In this issue of Blood, Miot et al report encouraging results in a retrospective analysis of hematopoietic stem cell transplantation (HSCT) in 29 patients with hypomorphic mutations in the IKBKG gene encoding nuclear factor κB essential modulator (NEMO).1
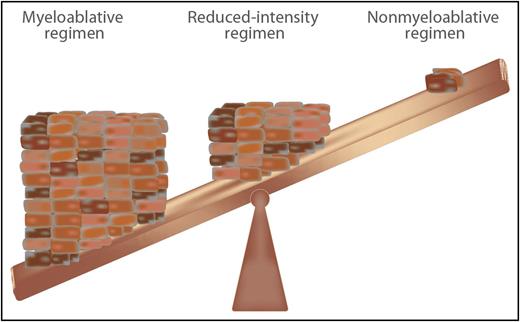
Intensity of pretransplant conditioning chemotherapy regimens required to facilitate engraftment but minimize toxicity for HSCT for PIDs. Professional illustration by Somersault18:24.
Intensity of pretransplant conditioning chemotherapy regimens required to facilitate engraftment but minimize toxicity for HSCT for PIDs. Professional illustration by Somersault18:24.
Several important nuggets can be mined from the results in this heterogeneous cohort of patients. First, the overall survival was a respectful 74%. Second, preexisting mycobacterial infections and colitis were associated with poor clinical outcome. Third, there was a trend toward better disease-free survival with a nonmyeloablative conditioning regimen. Thus, this report contributes to the important question of when and how to transplant these patients.
Perhaps equally important, this study brings up all the questions that confront the issue of HSCT for patients with primary immunodeficiency diseases (PIDs): the patient’s genotypes were quite heterogeneous, with 23 different mutations in the 29 patients; the conditioning regimen and donor sources were variable; and the extent of donor chimerism required to reverse the phenotype was not clear. Finally, a significant proportion of patients experienced serious complications, including graft failure, graft-versus host-disease (GVHD), persistence or even de novo development of severe inflammatory bowel disease, serious infections, and conditioning regimen–associated toxicities.
Distilling the key ingredients responsible for successful HSCT for PIDs represents a challenging task. Nevertheless, for the field to progress, some attempt must be made to identify and formulate the rules of engagement for HSCT in this setting. First, the natural history of the disease should determine whether and when to proceed to HSCT. For instance, X-linked severe combined immunodeficiency disease (X-SCID) is typically fatal in the first years of life, and the best results are achieved with HSCT in the first 3 months of life.2 In contrast, patients with mutations in GATA2 may remain asymptomatic into old age; thus, HSCT can be deferred until patients develop symptoms or signs of disease.3 In the study by Miot et al, age at transplantation had no impact on survival, indicating that other variables play a more important role. In the case of NEMO deficiency, HSCT has often been reserved for patients with serious clinical complications, and yet this study demonstrates that the presence of mycobacterial disease and colitis at the time of transplantation may represent negative risk factors for a successful HSCT outcome of the procedure. This observation should set the benchmarks for future studies aimed at better defining how to control infections and inflammation pretransplant. It will also be important to assess whether the use of more aggressive regimens to dampen gut inflammation before transplant may be beneficial. The role of abnormalities in the microbiome should also be investigated in greater detail, and interventions aimed at improving the composition of the gut flora should be considered.
The second issue in HSCT for PIDs revolves around the level of chimerism and the leukocyte subset–specific chimerism necessary to reverse the disease phenotype. In some PIDs, this is known, and in some PIDs, this remains unclear. For instance, in canine models of leukocyte adhesion deficiency type 1 (LAD-1), even 5% of CD18+ neutrophils results in reversal of the phenotype.4 If the donor cells have a competitive advantage, such as normal donor T cells in X-SCID, these cells should accumulate to normal levels over time resulting in reversal of the phenotype.2 Skewed X-chromosome inactivation has been reported in multiple blood lineages from most carrier females of NEMO deficiency suggesting selective advantage for cells with normal NEMO function.5 However, random X-inactivation has been reported in a few carrier females.6
Possibly no question divides the field of HSCT for PIDs more than the third issue: the type of pretransplant conditioning to use. The intensity of conditioning for PIDs prior to HSCT ranges from immunosuppressive only to nonmyeloablative, to reduced intensity, to myeloablative. The level of conditioning is influenced by whether the T-cell arm of the immune system is intact. If the T-cell compartment is either depleted (SCID) or functionally defective, no conditioning or a reduced-intensity regimen may be effective in enabling engraftment. However, if the T-cell compartment is intact (eg, chronic granulomatous disease7 or GATA2 deficiency8 ), some degree of myeloablation is required for engraftment. Many studies are now addressing the optimal level of myeloablation and immunoablation in HSCT for different PIDs (see figure). Although statistical significance was not reached in the study by Miot et al, there was a clear trend toward improved survival among recipients of reduced-intensity conditioning. This may be a better choice, also considering that the vast majority of patients attained more than 90% donor chimerism.
The best type of donor and donor graft source for HSCT for PIDs represents the fourth challenge. Matched related donors are clearly the first choice. However, haploidentical related donors are currently neck and neck with matched unrelated donors in many HSCT scenarios.9 In the 29 NEMO patients, 7 were matched related donors, 12 were matched unrelated donors, 8 were mismatched unrelated donors (mostly umbilical cord blood), and only 2 were haploidentical related donors.1 Moreover, 4 of the 5 patients who received HSCT from a related carrier female donor developed complications post-HSCT, including recurrent infections and persistence or de novo development of colitis. Additional studies are needed to establish whether HSCT from carrier females (and especially from symptomatic females) should be avoided in this disease.
Lastly, strategies to prevent GVHD are paramount in HSCT for PIDs because there is no advantage to GVHD where there is no preexisting malignancy, GATA2 deficiency representing an exception to this rule. NEMO patients had a 50% incidence of GVHD in this study. The latest development in GVHD prophylaxis uses posttransplant cyclophosphamide in matched related and unrelated donors, as well as haploidentical related donors.
The field of HSCT, and especially HSCT for PIDs, has come a long way in 50 years. Studies such as the report by Miot et al are critical for the field to advance.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal