In this issue of Blood, Madhusudhan et al elegantly demonstrate a novel role of the coagulation protease activated protein C (aPC) in maintaining endoplasmic reticulum (ER) homeostasis, and thus cellular metabolism, in the context of diabetic kidney disease (DKD).1
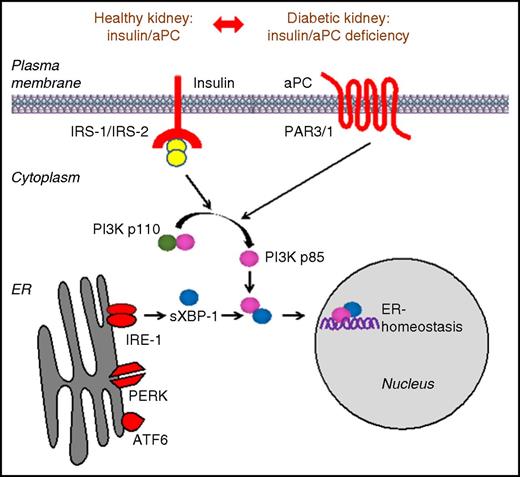
Schematic representation demonstrating that aPC can compensate for insulin deficiency in the context of DKD. Downstream signals from the insulin and PAR1/3 receptor converge via the PI3K pathway to regulate the cytoprotective ER transcription factor sXBP1 in podocytes. See Figure 6 in the article by Madhusudhan et al that begins on page 1445.
Schematic representation demonstrating that aPC can compensate for insulin deficiency in the context of DKD. Downstream signals from the insulin and PAR1/3 receptor converge via the PI3K pathway to regulate the cytoprotective ER transcription factor sXBP1 in podocytes. See Figure 6 in the article by Madhusudhan et al that begins on page 1445.
aPC is a critical regulator of hemostasis, and it possesses several important anti-inflammatory and cytoprotective functions.2 aPC is generated by the enzymatic cleavage of protein C by thrombin and, with the assistance of its cofactor protein S, acts to inhibit factor Va (FVa) and FVIIIa to curb the production of thrombin. This process is accelerated on the endothelium in the presence of the endothelial protein C receptor (EPCR) and thrombomodulin. More recently, the anti-inflammatory and cytoprotective functions of aPC have become the focus of investigation by numerous groups. Indeed, the role of aPC and the EPCR in the context of cancer biology, innate immunity, and malaria pathogenesis remains a source of ongoing investigation.2,3
In keeping with these observations, aPC has previously been shown by Isermann et al4 to prevent endothelial and podocyte apoptosis and thus plays a protective role against the development of DKD. DKD is a major cause of morbidity and mortality in patients with diabetes and represents the leading cause of end-stage renal failure globally.5 Importantly, one of the earliest signs of DKD is the loss of the specialized epithelial cells (podocytes) in the renal glomerulus.6 Therefore, greater understanding of the molecular mechanisms underpinning the premature loss of podocytes seen in diabetic nephropathy may hold the key to developing novel therapeutic strategies. Intriguingly, in addition to aPC, insulin has also been demonstrated to be a key regulator of podocyte function because mice specifically lacking the insulin receptor on podocytes develop a phenotype similar to DKD in the absence of hyperglycemia.7
In their study, by using multiple transgenic mouse lines, Madhusudhan et al link these 2 seminal observations by demonstrating that aPC initiates cytoprotective signaling in podocytes in a pattern that is very similar to that described for insulin. Indeed, by using a streptozotocin mouse model of diabetes (considered to be a model of type 1 diabetes), the authors show that mice with impaired aPC activation displayed exacerbated DKD, which was associated with a more prominent maladaptive renal unfolded protein response (UPR), predominantly characterized by impaired nuclear translocation of spliced-X-box binding protein-1 (sXBP1). In contrast, these effects could be rescued by restoration of aPC levels, whereas high-expressing aPC mice were protected. Therefore, to test the effect of aPC on the development of diabetic nephropathy in the absence of insulin signaling, mice lacking the insulin receptor specifically on podocytes were used. Insulin receptor–deficient mice developed overt DKD but, strikingly, transgenic overexpression or exogenous administration of aPC protected these mice from developing DKD and restored nuclear levels of sXBP1. These findings were recapitulated in a model of type 2 diabetes (db/db mice), thus confirming that aPC can confer potent nephroprotective effects and restore the UPR in the setting of insulin deficiency or resistance. Complementary in vitro assays demonstrated that aPC or insulin promote the nuclear translocation of sXBP1 via heterodimerization with p85α/p85β in podocytes. Consistent with this postulate, the renoprotective effect of high aPC expression was ameliorated in the context of XBP1, p85α, or p85β deficiency. Thus, despite having distinct receptors, podocytes share common downstream signaling events in response to either stimulus, which act to regulate the cytoprotective ER transcription factor sXBP1 (see figure).
These findings have broad-ranging implications. Much enthusiasm remains for the development of novel anticoagulants. Foremost among these are the inhibitors of FXII and FXI in preclinical and early-phase development. These approaches do not seem to cause bleeding and have been shown to confer benefit in preclinical models in a range of inflammatory conditions such as multiple sclerosis and atherosclerosis.8 Likewise, there is now a plethora of new agents in development for the treatment of hemophilia, which include inhibitors of aPC and small interfering RNAs that knock down the expression of antithrombin.9 Whether such approaches are associated with any unforeseen metabolic effects remains to be explored. In this context, it is intriguing that coagulation signaling, via tissue factor–FVIIa and the PAR2 receptor, promotes weight gain, insulin resistance, and adipose inflammation.10 Conversely, given the global diabetes and obesity epidemic, the ability to potentially bypass the effects of insulin resistance or deficiency by targeting common downstream signaling pathways may open new therapeutic avenues that could represent novel approaches to reduce the huge burden of diabetes complications seen globally, and in particular, a major cause of morbidity and premature mortality, DKD.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal