Key Points
P2Y12 is important in the interaction between platelets and cancer cells.
A P2Y12 inhibitor or P2Y12 deficiency reduces tumor growth in murine models of ovarian cancer.
Abstract
We investigated the effect of platelets on ovarian cancer and the role of adenosine diphosphate (ADP) receptors (P2Y12 and P2Y1) on platelets in the growth of primary ovarian cancer tumors. We showed that in murine models of ovarian cancer, a P2Y12 inhibitor (ticagrelor) reduced tumor growth by 60% compared with aspirin and by 75% compared with placebo. In P2Y12−/− mice, the growth of syngeneic ovarian cancer tumors was reduced by >85% compared with wild-type (WT) mice. In contrast, there was no difference in tumor growth between P2Y1−/− and WT mice. Reconstitution of hematopoiesis in irradiated P2Y12−/− mice by hematopoietic progenitor cells from WT mice (WT→P2Y12−/−) restored tumor growth in P2Y12−/− mice. Finally, knockdown of ecto-apyrase (CD39) on ovarian cancer cells increased tumor growth in tumor-bearing mice. Although in the absence of platelets, ADP, the P2Y12 inhibitor, recombinant apyrase, or knockdown of CD39 did not affect cancer cell proliferation, in the presence of platelets, the P2Y12 inhibitor and recombinant apyrase reduced and knockdown of CD39 increased platelet-enhanced cancer cell proliferation. These results suggest that P2Y12 on platelets and ADP concentration at the interface between cancer cells and platelets affect the growth of primary ovarian cancer tumors in mice. If additional studies in mice and in pilot human trials confirm our results, inhibition of P2Y12 might be a new therapeutic option that can be used in adjuvant to the traditional surgery and chemotherapy in patients with ovarian cancer.
Introduction
Many cancer patients, including one-third of patients with ovarian cancer, have elevated platelet counts, which predict a poor prognosis.1-6 Platelet activation is important in the interaction between platelets and cancer cells. The ability of cancer cells to activate platelets in vitro, or tumor cell–induced platelet aggregation (TCIPA), predicts their in vivo aggressiveness.7 We have previously shown that ovarian cancer cells secrete adenosine diphosphate (ADP),8,9 a major mediator of TCIPA9-11 and that activated platelets enhance the proliferation of cancer cells and tumor growth, partially by releasing transforming growth factor-β (TGF-β).12
In this study, we investigated the importance of ADP receptors on platelets in the growth of ovarian cancer. There are 2 ADP receptors on platelets, P2Y1 and P2Y12. ADP binding to these receptors results in the activation and degranulation of platelets and the release of multiple growth factors.13-15 Currently, several P2Y12 inhibitors are used in the management of patients with cardiovascular diseases.16,17 Ticagrelor is a nucleoside analog and an oral reversible inhibitor of P2Y12 that does not need to be premetabolized to an active form in the body.16,18,19 Using murine models of ovarian cancer, we investigated the effect of ticagrelor, P2Y1, and P2Y12 on tumor growth. We also conducted in vitro studies to differentiate between a direct effect of ADP on cancer cells and an indirect effect mediated by platelets. We compared the effect of ticagrelor, ADP, recombinant apyrase, and knockdown of P2Y12 or ecto-apyrase genes on the proliferation of cancer cells in the absence and presence of platelets.
Materials and methods
Reagents
Cell culture media (GE Healthcare Life Sciences), fetal bovine serum (GE Healthcare Life Sciences), and gentamicin (Thermo Fisher Scientific), ADP (Sigma Aldrich), apyrase (Sigma Aldrich), aspirin (Sigma Aldrich), and ticagrelor (AstraZeneca) were purchased from the indicated commercial sources.
Cell lines and culture conditions
The detailed information of cell lines is provided in the supplemental Methods, available on the Blood Web site.
Animals
P2Y12- and P2Y1-deficient mice were gifts from S. Kunapuli (Temple University, Philadelphia, PA) and were genotyped according to a previously published protocol.20 Female athymic nude, nu/nu mice were purchased from Taconic, Inc., and wild-type (WT) C57BL/6 mice were purchased from The Jackson Laboratory.
Murine models of ovarian cancer
All of the studies on mice were conducted according to the protocols approved by the Institutional Review Board and Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
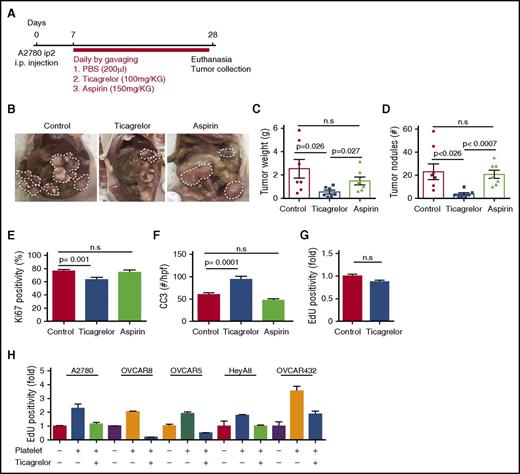
Orthotopic murine models of ovarian cancer were generated by intraperitoneal injection of cancer cells. In the athymic nude model, 1 × 106 (A2780, A2780-CD39-CRISPR-Cas9, and OVCAR8) human ovarian cancer cells were resuspended in 200 µL of Hanks balanced salt solution and injected into the peritoneum of 6- to 8-week-old female nude mice. In some experiments, P2Y12 small interfering RNAs (siRNAs) conjugated to 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC)-based liposomes were injected into the tumor-bearing mice at a dose of 150 µg/kg per mouse twice a week for 4 to 6 weeks starting 1 week after the injection of cancer cells. In another group of experiments, 7 days after the injection of cancer cells, mice were divided into 3 treatment subgroups receiving placebo or medications by gavage until they were sacrificed at the end of the experiment. One group received ticagrelor (100 mg/kg), the other received aspirin (150 mg/kg), and the last group received phosphate-buffered saline (PBS [placebo], 200 μL) (Figure 1A).
Ticagrelor reduces the growth of tumors in murine models of ovarian cancer. (A) Therapeutic schema for treatment with antiplatelet agents. (B) Representative images of tumors from each treatment groups. (C) Mean aggregate tumor weight. (D) Number of tumor nodules induced by intraperitoneal injection of A2780ip2 human ovarian cancer cells into nude mice treated with antiplatelet agents (n = 10 mice per group). (E) Quantification of Ki67-positive cells in resected tumors (n = 15 HPFs/5 mice per group). (F) CC3 quantification in control mice (red bars), mice treated with ticagrelor (blue bars), and mice treated with aspirin (green bars) (n = 15 HPFs/5 mice per group). (G) Effect of ticagrelor on the in vitro proliferation rate of A2780ip2 human ovarian cancer cells in the absence of platelets. (H) The effect of ticagrelor on the in vitro proliferation rate of A2780ip2, OVCAR8, OVCAR5, HeyA8, and OVCAR432 ovarian cancer cells in the absence and presence of platelets. Results are normalized to the proliferation rate of A2780ip2, OVCAR8, OVCAR5, HeyA8, and OVCAR432 in the absence of platelets and ticagrelor (n = 3 triplicate experiments).
Ticagrelor reduces the growth of tumors in murine models of ovarian cancer. (A) Therapeutic schema for treatment with antiplatelet agents. (B) Representative images of tumors from each treatment groups. (C) Mean aggregate tumor weight. (D) Number of tumor nodules induced by intraperitoneal injection of A2780ip2 human ovarian cancer cells into nude mice treated with antiplatelet agents (n = 10 mice per group). (E) Quantification of Ki67-positive cells in resected tumors (n = 15 HPFs/5 mice per group). (F) CC3 quantification in control mice (red bars), mice treated with ticagrelor (blue bars), and mice treated with aspirin (green bars) (n = 15 HPFs/5 mice per group). (G) Effect of ticagrelor on the in vitro proliferation rate of A2780ip2 human ovarian cancer cells in the absence of platelets. (H) The effect of ticagrelor on the in vitro proliferation rate of A2780ip2, OVCAR8, OVCAR5, HeyA8, and OVCAR432 ovarian cancer cells in the absence and presence of platelets. Results are normalized to the proliferation rate of A2780ip2, OVCAR8, OVCAR5, HeyA8, and OVCAR432 in the absence of platelets and ticagrelor (n = 3 triplicate experiments).
In the syngeneic immune-competent model, the same number of murine ovarian cancer cells (ID8-VEGF) were injected intraperitoneally to C57BL/6 WT, P2Y12−/−, P2Y1−/−, WT→P2Y12−/−, WT→WT mice.
In both models, 6 to 8 weeks after the injection of cancer cells, mice became moribund and were sacrificed. Tumor nodules were resected from the peritoneum, counted, and weighed. Some tumor nodules were fixed in formalin and some were saved as fresh frozen samples by embedding in optimal cutting temperature compound.
Adoptive transfer of hematopoietic progenitor cells
Bone marrow cells were harvested from C57BL/6 mice by flushing the marrow from the femurs and tibias. Bone marrow suspensions were strained through 70-μm cell strainers (Becton Dickinson, Franklin Lakes, NJ) and then washed once with cold PBS. Bone marrow cells were resuspended in cytotoxicity medium (Cedarlane, Hornby, Ontario, Canada) at a concentration of 1 × 107/mL. Purified rat anti-mouse Thy1.2 monoclonal antibody (Becton Dickinson) was added and mixed at a concentration of 0.1 μg per 1 × 107 cells. After 1 hour of incubation on ice, the cells were washed once with PBS and then suspended in Cedarlane Cytotoxicity Medium containing 1:10 Low-Tox-M Rabbit Complement (Cedarlane). The cells were incubated at 37°C for 1 hour and washed twice with cold PBS before use. Recipient mice (8-week-old C57BL/6 mice or P2Y12−/− mice) were lethally irradiated with 1200 cGy. After a 1-hour rest, the recipient mice were injected IV via the tail vein with 7 × 106 T-cell–depleted bone marrow cells.
Platelet isolation
Whole blood was drawn from the inferior vena cava of anesthetized C57BL/6 mice into a 1-mL syringe preloaded with 100 μL of acid citrate dextrose (2.5 g sodium citrate, 1.5 g citric acid, and 2 g glucose in 100 mL deionized water) and centrifuged at 1100 rpm (200g) for 10 minutes at room temperature to isolate platelet-rich plasma. Platelets were purified from platelet-rich plasma by using a sepharose column as described previously.12 Twenty million platelets were used for in vitro incubation with cancer cells.
Cell proliferation assay (EdU assay)
Cell proliferation was quantified by measuring the incorporation of fluorescence-conjugated 5-ethynyl-2′-deoxyuridine (EdU) (Click-iT EdU Alexa Fluor; Invitrogen) to the newly synthesized DNA, as described previously.12 Briefly, 5 × 104 ovarian cancer cells were plated in 6-well plates. Two days after coincubation with ticagrelor (10 μM), in the presence or absence of platelets (20 × 106), cancer cells were treated with 10 mM EdU for 2 hours, washed 3 times with PBS, detached with 0.25% EDTA-trypsin, fixed with 4% paraformaldehyde for 15 minutes, stained with Alexa Fluor 647 (Invitrogen), and analyzed by flow cytometry (BCI Gallios Analyzer; Beckman Coulter).
CRISPR-Cas9–mediated P2Y12 and CD39 gene knockdown
Murine (m) P2Y12 CRISPR-Cas9 and homology-directed repair (HDR) mP2Y12 plasmids (Santa Cruz Biotechnology) were cotransfected into ID8-VEGF murine ovarian cancer cells by using lipofectamine. Human (h) CD39 CRISPR-Cas9 and HDR hCD39 plasmids (Santa Cruz Biotechnology) were cotransfected into A2780 human ovarian cancer cells by using lipofectamine. Briefly, 5 µg of mP2Y12 or hCD39-CRISPR plasmid was transfected into ID8-VEGF cells or A2780 along with 5 µg of mP2Y12 or hCD39-HDR by using Lipofectamine 3000 Transfection Reagent (Invitrogen) according to the manufacturer’s protocol. Twenty-four hours later, 4 µg/mL of puromycin was added to the media for 7 days. Puromycin-resistant cells were analyzed by quantitative reverse transcription polymerase chain reaction to determine their level of P2Y12 or hCD39 messenger RNAs (mRNAs).
siRNA transfection
Predesigned human (h)P2Y12- and hCD39-specific siRNAs were purchased from Sigma Aldrich. Two micrograms of siRNA was incubated in serum-free media (SFM) with 3 μL of lipofectamine (Invitrogen) for 30 min, and the mixture was added into 5 × 104 cells in 6-well plates and incubated in SFM for 6 hours. Transfected cells were coincubated in the presence or absence of platelets (20 × 106) for 2 days and used in cell proliferation assays.12
Immunostaining
Immunostaining of resected tumor nodules for Ki67, cleaved caspase 3 (CC3), and CD39 was performed on 4-μm thick, formalin-fixed, paraffin-embedded tumor nodules by using the method previously described.21 Briefly, slides were deparaffinized, antigen retrieval was performed, and endogenous peroxidases and nonspecific binding were blocked. After overnight incubation with the primary antibodies (1:50-1:200 dilution), slides were washed and incubated with horseradish peroxidase–conjugated secondary antibodies for 20 minutes and subsequently with horseradish peroxidase substrate.
Statistics
Statistical analysis was performed by using Graph-Pad Prism 6 software. Two-tailed Student t test was used to evaluate the differences between groups. Results are presented as mean ± standard error of the mean. For all statistical analyses, P < .05 was considered statistically significant.
Results
Ticagrelor reduced the growth of orthotopic ovarian tumors in mice
We compared the effect of ticagrelor (100 mg/kg), aspirin (150 mg/kg), or PBS (200 μL; placebo) given by daily gavage on the growth of orthotopic tumors induced by A2780 human ovarian cancer cells in nude mice (Figure 1A). The growth of ovarian tumors was dramatically decreased in the ticagrelor-treated mice compared with the aspirin- or placebo-treated mice (average tumor weight, 0.61 ± 0.166 g in ticagrelor-treated mice vs 1.57 ± 0.394 g in aspirin-treated mice vs 2.52 ± 0.805 g in placebo-treated mice; n = 10 per group; P = .027; Figure 1B-C). There was no statistically significant difference in the growth of orthotopic tumors between the placebo- and aspirin-treated groups (P = .33). The number of tumor nodules was also significantly lower in the ticagrelor group compared with the placebo and aspirin groups (4.1 ± 1.24 nodules per mouse in ticagrelor vs 24.4 ± 8.53 and 22.6 ± 3.66 nodules per mouse in the placebo group and the aspirin group, respectively; P = .026; Figure 1D).
To examine the effect of ticagrelor administration to mice on platelet function, we measured ADP-induced aggregation in platelets isolated from ticagrelor-treated (100 mg/kg for 3 days), placebo-treated, and P2Y12−/− mice. Ticagrelor reduced ADP-induced aggregation of WT platelets significantly to a level similar to that of P2Y12−/− platelets (placebo-treated group: 41.3% ± 4.3%; ticagrelor-treated group: 6.33% ± 0.8%; and P2Y12−/−: 6.67% ± 0.3%; n = 3, P = .001; supplemental Figure 1A). We did not detect any significant difference in collagen-induced aggregation in platelets isolated from ticagrelor-treated, placebo-treated, and P2Y12−/− mice (supplemental Figure 1B).
Ticagrelor reduced proliferation and increased apoptosis in ovarian cancer cells in vivo and in vitro
To study the effect of P2Y12 inhibition on the proliferation of cancer cells, we measured cell proliferation indices in the resected tumor nodules from ticagrelor-, aspirin-, or placebo-treated mice by using Ki67 immunostaining. We found that tumors resected from Ticagrelor-treated mice had a significantly lower percentage of Ki67 positivity compared with those from placebo-treated mice (78.5% ± 2.6% vs 60.3% ± 3.68%; P = .001, Student t test;Figure 1E). CC3 staining was performed to measure apoptosis indices in these tumors. Inhibition of the ADP receptor by using ticagrelor increased apoptosis in cancer cells (95 ± 9.67 cells per high-power field [HPF] in ticagrelor-treated vs 60 ± 3.65 cells per HPF in placebo-treated mice; n = 15 HPFs per mouse, n = 5 mice per group, P = .0001; Figure 1F). There were no significant differences in the apoptotic indices in tumors resected from aspirin-treated mice and placebo-treated mice (52 ± 4.05 cells per HPF in the aspirin group; P = not significant [n.s.]; Figure 1F). These data suggested that platelet activation through the P2Y12 receptor had pro-proliferative and antiapoptotic effects on ovarian cancer cells.
To investigate whether ticagrelor had any direct effect on cancer cell proliferation, we incubated human ovarian cancer cells (A2780ip2) with ticagrelor for 48 hours and measured cell proliferation by using the EdU incorporation assay (Figure 1G). Although, in the absence of platelets, the proliferation rates of buffer- and ticagrelor-treated cancer cells were not different (1.0 ± 0.06-fold increase in the control group vs 0.87 ± 0.07-fold increase in the ticagrelor-treated group; P = n.s.; Figure 1G), platelet-enhanced proliferation in A2780ip2, OVCAR8, OVCAR5, HeyA8, and OVCAR432 ovarian cancer cells (2.3 ± 0.5-, 2.03 ± 0.06-, 1.90 ± 0.23-, 1.76 ± 0.11-, and 3.54 ± 0.6-fold increases, respectively, compared with control cancer cells; n = 3) was abolished in the presence of ticagrelor (1.15 ± 0.2-, 0.2 ± 0.03-, 0.5 ± 0.03-, 0.99 ± 0.15-, and 1.86 ± 0.4-fold, respectively; n = 3) (Figure 1H).
To investigate whether aspirin had a similar inhibitory effect on platelet-enhanced cancer cell proliferation, we incubated human ovarian cancer cells (A2780ip2) with different concentrations of aspirin (0, 1, 3, or 5 mg/mL) in the presence or absence of platelets (20 × 106), and after 48 hours, we measured cell proliferation by using the EdU incorporation assay (supplemental Figure 1C). We found that aspirin did not reduce the platelet-enhanced cancer cell proliferation.
Ovarian cancer cells secreted ADP to the culture media. In 48 hours, A2780 cells increased the ADP concentration in SFM by 2.3 µM, and SKOV3ip1 increased it by 2.4 µM. Platelets became activated after incubation with various ovarian cancer cell lines (A2780, OVCAR8, OVCAR5, HeyA8, and OVCAR432), as was evident by an increase in the expression of CD62P (P-selectin) on platelets coincubated with cancer cells as compared with buffer-incubated platelets (supplemental Figure 1D).
Purinergic receptor P2Y12 on platelets mediated the progrowth effect of platelets on ovarian cancer
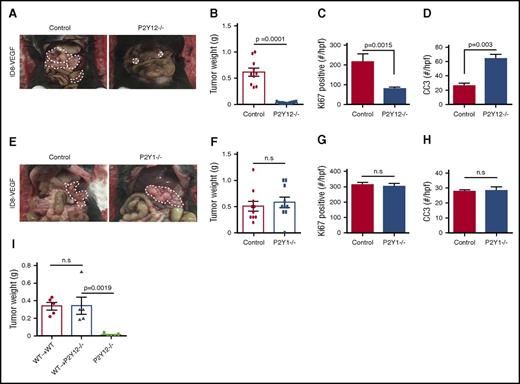
To further investigate the role of platelet ADP receptors on tumor growth and to distinguish between the effect of P2Y1 and P2Y12 receptors, we compared the growth of syngeneic ovarian tumors in P2Y12−/−, P2Y1−/−, and WT (C57BL/6 WT) mice. One million ID8-VEGF murine ovarian cancer cells were injected into the peritoneum of P2Y12−/−, P2Y1−/−, and WT mice, and tumors were resected 8 weeks after cancer cell injection. P2Y12 deficiency reduced tumor growth by 93% (average tumor weight, 0.61 ± 0.005 g in WT vs 0.04 ± 0.07 g in P2Y12−/− mice; n = 10 mice per group, P = .0001; Figure 2A-B). Proliferation and apoptosis indices were assessed in tumors resected from P2Y12−/− and WT mice. Tumors resected from P2Y12−/− mice showed lower proliferation and higher apoptosis indices compared with those from WT mice (proliferation: 78 ± 38.22 vs 217 ± 9.07 cells per HPF, P = .0015; apoptosis: 64 ± 8.63 per HPF vs 26 ± 3.69 cells per HPF, P = .003, respectively) (Figure 2C-D). We also compared tumor growth in P2Y1−/− and WT mice, but did not detect any significant difference (average tumor weight, 0.58 ± 0.09 g in P2Y1−/− vs 0.52 ± 0.13 g in WT mice; n = 10 per group, P = .72; Figure 2E-F). Proliferation and apoptosis indices were also not different between tumors resected from P2Y1−/− and WT mice (proliferation: 312 ± 14.17 per HPF vs 301 ± 19.29 per HPF, P = n.s.; Figure 2G; apoptosis: 27 ± 1.32/HPF vs 28 ± 2.55/HPF, P = n.s.; Figure 2H).
P2Y12 on platelets plays an important role in the growth of ovarian cancer. (A) Representative images of a necropsy on WT and P2Y12−/− tumor-bearing mice that carried tumors induced by intraperitoneal injection of ID8-VEGF murine ovarian cancer cells. (B) Mean aggregate tumor weight in WT and P2Y12−/− tumor-bearing mice (n = 10 mice per group). (C) Proliferation rate as quantified by the percentage of Ki67-positive cells in tumors resected from WT and P2Y12−/− tumor-bearing mice (n = 15 HPFs/5 mice per group). (D) Apoptosis rate as quantified by CC3 positivity in tumors from WT and P2Y12−/− mice (n = 15 HPFs/5 mice per group). (E) Representative images of a necropsy on WT and P2Y1−/− tumor-bearing mice that carried tumors induced by intraperitoneal injection of ID8-VEGF murine ovarian cancer cells. (F) Mean aggregate tumor weight in WT and P2Y1−/− tumor-bearing mice (n = 10 mice per group). (G) Proliferation rate as quantified by the percentage of Ki67-positive cells in tumors resected from WT and P2Y1−/− tumor-bearing mice (n = 15 HPFs/5 mice per group). (H) Apoptosis rate as quantified by CC3 positivity in tumors from WT and P2Y1−/− mice (n = 15 HPFs/5 mice per group). (I) Mean aggregate tumor weight after adoptive transfer of WT hematopoietic progenitor cells to lethally irradiated P2Y12−/− and WT recipient mice (WT→P2Y12−/− and WT→WT, respectively). Nontransplanted P2Y12−/− mice served as a negative control (n = 5 mice per group, P = .0019).
P2Y12 on platelets plays an important role in the growth of ovarian cancer. (A) Representative images of a necropsy on WT and P2Y12−/− tumor-bearing mice that carried tumors induced by intraperitoneal injection of ID8-VEGF murine ovarian cancer cells. (B) Mean aggregate tumor weight in WT and P2Y12−/− tumor-bearing mice (n = 10 mice per group). (C) Proliferation rate as quantified by the percentage of Ki67-positive cells in tumors resected from WT and P2Y12−/− tumor-bearing mice (n = 15 HPFs/5 mice per group). (D) Apoptosis rate as quantified by CC3 positivity in tumors from WT and P2Y12−/− mice (n = 15 HPFs/5 mice per group). (E) Representative images of a necropsy on WT and P2Y1−/− tumor-bearing mice that carried tumors induced by intraperitoneal injection of ID8-VEGF murine ovarian cancer cells. (F) Mean aggregate tumor weight in WT and P2Y1−/− tumor-bearing mice (n = 10 mice per group). (G) Proliferation rate as quantified by the percentage of Ki67-positive cells in tumors resected from WT and P2Y1−/− tumor-bearing mice (n = 15 HPFs/5 mice per group). (H) Apoptosis rate as quantified by CC3 positivity in tumors from WT and P2Y1−/− mice (n = 15 HPFs/5 mice per group). (I) Mean aggregate tumor weight after adoptive transfer of WT hematopoietic progenitor cells to lethally irradiated P2Y12−/− and WT recipient mice (WT→P2Y12−/− and WT→WT, respectively). Nontransplanted P2Y12−/− mice served as a negative control (n = 5 mice per group, P = .0019).
Adoptive transfer of hematopoiesis from WT mice to P2Y12−/− mice restored the growth of syngeneic ovarian cancer tumors
We transplanted lethally irradiated P2Y12−/− and WT (control) recipient mice with hematopoietic progenitor cells collected from the bone marrow of WT mice (WT→P2Y12−/− and WT→WT). Nontransplanted P2Y12−/− mice served as negative controls. Three weeks after bone marrow transplantation, platelet recovery was similar in WT→ WT and WT→ P2Y12−/− mice (platelet counts of 395.5 ± 64.28 × 109/L and 295.3 ± 56.73 × 109/L, respectively, P = .27; n = 5 mice per group). One week later (4 weeks after bone marrow transplant), 1 × 106 ID8-VEGF murine ovarian cancer cells were injected into the peritoneum of transplanted P2Y12−/− and WT mice (n = 5 mice per group). Seven weeks after cancer cell injection, orthotopic tumors were resected from moribund mice and compared among WT→P2Y12−/−, WT→WT, and P2Y12−/− mice. P2Y12−/− mice without transplantation developed very small tumors (0.02 ± 0.01 g), but tumor sizes in WT→P2Y12−/− and WT→WT mice were similar (0.34 ± 0.09 g in WT→P2Y12−/− and 0.34 ± 0.04 g in WT→WT mice; n = 5 mice per group, P = .97), and were significantly larger than those of P2Y12−/− mice (P = .0019; Figure 2I).
P2Y12 knockdown in cancer cells does not affect tumor growth
P2Y12 mRNA is expressed in many cell types, but P2Y12 protein has been detected only in platelets and glial cells.22,23 We investigated the ectopic expression of P2Y12 mRNA and protein in ovarian cancer cells. P2Y12 mRNA was detectable in ovarian cancer cells, although in a much smaller quantity than platelets (supplemental Figure 1E), but we could not detect any P2Y12 protein in ovarian cancer cells (data not shown). To confirm that P2Y12 on platelets, and not on ovarian cancer cells, is responsible for platelet-induced cancer cell proliferation and platelet-enhanced tumor growth, we reduced the P2Y12 mRNA level using CRISPR-Cas9–mediated gene knockdown in ID8-VEGF cells by 95% (n = 3 triplicates, P < .0001; Figure 3A). P2Y12 knockdown or WT ID8-VEGF cells were used in the syngeneic murine model of ovarian cancer. There was no significant difference in the aggregate weight of tumor nodules induced by P2Y12 knockdown or WT ID8-VEGF cells in WT C57BL/6 mice (tumors induced by P2Y12 knockdown ID8-VEGF cells, 0.84 ± 0.97g vs WT cells, 0.69 ± 0.11 g; n = 10 mice per group, P = .3; Figure 3B-C). We also investigated the effect of siRNA-mediated P2Y12 knockdown in human ovarian cancer cells on the behavior of these cells in vivo and in vitro. We used hP2Y12 siRNA to knockdown any possible expression of P2Y12 in A2780ip2 and OVCAR8 human ovarian cancer cell lines. hP2Y12 siRNA was human-specific and did not affect murine P2Y12 gene expression (supplemental Figure 1F-G). One million A2780ip2 or OVCAR8 human ovarian cancer cells were injected into nude mice (10 mice per group). Starting 1 week after injection of ovarian cancer cells, hP2Y12 siRNA conjugated with DOPC nanoliposomes was injected into the peritoneal cavity of tumor-bearing mice twice per week for 5 weeks. Control mice underwent the same procedures, except that they received scrambled siRNA-DOPC. There was no difference in the final tumor size between mice receiving hP2Y12 siRNA and mice receiving scrambled siRNA (in A2780ip2-induced tumors: average tumor weight, 1.95 ± 0.74 g in hP2Y12 siRNA vs 2.29 ± 0.57 g in scrambled siRNA; n = 10 mice per group; supplemental Figure 1H-I; and in OVCAR8-induced tumors: average tumor weight, 0.27 ± 0.09 g in hP2Y12 siRNA vs 0.22 ± 0.05 g in scrambled siRNA; n = 10 mice per group; supplemental Figure 1J-K). Incubation of human ovarian cancer cells in vitro with ADP, ticagrelor, or apyrase (in the absence of platelets), or hP2Y12 siRNA did not change the proliferation rate in cancer cells (supplemental Figure 1L).
Effect of P2Y12 gene knockdown in murine ovarian cancer cells on the growth of orthotopic ovarian tumors. (A) Expression of P2Y12 mRNA in control and mP2Y12 CRISPR-Cas9 ID8-VEGF cells before the injection into mice (n = 3 triplicate experiments). (B) Representative images of necropsy in mice carrying control or mP2Y12 CRISPR-Cas9 ID8-VEGF cell–induced tumors. (C) Mean aggregate tumor weight in mice carrying control or mP2Y12 CRISPR-Cas9 ID8-VEGF cell–induced tumors (n = 10 per group). (D-E) Effect of ecto-apyrase (CD39) gene knockdown using hCD39 siRNA on the platelet-induced increase in the proliferation rate in A2780ip2 (D) and SKOV3ip1 (E) human ovarian cancer cells (n = 3 triplicate experiments). (F) Expression of CD39 in tumor nodules induced by WT A2780ip2 or A2780ip2-CD39 CRISPR-Cas9 cells. (G) Mean tumor weight aggregate in mice carrying tumors induced by WT or A2780ip2-CD39 CRISPR-Cas9 cells (n = 6 mice per group).
Effect of P2Y12 gene knockdown in murine ovarian cancer cells on the growth of orthotopic ovarian tumors. (A) Expression of P2Y12 mRNA in control and mP2Y12 CRISPR-Cas9 ID8-VEGF cells before the injection into mice (n = 3 triplicate experiments). (B) Representative images of necropsy in mice carrying control or mP2Y12 CRISPR-Cas9 ID8-VEGF cell–induced tumors. (C) Mean aggregate tumor weight in mice carrying control or mP2Y12 CRISPR-Cas9 ID8-VEGF cell–induced tumors (n = 10 per group). (D-E) Effect of ecto-apyrase (CD39) gene knockdown using hCD39 siRNA on the platelet-induced increase in the proliferation rate in A2780ip2 (D) and SKOV3ip1 (E) human ovarian cancer cells (n = 3 triplicate experiments). (F) Expression of CD39 in tumor nodules induced by WT A2780ip2 or A2780ip2-CD39 CRISPR-Cas9 cells. (G) Mean tumor weight aggregate in mice carrying tumors induced by WT or A2780ip2-CD39 CRISPR-Cas9 cells (n = 6 mice per group).
Knockdown of the ecto-apyrase (CD39) gene in ovarian cancer cells enhanced platelet-induced cancer cell proliferation in vitro and increased tumor growth in vivo
We found that ovarian cancer cells express ecto-apyrase (CD39; ENTPD1). SKOV3ip1 human ovarian cancer cells expressed 18-fold higher and A2780ip2 human ovarian cancer cells expressed 35-fold higher CD39 mRNA levels compared with the endothelial cell line (RF24 cells) (supplemental Figure 2A). We reduced expression of the CD39 gene in SKOV3ip1 and A2780ip2 human ovarian cancer cells using hCD39 siRNAs (supplemental Figure 2B-C) and investigated whether CD39 gene knockdown in cancer cells increases platelet-induced cancer cell proliferation. After incubation with platelets, hCD39 siRNA-transfected A2780ip2 cells had a 4.12. ± 0.55-fold increase and scrambled siRNA-transfected cells had a 2.67 ± 0.22-fold increase in the cell proliferation rate (P < .02, Figure 3D). The platelet-enhanced proliferation rate for CD39 siRNA-transfected and scrambled siRNA-transfected SKOV3ip1 cells increased by 4.19 ± 0.52-fold and 2.85 ± 0.24-fold, respectively (P < .05, Figure 3E). In the absence of platelets, CD39 gene knockdown in ovarian cancer cells did not affect cell proliferation.
We investigated the effect of CRISPR-Cas9-mediated CD39 gene knockdown in A2780ip2 ovarian cancer cells on the growth of the orthotopic tumors in mice. Tumor nodules induced by A2780ip2-CD39 CRISPR-Cas9 cells in nude mice did not express CD39 (Figure 3F) and were 4 times larger than those induced by WT A2780ip2 cells (Figure 3G) (1.23 ± 0.33 g vs 0.26 ± 0.11 g, respectively; n = 6; P = .019). These data suggest that knockdown of ecto-apyrase in ovarian cancer cells enhances the pro-proliferative effect of platelets on cancer cells.
Discussion
Many cancer patients, including one-third of patients with ovarian cancer, develop paraneoplastic thrombocytosis, which is associated with a worse prognosis.6 Several studies have shown that platelets and cancer cells interact, and elevated platelet counts may affect cancer behavior and aggressiveness. In fact, we have shown that lowering platelet counts, in the absence of any anticancer reagents, reduced tumor growth in murine models of ovarian cancer6 and, conversely, that increasing platelets counts enhanced tumor growth in these mice.8 The effect of platelet number on ovarian cancer persisted in the presence of chemotherapy (ie, lower platelet counts increased chemosensitivity and higher platelet counts caused chemoresistance).8
Several ovarian cancer cell lines activate platelets, and, among them, highly metastatic cell lines activate platelets the most.24 The mechanism of TCIPA has been extensively studied, and ADP was found to be a major mediator of TCIPA10,25-28 and to play an important role in metastasis.29,30 We found that ovarian cancer cells secrete a significant amount of ADP. Scavenging ADP by apyrase and blocking ADP receptors on platelets inhibit TCIPA.10,24,31,32
Activated platelets in turn enhance epithelial–mesenchymal transition in cancer cells33 and guide the formation of an early metastatic niche with the help of neutrophils,34 both promoting metastasis.35 ATP released from activated platelets, acting through P2Y2 receptors on endothelial cells, promotes extravasation of cancer cells during metastasis.36 We have shown that platelets not only promote metastasis, but also enhance the growth of the primary tumors.6,12 Both prometastasis and progrowth effects of platelets on cancer cells depend on the secretion of cytokines, such as TGF-β,12,33 CXCL5, and CXCL7,34 that are released from degranulating platelets. We have shown that blocking Tgf-β1 secreted from platelets or reducing Tgf-β1 receptors on ovarian cancer cells decreased the pro-proliferative effect of platelets on cancer cells.12 The presence of a positive feedback loop between cancer cell–induced platelet activation and activated platelet–induced cancer cell proliferation and epithelial–mesenchymal transition explains the protumor consequences of the interaction between platelets and cancer cells and points to potential therapeutic targets in this interaction. Because of the important role of ADP as a platelet agonist in TCIPA and because our findings point to ovarian cancer cells as the source of ADP, we hypothesized that blocking ADP receptors on platelets might reduce the progrowth effect of platelets on cancer cells.
ADP receptors on platelets, P2Y12 and P2Y1, are G protein–coupled receptors and mediate ADP-induced platelet activation. The P2Y1 receptor couples to the Gq protein that activates phospholipase Cβ, increases cytosolic calcium levels, and activates protein kinase C. P2Y12 couples to the Gi protein that negatively regulates adenylyl cyclase and activates phosphatidylinositol 3-kinase.37-40 Activation of P2Y12 results in degranulation, and activation of P2Y1 results in shape changes in platelets.41,42 In this study, we showed that ticagrelor, a reversible P2Y12 inhibitor, decreased proliferation and increased apoptosis in ovarian cancer cells in vitro and reduced the growth of primary tumors in murine models of ovarian cancer. We compared the antitumor effect of ticagrelor with that of aspirin and found that ticagrelor-treated mice developed smaller tumors than aspirin-treated mice. To rule out the possibility of a P2Y12-independent ticagrelor effect, we compared the growth of syngeneic ovarian cancer in P2Y12-deficient mice and WT mice and found that P2Y12 deficiency was associated with a reduction in tumor growth, supporting a specific role for P2Y12 receptors in the growth of ovarian cancer. Next, we examined whether the other ADP receptor on platelets (P2Y1) has a similar effect on tumor growth, but we found that the lack of P2Y1 did not have any impact on the growth of tumor nodules induced by syngeneic ovarian cancer cells in mice. We confirmed the importance of platelet P2Y12 by showing reestablished tumor growth in P2Y12 −/− mice after adoptive transfer of WT hematopoietic progenitor cells. The importance of ADP in platelet–cancer cell interaction was further supported by our data that showed an additional increase in platelet-induced proliferation after knockdown of the ecto-apyrase (CD39) gene43,44 in ovarian cancer cells, a reduction in platelet-induced proliferation after exposure of ovarian cancer cells to recombinant apyrase, and an increase in the size of tumors after knockdown of the CD39 gene in ovarian cancer cells. Interestingly, the spontaneous development of hepatocellular carcinoma in CD39-deficient mice has been reported.45
In the absence of platelets, exposure of ovarian cancer cells to ADP, ticagrelor, or apyrase and knockdown of P2Y12 or ecto-apyrase genes in cancer cells did not affect cancer cell proliferation in vitro or tumor growth in vivo, pointing toward the importance of a P2Y12-dependent ADP role in the interaction between platelets and cancer cells and in tumor growth.
Ectopic expression of P2Y12 could not be detected at the protein level in ovarian cancer cells, and even expression of P2Y12 at a level undetectable by Western blotting has no role in tumor growth because P2Y12 knockdown (siRNA or CRISPR-Cas9) in ovarian cancer cells did not affect their proliferation in vitro and the growth of their induced tumors in vivo.
Our finding on the anticancer effect of ticagrelor is not the first observed anticancer effect of a platelet antagonist. The effect of daily, low-dose aspirin (<100 mg) in primary and secondary cancer prevention has been shown in several meta-analyses using large epidemiologic data.46,47 The anticancer effect of aspirin has been attributed to its inhibitory effect on COX2 in epithelial cells.48 However, because of the short half-life of aspirin (t1/2 = 20 min) and rapid regeneration of COX1 and COX2 in nucleated cells, the main effect of aspirin is on platelets. Inhibition of COX2 requires a high dose of aspirin (∼1000 mg daily), whereas the anticancer effect of aspirin is mainly evident at a lower dose (<100 mg daily) that is only capable of inhibiting COX1 in platelets. From the abovementioned observations, one can conclude that the anticancer effect of aspirin might be due to its antiplatelet effect. There are several caveats that epidemiologic studies on the effect of aspirin in cancer prevention might teach us about studying the effect of other antiplatelet agents in cancer. One important point is that the anticancer effect of aspirin becomes evident after 5 years of aspirin therapy, and studies of a shorter duration might not show any benefit.46 Another important point is that the beneficial effect of aspirin is more evident in certain types of cancer and for certain genetic backgrounds.47,49
We should be cautious in reaching any clinical conclusions based on the results of studies in murine models of cancer. We propose that if additional studies confirm our data on the beneficial effect of ticagrelor in murine models of ovarian cancer, it is reasonable to investigate the therapeutic use of ticagrelor in patients who are at high risk of developing ovarian cancer (primary prevention) or in patients with ovarian cancer (secondary prevention or treatment) to carefully examine the balance between the possible antitumor effects vs the potential increase in the risk of bleeding. Although we did not observe any visceral or intratumor bleeding in tumor-bearing mice treated with ticagrelor, one should consider that hemostasis and thrombosis in mice are significantly different than in humans. The results of these studies will be useful in targeting the platelet–ovarian cancer interaction as a possible new preventive or therapeutic strategy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health, National Cancer Institute grant R01CA177909 (V.A-K. and A.K.S.) and the Ovarian Cancer Research Fund (grant 258813) (V.A-K. and A.K.S.), National Institutes of Health, National Cancer Institute grants CA083639 and CA016672, the American Cancer Society Research Professor Award, and the Frank McGraw Memorial Chair in Cancer Research (A.K.S.).
Authorship
Contribution: M.S.C. designed and conducted the experiments, analyzed and interpreted the data, and wrote the manuscript; K.N., M.H., H.P., Q.H., T.H., T.M., and S.L.C.M. performed the experiments and analyzed the data; D.L. and Q.M. performed the adoptive transplantation and interpreted the data; S.K. provided valuable reagents and participated in designing the experiments; A.K.S. designed the experiments, interpreted the data, and supervised the study; V.A.-K. designed the experiments, analyzed and interpreted the data, wrote the manuscript, and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vahid Afshar-Kharghan, Department of Benign Hematology, University of Texas MD Anderson Cancer Center, 2121 West Holcombe Blvd, Suite 7.16, Unit 1100, Houston, TX 77030; e-mail: vakharghan@mdanderson.org; and Anil K. Sood, Department of Gynecologic Oncology and Reproductive Medicine, University of Texas MD Anderson Cancer Center, Dan L. Duncan Building (CPB6.3275), 1515 Holcombe Blvd, Unit 1362, Houston, TX 77030; e-mail: asood@mdanderson.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal