Abstract
The deregulation of polycomb repressive complexes (PRCs) has been reported in a number of hematological malignancies. These complexes exert oncogenic or tumor-suppressive functions depending on tumor type. These findings have revolutionized our understanding of the pathophysiology of hematological malignancies and the impact of deregulated epigenomes in tumor development and progression. The therapeutic targeting of PRCs is currently attracting increasing attention and being extensively examined in clinical studies, leading to new therapeutic strategies that may improve the outcomes of patients with hematological malignancies.
Introduction
Epigenetic regulation is important in gene expression because of its modulation of the chromatin structure and function.1 Recent advances in genomic and epigenetic research have revealed a central role for aberrant epigenetic regulation in the pathogenesis of hematological malignancies.2,3 Among epigenetic regulators, many of the genes encoding DNA and histone modifiers are targeted by somatic gene mutations and deletions or are involved in chromosomal abnormalities, highlighting their causative role in tumor development. Polycomb group (PcG) genes encoding histone modifier proteins are representative epigenetic genes that are deregulated in many hematological malignancies. In this review, I summarize the current knowledge on polycomb dysfunctions in hematological malignancies as well as therapeutic approaches that target PcG complexes.
Function of PRCs in hematopoiesis
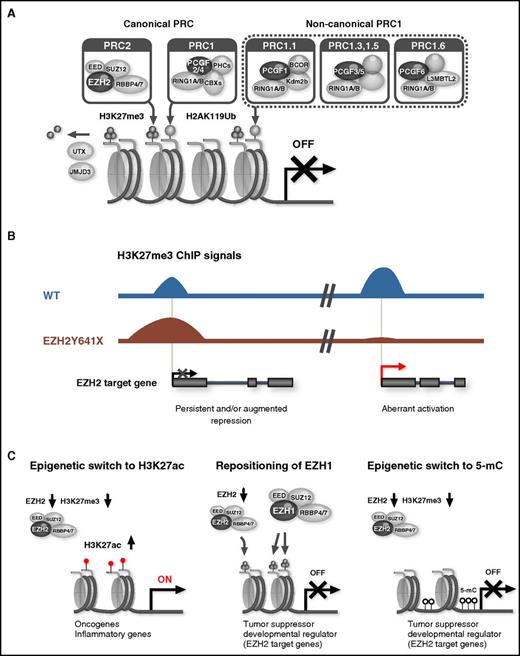
PcG proteins form multiprotein complexes that play an important role in maintaining the transcriptional repression of target genes through chromatin modifications. Canonical polycomb repressive complex 1 (PRC1) and PRC2 have been characterized in detail.4 PRC1 and PRC2 exhibit catalytic activities that are specific to the monoubiquitination of histone H2A at lysine 119 (H2AK119ub1) and the mono-, di-, and trimethylation of histone H3 at lysine 27 (H3K27me1/2/3), respectively. After the recruitment of PRC2 to chromatin, PRC2 trimethylates H3K27 (H3K27me3), which, in turn, recruits canonical PRC1 via the CBX subunit that binds to H3K27me3. In contrast, variant PRC1 complexes, which have recently been identified, are proposed to catalyze H2AK119ub1 modifications independently of PRC2 activity or the H3K27me3 mark. H2AK119ub1 then promotes the recruitment of PRC2 and potentiates its catalytic activity (Figure 1A).5 Thus, PRC1 and PRC2 function in concert or independently to establish and maintain gene silencing. However, PRC2 may activate transcription in a specific setting. During erythroid differentiation, EZH1 and SUZ12 have been shown to assemble a noncanonical PRC2 complex independent of EED and positively regulate gene expression.6 EZH2 also has nonhistone targets and regulates cellular processes such as actin polymerization in the cytoplasm through its methyltransferase activity.7
PcG complexes and aberrant polycomb functions in hematological malignancies. (A) Composition of canonical and noncanonical PcG complexes. (B) Oncogenic functions of activating EZH2 mutants. EZH2Y641 mutants (Y641E, Y641F, Y641N, Y641S, Y641C, and Y641H) increase the global abundance of H3K27me3 and reinforce the repression of EZH2 target genes (left). They also cause the widespread redistribution of H3K27me3, inducing the aberrant activation of EZH2 target genes (right). ChIP, Chromatin ImmunoPrecipitation; WT, wild type. (C) Tumor suppressive function of PRC2. An EZH2 insufficiency induces the activation of PRC2 target genes via a methylation-to-acetylation switch at H3K27 at promoters, thereby conferring a growth advantage to malignant clones (left). Key tumor suppressor and developmental regulator genes are largely maintained in a transcriptionally repressed state in EZH2-insufficient hematopoietic stem and progenitor cells (HSPCs) via the locus-specific repositioning of EZH1 to the EZH2 target loci (middle) and an epigenetic switch from H3K27me3 to DNA methylation at EZH2 target genes (right).
PcG complexes and aberrant polycomb functions in hematological malignancies. (A) Composition of canonical and noncanonical PcG complexes. (B) Oncogenic functions of activating EZH2 mutants. EZH2Y641 mutants (Y641E, Y641F, Y641N, Y641S, Y641C, and Y641H) increase the global abundance of H3K27me3 and reinforce the repression of EZH2 target genes (left). They also cause the widespread redistribution of H3K27me3, inducing the aberrant activation of EZH2 target genes (right). ChIP, Chromatin ImmunoPrecipitation; WT, wild type. (C) Tumor suppressive function of PRC2. An EZH2 insufficiency induces the activation of PRC2 target genes via a methylation-to-acetylation switch at H3K27 at promoters, thereby conferring a growth advantage to malignant clones (left). Key tumor suppressor and developmental regulator genes are largely maintained in a transcriptionally repressed state in EZH2-insufficient hematopoietic stem and progenitor cells (HSPCs) via the locus-specific repositioning of EZH1 to the EZH2 target loci (middle) and an epigenetic switch from H3K27me3 to DNA methylation at EZH2 target genes (right).
Human additional sex combs like 1 (ASXL1) is one of the homologs of the additional sex combs (Asx) gene, which encodes a chromatin-binding protein that regulates the balance between trithorax and polycomb functions in Drosophila.8 ASXL1 has been shown to associate with PRC2, but not PRC1, and the loss of ASXL1 results in global reductions in H3K27me3 levels, suggesting its critical function associated with PRC2.9
Polycomb complexes function as general regulators of stem cells and maintain the self-renewal capacity and multipotency of hematopoietic stem cells (HSCs).10 Canonical PRC1 containing BMI1/PCGF4 and PRC2 play central roles in this process and transcriptionally repress the CDKN2A locus, the critical target of PcG complexes for maintaining the self-renewal capacity of HSCs.11-15 They also repress a cohort of developmental regulator genes to maintain the immature state and multipotency of HSPCs.16 The forced expression of Bmi1, Ezh2, Cbx7, or Kdm2b/Fbxl10 in mouse HSCs promotes their self-renewal and efficiently preserves HSC potential during serial transplantations.17-20 In contrast, mice with a heterozygous loss-of-function allele of Suz12, Ezh2, or Eed display enhanced HSC activity,21,22 suggesting that PRC2 also has a tumor-suppressive function.
Oncogenic function of PRCs
Because of their function in somatic stem cells, PcG genes have been characterized as oncogenes.10,23 Bmi1 was initially identified as an oncogene that collaborates with the Eμ-myc transgene in murine pre–B-cell lymphomagenesis.24 Its oncogenic function largely depends on its capacity to transcriptionally repress the Ink4a locus encoding p16Ink4a and p19Arf.25 Bmi1 is also essential for maintaining the proliferative activity of leukemic stem cells as well as the leukemic transformation of hematopoietic progenitor cells by the MLL-AF9 fusion gene in mice.26,27 BMI1 collaborates with BCR-ABL in the leukemic transformation of human CD34+ cells.28 The expression of BMI1 has been correlated with disease progression and prognosis of myelodysplastic syndrome (MDS) as well as the prognoses of acute myeloid leukemia (AML) and chronic myeloid leukemia.29-31 Although BMI1 gene amplification was previously reported in 4 (11%) of 36 cases of mantle cell lymphoma,32 alterations in the BMI1 gene on chromosome 10p13 are generally uncommon in hematological malignancies. Somatic BMI1 gene mutations have not yet been reported. The aberrant regulation of PRC2 functions and H3K27me3 modifications has been reported in various cancers.10,23,33 EZH2 was found to be overexpressed and/or amplified in prostate, breast, bladder, and colon cancers and its expression correlated with metastasis and poor prognosis. We and other groups have shown that PRC2 is required for MLL-AF9 leukemia in mice and demonstrated that Ezh2 inhibits differentiation programs in leukemic stem cells, thereby augmenting their leukemogenic activity.34,35
Next-generation sequencing has led to a paradigm shift in our understanding of PcG functions, revealing monoallelic gain-of-function mutations in EZH2 in germinal center (GC) B-cell–type diffuse large B-cell lymphoma and follicular lymphoma.36 EZH2 is strongly expressed in GC B cells and is required for the formation of GCs. EZH2 represses proliferation checkpoint genes and transcriptional programs required for exiting the GC reaction and terminal differentiation to transiently suppress GC B-cell differentiation.37 EZH2 cooperates with BCL6 to recruit a noncanonical PRC1-BCOR complex containing CBX8 in a GC B-cell–specific manner to repress differentiation gene expression.38 The conditional expression of mutant EZH2 (EZH2Y641N) in mice has been shown to induce GC hyperplasia and accelerate lymphomagenesis.37,39 Mutant EZH2 not only increased the global abundance of H3K27me3 but also caused the widespread redistribution of this repressive mark, suggesting that mutant EZH2 induces lymphoma through a vast reorganization of the chromatin structure, inducing the persistent repression and aberrant activation of EZH2 target genes (Figure 1B).39
The silencing of PRC2 targets is also reinforced in advanced stages of multiple myeloma cells.40,41 Furthermore, deletions and inactivating mutations in KDM6A (also known as UTX), which encodes a demethylating enzyme that removes methyl residues from H3K27me2/3, have been observed in 3.2% to 10% of patients with multiple myeloma and are associated with shorter overall survival.42,43 The t(4;14) translocation, which involves WHSC1/MMSET encoding an H3K36 methyltransferase, and KDM6A mutations are potentially mutually exclusive.42 Although activating EZH2 mutations have not yet been identified in multiple myeloma, the inactivation of KDM6A supports an oncogenic role for PRC2 in multiple myeloma. KDM6A is also targeted by loss-of-function mutations in 5% to 15% of T-cell acute lymphoblastic leukemia (T-ALL) and functions as a tumor suppressor by modulating H3K27me3 modifications at the promoters of tumor-suppressor genes.44,45 Mutations in KDM6A, which is located on the X chromosome, are exclusively present in male patients with T-ALL, and T-ALL driven by the inactivation of KDM6A exhibits collateral sensitivity to the pharmacological inhibition of H3K27me3.45 In contrast, another H3K27 demethylase, JMJD3, is essential for the initiation and maintenance of T-ALL, because it controls important oncogenic genes; thus, it is a potential therapeutic target in T-ALL.44 Of interest, UTX also functions as a pro-oncogenic cofactor of TAL1 in TAL1+ T-ALL and removes H3K27me3 at the TAL1 target gene loci, thereby promoting the establishment of a TAL1-mediated leukemic gene expression program.46 UTX is required specifically in TAL1+ TALL, whereas UTX overexpression suppresses cell growth in TAL1− T-ALL,46 which is consistent with its role as a tumor suppressor in T-ALL described previously in this paragraph.44,45 The function of PRC2 is also augmented in adult T-cell leukemia cells, in which EZH2 is upregulated by HTLV-1 Tax and an activated NFκB signal.47
Tumor-suppressive function of PRCs
EZH2, located at chromosome 7q36.1, is frequently involved in chromosomal abnormalities such as −7 and 7q−, which are associated with very poor prognosis in hematological malignancies.48 A functional mapping study using induced pluripotent stem cells derived from a patient with 7q− MDS revealed a causative role for an EZH2 haploinsufficiency in the defective hematopoiesis of 7q− MDS clones, suggesting a tumor-suppressive function for EZH2.49 Correspondingly, next-generation sequencing also identified deletions and inactivating mutations in EZH2 that abrogate its methyltransferase activity in patients with MDS (3% to ∼13%), myeloproliferative neoplasms (MPNs) (3% to ∼13%), and MDS/MPN-overlapping disorders (8% to ∼15.6%) (Table 1). 2,50-56 Other components of PRC2, EED and SUZ12, are also targeted by somatic-inactivating mutations, although the frequencies of their mutations are markedly lower than those of EZH2 mutations (Table 1).2,57,58 Collectively, these findings suggest that PRC2 also functions as a tumor suppressor. Loss-of-function mutations have been detected in both monoallelic and biallelic states.51 Patients with MDS or MDS/MPNs with mutations in EZH2 show significantly poorer outcomes than patients without mutations,51,52 and survival was found to be slightly shorter in patients with homozygous mutations than in those with heterozygous mutations.51 EZH2 mutations have also been shown to predict poor survival in patients with primary myelofibrosis regardless of the presence of JAK2 mutations.59 However, PRC2 mutations are rare in de novo AML, but they are present in secondary AML.2,60,61 Spliceosomal gene mutations are frequently identified in hematological malignancies, particularly in patients with MDS. Of note, SRSF2 and U2AF1 mutants were shown to cause the missplicing of EZH2.62,63 SRSF2 mutants promote the inclusion of a cassette exon that results in a premature terminal codon (PTC)–containing isoform of EZH2 messenger RNA. The PTC-containing isoform of EZH2 undergoes degradation by nonsense-mediated decay, resulting in decreased levels of EZH2 messenger RNA. The link between SRSF2 mutations and insufficient EZH2 expression may explain the mutual exclusivity between SRSF2 and EZH2 mutations in patients with MDS.62 Thus, multiple mechanisms deregulate EZH2 in myeloid malignancies. Indeed, EZH2 expression in CD34+ HSPCs is significantly weaker in patients with MDS, particularly those with chromosome 7 abnormalities, than in healthy individuals.15,64
Mutations in PcG and PcG-associated genes in hematological malignancies
| Malignancy . | EZH2, % . | EED, % . | SUZ12, % . | ASXL1, % . | Reference . |
|---|---|---|---|---|---|
| MDS | 3-13 | Rare | Rare | 10.6-18.5 | 50-55 |
| MPNs | 3 (PV) | Rare | Rare | 1-3 (ET/PV) | 51,56-58 |
| 5-13 (PMF) | 25 (PMF) | ||||
| MDS/MPNs | 8-15.6 | 1 | 1.4 | 15.6-43 | 51,58 |
| AML | Rare | Rare | Rare | 9-18 | 51,60,61 |
| DS-AMKL | 32.7 | 0 | 2 | 2 | 76 |
| T-ALL* | 18 | ND | 7 | 0 | 78 |
| ETP-ALL† | 12.7 | 12.7 | 17.5 | 0 | 77 |
| Non-ETP† | 3.3-4.8 | 3.3-7 | 0-2.4 | 0 | 77,79 |
| Malignancy . | EZH2, % . | EED, % . | SUZ12, % . | ASXL1, % . | Reference . |
|---|---|---|---|---|---|
| MDS | 3-13 | Rare | Rare | 10.6-18.5 | 50-55 |
| MPNs | 3 (PV) | Rare | Rare | 1-3 (ET/PV) | 51,56-58 |
| 5-13 (PMF) | 25 (PMF) | ||||
| MDS/MPNs | 8-15.6 | 1 | 1.4 | 15.6-43 | 51,58 |
| AML | Rare | Rare | Rare | 9-18 | 51,60,61 |
| DS-AMKL | 32.7 | 0 | 2 | 2 | 76 |
| T-ALL* | 18 | ND | 7 | 0 | 78 |
| ETP-ALL† | 12.7 | 12.7 | 17.5 | 0 | 77 |
| Non-ETP† | 3.3-4.8 | 3.3-7 | 0-2.4 | 0 | 77,79 |
DS-AMKL, acute megakaryoblastic leukemia associated with Down syndrome; ET, essential thrombocythemia; ETP-ALL, early T-cell precursor acute lymphoblastic leukemia; ND, not determined; PMF, primary myelofibrosis; PV, polycythemia vera.
Adults
Children.
The tumor-suppressive function of EZH2 has been analyzed using Ezh2-deficient mice. We have shown that the hematopoietic cell–specific deletion of Ezh2 results in the development of heterogeneous myeloid malignancies with long latencies, including MDS and MDS/MPNs.15 EZH2 mutations were found to co-occur with TET2, RUNX1, and ASXL1 mutations.63 The loss of Ezh2 cooperated with a Tet2 hypomorph to induce MDS and MDS/MPNs in mice.65 The loss of Ezh2 also enhanced the initiation and progression of RUNX1 mutant–induced MDS but attenuated the predisposition to leukemic transformation.66 Furthermore, the loss of Ezh2 significantly promoted the development of JAK2V617F mutant–induced myelofibrosis, resulting at least in part from the enhancement of aberrant megakaryocytopoiesis.67-69 These findings clearly indicate that EZH2 plays a tumor-suppressive role in myelodysplastic and myeloproliferative disorders that originate from HSCs. Eμ-myc lymphomagenesis was also accelerated by a heterozygous loss-of-function allele of Suz12 or by short hairpin RNA-mediated knockdown of Suz12 or Ezh2, suggesting that PRC2 restricts the self-renewal of B-lymphoid progenitors.70
A series of analyses of these mouse models revealed that an Ezh2 insufficiency induced the activation of oncogenic genes of direct and indirect polycomb targets (Figure 1C).65-69,71 These genes include a cohort of fetal-specific genes, such as let-7 microRNA target genes (such as Lin28, Hmga2, and Igfbp3), which are so-called oncofetal genes, suggesting that Ezh2 restricts the transformation of HSPCs by repressing a cohort of oncogenic genes.67-69,71 Ezh2 insufficiency also activates the production of inflammatory cytokines and proteins such as interleukin-6, S100a8, and S100a9 from malignant hematopoietic clones, creating an inflammatory bone marrow environment that confers a growth advantage on malignant clones over the residual normal clones while affecting the production of mature progenies.66,69,71,72
In contrast, key tumor-suppressor and developmental regulator genes are largely maintained in a transcriptionally repressed state in malignant HSPCs, even in Ezh2-insufficient states.15,65 A Chromatin ImmunoPrecipitation sequence analysis of H3K27me3 revealed a locus-specific compensatory function for Ezh1, another enzymatic component of PRC2, which repositions to the Ezh2 target loci to restrict the activation of tumor-suppressor and developmental regulator genes (Figure 1C).15 Correspondingly, no recurrent somatic mutations have been reported in EZH1. Furthermore, an Ezh2 insufficiency promoted the propagation of aberrant DNA methylation in a mouse MDS model hypomorphic for Tet2 combined with the loss of Ezh2.73 In this model, Ezh2 target genes largely lost the H3K27me3 mark while acquiring a significantly higher level of DNA methylation than Ezh1 target genes that retained the mark. These findings indicate that Ezh2 target genes are the main targets of the epigenetic switch from H3K27me3 to DNA methylation in MDS with an Ezh2 insufficiency. An Ezh2 insufficiency also induces a switch from methylation (H3K27me3) to acetylation (H3K27ac) at H3K27 at promoters, which is closely associated with the activation of PRC2 target genes (Figure 1C). The epigenetic switch conferred an oncogenic addiction to the H3K27ac modification, while it sensitized tumor-initiating cells to bromodomain inhibition in a mouse model of myelofibrosis with JAK2V617F combined with the loss of Ezh2.67 In juvenile myelomonocytic leukemia (JMML), genetic alterations impairing PRC2 functions, such as −7/7q− and deletions and mutations in EZH2, SUZ12, and ASXL1, have been reported in 33% of sporadic JMML patient cases with alterations in the RAS pathway.74 A cooperative effect of the activation of RAS and impairment of PRC2 has also been reported in NF1-associated cancers, in which the loss of PRC2 augments Ras-regulated transcription by enhancing acetylation at H3K27.75 A similar molecular mechanism may also be relevant in JMML. EZH2 has also been targeted with deletions and inactivating somatic mutations in childhood acute megakaryoblastic leukemia associated (33%) or not associated (16%) with Down syndrome and is frequently comutated with genes encoding cohesin components (Table 1).76
Loss-of-function mutations in EZH2 and SUZ12 have been detected in ETP (42.2%) and non-ETP (11.9%) pediatric T-ALL and in 25% of adult T-ALLs (Table 1).77-79 Moreover, in several mouse models, Ezh2-deficient hematopoietic cells have been reported to induce T-ALL.15,80,81 In a mouse model and human T-ALL cells, oncogenic NOTCH1 mutations specifically induced the loss of H3K27me3 modifications by antagonizing the functions of PRC2, leading to the activation of the NOTCH1 transcriptional program.78 These findings indicate that PRC2 functions as a tumor suppressor in T-ALL. Activating NOTCH mutations are much less common in ETP-ALL than in regular T-ALL. In contrast, RAS pathway mutations are common in ETP-ALL. Given the high frequencies of inactivating mutations in PRC2 genes in ETP-ALL, a cooperative effect of Ras activation and PRC2 dysfunction may contribute to the pathogenesis of ETP-ALL as in JMML. PRC2 inactivation also caused derepression of Il6ra, resulting in the activation of JAK/STAT signaling in a mouse model of ETP-ALL.81
ASXL1, the loss of which causes reductions in H3K27me3 levels, is also targeted by deletions and somatic mutations in patients with MDS, MPNs, and MDS/MPNs and also in those with de novo AML (Table 1).2,82 The loss of ASXL1 was shown to activate the expression of posterior HOXA genes, and the conditional deletion of Asxl1 in mouse hematopoietic cells resulted in a myelodysplastic phenotype.9,83 These findings suggest that ASXL1 functions as a tumor suppressor in myeloid malignancies. However, the molecular mechanisms responsible for the pathological impact of ASXL1 mutations in hematological malignancies as well as functional differences from PRC2 component mutations have not yet been elucidated in detail.
Recurrent inactivating mutations have been identified in various hematological malignancies in the X-linked BCOR gene encoding the BCL6 corepressor (BCOR) and its closely related homolog, BCOR-like 1 (BCORL1).84 Although BCOR and BCORL1 have recently been identified as components of the noncanonical PRC1.1, limited information is available on their role in hematological malignancies, and it currently remains unclear whether BCOR and BCORL1 mutants are relevant to the functions of PRC1.1.85
Therapeutic targeting of PRCs
EZH2 inhibitors that target the oncogenic function of EZH2 have been developed, and preclinical and clinical trials are now under way.86,87 The efficacy of EZH2 inhibitors was initially demonstrated in lymphoma,88 and lymphoma and multiple myeloma are now the major targets in clinical trials.87 Of interest, cancers with mutations in SWI/SNF subunits, such as ARID1A, PBRM1, and SMARCA4, are dependent on EZH2 activity.89 A synthetic lethality between ARID1A mutation and EZH2 inhibition has been reported in ARID1A-mutated ovarian cancer cells,90 and EZH2 inhibition sensitizes SMARCA4-mutant lung tumors to topoisomerase II inhibitors.91 However, SWI/SNF-mutant cancer cells are primarily dependent on a noncatalytic role of EZH2 and are only partially dependent on EZH2 histone methyltransferase activity, raising a concern about the efficacy of EZH2 enzymatic inhibitors in these cancers, including leukemia.89 In addition to the EZH2-specific inhibitors, UNC1999, a dual EZH1 and EZH2 inhibitor, has been developed. Because EZH1 complements EZH2 functions, targeting EZH2 and EZH1 may represent a promising approach.92 Furthermore, PRC2 inhibitors have the potential to be used in combination with other agents to enhance their therapeutic value. The leukemic stem cells of chronic myeloid leukemia were shown to be sensitive to the combined effects of an EZH2 inhibitor and tyrosine kinase inhibitor.93 Many agents are now being evaluated to obtain synergistic effects in combination with EZH2 inhibitors.
In contrast, therapeutic approaches that target the tumor-suppressive functions of PRC2 are currently limited. As described earlier in this article, PRC2 insufficiencies induce a methylation-to-acetylation switch at H3K27, thereby sensitizing tumor cells to bromodomain inhibitors.67,75 JAK/STAT inhibition is a potential therapeutic strategy for hematological malignancies with PRC2 insufficiency such as ETP-ALL.81 In addition, the ablation of the residual PRC2 activity may be an alternative approach to eradicate tumor cells with PRC2 insufficiency.94 Therapeutic breakthroughs are needed to treat PRC2-insufficient tumors. PTC596, a small compound that induces the degradation of the BMI1 protein, has entered a phase 1 clinical trial for patients with advanced solid tumors (www.clinicaltrials.gov #NCT02404480).95
Finally, recent analyses have linked PRC2 insufficiency to drug resistance in leukemia. Low levels of EZH2 protein induce resistance to multiple drugs in AML partly because of the derepression of HOX genes, representative EZH2 targets.96 Inactivating PRC2 mutations also induce resistance to conventional chemotherapy by inhibiting mitochondrial apoptosis in T-ALL.97 These findings may represent complex functions of PRC2 in hematological malignancies and underscore the importance of PRC2 status in the treatment of hematological malignancies.
Conclusions and perspectives
The dysregulation of PcG proteins has been implicated in various aspects of the ontogeny of hematological malignancies. Although a lot of detail is known about PcG proteins, we may not yet have the full picture because of their complex functions and broad targets. Noncanonical PRC1 may also be involved in the pathogenesis of hematological malignancies; however, its role remains largely uncharacterized. Recent advances in epigenetic research are contributing to a new stage of translation, and clinical trials on polycomb-targeting agents, which may have both beneficial and adverse effects in patients, are providing important information. Continuous and seamless efforts from basic to clinical research will undoubtedly promote advances in epigenetic therapy.
Acknowledgments
The author thanks Motohiko Oshima for his assistance in making figures.
This work was supported in part by Grants-in-Aid for Scientific Research (#15H02544) and Scientific Research on Innovative Areas Stem Cell Aging and Disease (#25115002) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Authorship
Contribution: A.I. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Atsushi Iwama, 1-8-1 Inohana, Chuo-ku, Chiba, 260-8670 Japan; e-mail: aiwama@faculty.chiba-u.jp.