In this issue of Blood, Cho et al demonstrate that blocking adenosine 5′-diphosphate (ADP) signaling to the P2Y12 receptor on platelets reduces the growth of ovarian cancer cells, implicating the P2Y12 inhibitor ticagrelor as a potential therapeutic option for primary and secondary prevention of ovarian cancer.1
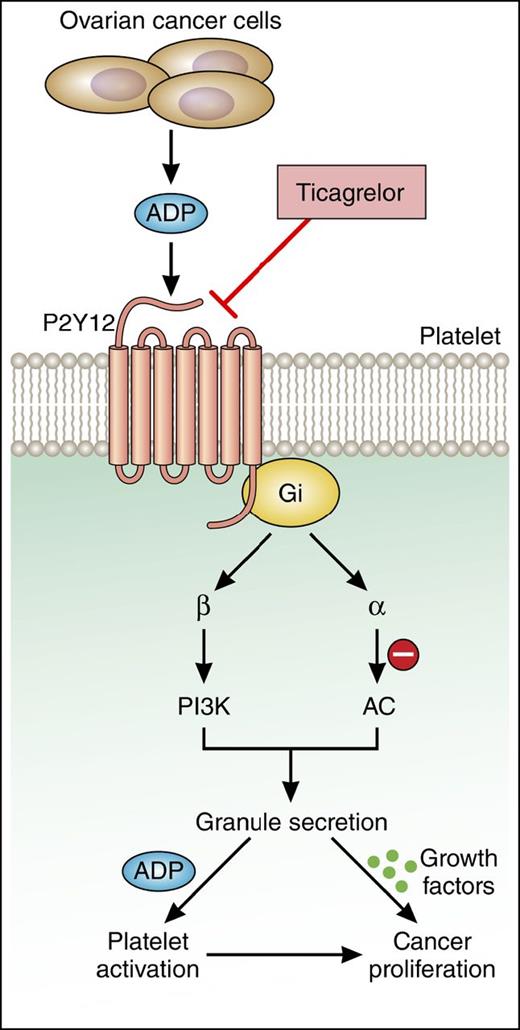
The central role of P2Y12 receptor in platelet-induced cancer cell proliferation. Cho et al demonstrate that platelet-induced proliferation and growth of ovarian cancer cells requires platelet activation mediated by ADP-P2Y12 signaling. The use of the P2Y12 antagonist ticagrelor may be a useful therapeutic approach to limit ovarian cancer growth. AC, adenylyl cyclase; Gi, inhibitory regulative G-protein; PI3K, phosphatidylinositol 3-kinase. Professional illustration by Patrick Lane, ScEYEnce Studios.
The central role of P2Y12 receptor in platelet-induced cancer cell proliferation. Cho et al demonstrate that platelet-induced proliferation and growth of ovarian cancer cells requires platelet activation mediated by ADP-P2Y12 signaling. The use of the P2Y12 antagonist ticagrelor may be a useful therapeutic approach to limit ovarian cancer growth. AC, adenylyl cyclase; Gi, inhibitory regulative G-protein; PI3K, phosphatidylinositol 3-kinase. Professional illustration by Patrick Lane, ScEYEnce Studios.
It is now well established that blood platelets play a key role in facilitating cancer metastasis, enabling cancer cells to successfully progress from primary tumors to secondary niches in distant tissues. Moreover, proliferation and growth of cancer cells has also been demonstrated to be driven, in part, by activated platelets.2,3 Indeed, various studies have shown that upon activation platelets release a number of factors that stimulate cancer cell proliferation and survival, including transforming growth factor β1 and platelet-derived growth factor.2-4 Accordingly, when cocultured in vitro with tumor cells, platelets stimulate cancer cell proliferation as measured by Ki67 levels in ovarian, colon, and pancreatic cancer cells.2,3 In addition, platelet depletion studies suggest that platelets are essential for the growth of select tumors in vivo.5 It appears that platelets promote cancer proliferation via both direct and indirect routes through stimulation of oncoproteins and tumor angiogenesis, respectively.3,4,6 The fact that cancer proliferation is in part dependent on platelet-derived bioactive molecules provides rationale for the use of antiplatelet therapies to interdict or prevent the signaling events that drive cancer growth.
The ability of platelets to promote cancer proliferation and metastasis is highly dependent on the state of platelet activation. Perhaps akin to a self-fulling prophecy, cancer cells themselves can activate platelets both via direct platelet–tumor cell interactions7-9 and indirectly through the release of soluble molecules such as ADP, as elegantly shown by Cho et al. Their study demonstrates that ovarian cancer cells secrete ADP to induce platelet activation downstream of the platelet ADP receptor, P2Y12 (see figure). In turn, activated platelets promote ovarian tumor cell growth. In an orthotopic mouse model, treatment with the P2Y12 inhibitor ticagrelor suppressed the growth of primary ovarian tumors. Similarly, mice deficient in the platelet receptor P2Y12 exhibited an 85% reduction in ovarian tumor growth relative to wild-type mice. To confirm the role of P2Y12 in platelet-induced cancer proliferation, the authors demonstrated that tumor growth could be rescued in irradiated P2Y12−/− mice by reconstituting hematopoiesis. Collectively, these data provide promising insights into the potential therapeutic benefit of ticagrelor in reducing the ability of platelets to promote ovarian cancer cell growth.
By identifying a central role for ADP in platelet-induced cancer proliferation and growth, this study then raises several new questions: (1) What is the nature of the molecular signals and underlying pathways that induce cancer cells to release ADP? (2) At what concentration is ADP released by cancer cells sufficient to activate platelets in the bloodstream? (3) What is the nature of the bioactive cargo deriving from ADP-P2Y12-activated platelets that drive and sustain proliferation programs in cancer cells?
Platelets have been shown to trigger an epithelial-mesenchymal–like transition in cancer cells while in the circulation.4,10 ADP is considered a weak platelet agonist necessary for the amplification and positive feedback required to form a stable platelet clot. It is unclear what minimum number of ovarian cancer cells is required to generate sufficient concentrations of ADP to activate platelets, or whether platelet activation occurs systemically in the circulation or locally at primary or secondary sites of tumor growth. Along these lines, it is unknown whether secretion of ADP by either single circulating tumor cells (CTCs) or CTC clusters is sufficient to promote platelet activation and what effect this might have on the survival and metastatic success of CTCs. Perhaps the rate of ADP secretion by CTCs may serve as a useful biomarker for deciding which patients may benefit from the use of P2Y12 inhibitors as part of their cancer therapy. Alternatively, uncovering the mechanisms underlying the secretion of ADP from tumor cells may help identify novel druggable targets to prevent ADP-mediated platelet activation by targeting the tumor cells rather than systemically inhibiting platelet function, which carries the inherent risk of causing bleeding. In conclusion, the novel findings presented by Cho et al provide rationale for the development of new approaches and therapies specifically designed to prevent platelet-driven cancer proliferation and highlight the key role that platelets play in oncogenesis and metastasis.
Conflict-of-interest disclosure: The authors declare no competing financial interests

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal