Ca2+ is released into the cytoplasm, leading to ER-store depletion. STIM1 and STIM2 sense changes in ER-Ca2+ concentration and induce SOCE via the interaction with calcium-release activated channels (CRAC) in the plasma membrane. Additionally, STIM1 induces production of ROS, which in turn inhibit the activation of the transcription factor NF-κB and cytokine synthesis. In contrast, STIM2 activates the calcium-dependent translocation of NF-κB, leading to increased cytokine synthesis. IP3R, IP3 receptor.
Calcium-dependent signaling pathways are indispensable for many processes in different cell types. In immune cells, processes like migration, degranulation, and phagocytosis, which are central functions within the innate immune response, are calcium dependent. Thus, the tight regulation of changes in cytosolic calcium concentrations as a result of the engagement of cell surface receptors is crucial for accurate immune reactions.
Following immune cell stimulation, SOCE is the main mechanism allowing calcium influx into the cell. Ligand binding to different cell receptors, like G-protein–coupled receptors, activates signaling cascades, leading to phospholipase C β2/3 activation. As a result, phosphatidylinositol 4,5-bisphosphate (PIP2) gets hydrolyzed into diacylglycerol (DAG) and the second messenger inositol triphosphate (IP3). IP3 binds to its receptors on the endoplasmic reticulum (ER), inducing calcium efflux from ER stores into the cytoplasm.2 STIM1 and STIM2 are structurally similar calcium-sensing molecules located within the membrane of the ER. The ER-luminal part of STIM1 and STIM2 binds Ca2+ within the ER with different affinities.3 Dependent on the calcium concentration within the ER, STIM molecules undergo conformational changes and can directly interact with their cytoplasmic domains with calcium channels in the plasma membrane. This interaction induces SOCE from the extracellular space into the cytoplasm.4 The role of STIM molecules, especially STIM1, in different immune cells was extensively investigated. A previous study demonstrated in an in vivo experiment that STIM1 in neutrophils plays a role in bacterial clearance and is involved in ischemia-reperfusion injury.5 However, several studies demonstrated differential roles for STIM1 and/or STIM2 depending on the immune cell type. The relative expression levels of both molecules are cell type dependent, indicating distinct functions. In neutrophils, STIM2 expression is 10 times higher than STIM1 expression.1 Even though extensive research has been conducted in recent years, the exact roles of STIM1 and STIM2 in neutrophils remain elusive.
Clemens et al generated and compared conditional knockout mice deficient for STIM1, STIM2, or both molecules in hematopoietic cells. In this very elegant study, specific functions of STIM1 and STIM2 in neutrophils were investigated via direct comparison of in vitro and in vivo phenotypes in conditional knockout mice. By performing different in vitro experiments, they confirmed that neutrophil SOCE is exclusively mediated by cooperating functions of STIM1 and STIM2. STIM2 deficiency led to a modest reduction in sustained calcium entry at lower agonist concentrations, in contrast to a more drastic and immediate reduction in STIM1-deficient neutrophils. The differences in the response time and strength indicate an involvement in different cellular processes. Bactericidal functions like reactive oxygen species (ROS) production and phagocytosis are reduced in STIM1-, but not STIM2-, deficient neutrophils, demonstrating differences in the physiological roles of the 2 molecules.
The most surprising result of the underlying work demonstrates that STIM2-, but not STIM1-, deficient neutrophils exhibit a defect in cytokine synthesis downstream of different receptor stimulations. Cytokine synthesis modulates neutrophil-mediated immune response to different stimuli. By identifying this specific phenotype of STIM2 deficiency, the authors provide a new approach to modulate neutrophil functions via the manipulation of STIM2 signaling. Additionally, the authors demonstrate that this pathway is induced via STIM2-mediated Ca2+-dependent activation of the kinases IκB kinase α/β and calmodulin-dependent kinase II, leading to the activation and translocation of the transcription factor NF-κB into the nucleus (see figure). The missing phenotype in STIM1-deficient neutrophils is explained by the lack of redox signaling, which inhibits cytokine synthesis in wild-type cells. By demonstrating the protective effect of STIM2 deficiency in a mouse model of systemic inflammatory response syndrome, Clemens and colleagues identify a unique function of STIM2 in a complex disease model.
Neutrophil recruitment to sites of inflammation is a very important part of the immune defense against invading pathogens or following injury.6 However, under distinct conditions like ischemia-reperfusion injury or sterile inflammation, recruited neutrophils and their elicited immune reactions can also destroy host cells and tissues, leading to severe complications.7 To keep the balance between positive and negative effects, neutrophil recruitment as well as effector functions have to be tightly controlled.8 Mutations or deficiencies in the 2 STIM molecules exhibit distinct phenotypes. Mutation in STIM1 manifests in children symptoms of immunodeficiency in combination with autoimmunity.9 The elimination of STIM2 in neutrophils protects against cytokine-mediated systemic inflammation in a mouse model.1
So far, most research projects and clinical reports have focused on consequences of STIM1 or Orai mutations or deficiency.10 The distinct description of STIM1 and STIM2 functions in neutrophils can enable the development of new therapeutic approaches as well as the explanation of clinical symptoms. However, there are still many open questions about the role of STIM1 and STIM2 during neutrophil-mediated immune responses. Future studies have to elucidate which steps of the neutrophil recruitment cascade are dependent on STIM1 and/or STIM2. Additionally, the involvement of STIM molecules in signaling pathways downstream of different receptors has to be characterized in more detail.
The work by Clemens and colleagues reveals different physiological roles of the homologous molecules STIM1 and STIM2 in neutrophils and also identifies possible signaling pathways involved in the cellular processes. In summary, this study helps to create new approaches for therapeutic interventions by targeting STIM1 or STIM2 in different diseases. However, because the expression patterns as well as the functional behavior of STIM molecules show differences between the murine and the human system, the obtained results have to be confirmed in human primary cells in future studies.
Conflict-of-interest disclosure: A.Z. received unrestricted research grants from Deutsche Forschungsgemeinschaft (DFG), Else-Kröner Fresenius Stiftung, Fresenius, and Astute Medical, and lecture fees from Astute Medical, Braun, Fresenius, and Astellas. A.S. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal