To the editor:
In a large international cohort of contemporary polycythemia vera (PV) patients1 recruited at diagnosis and treated according to European LeukemiaNet recommendations,2 the postdiagnosis total major thrombosis rate was 2.62% patients per year, lower than that reported in the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) trial3 (4.4% patients per year) but comparable to the rate in the Cyto-PV study4 (2.7% patients per year) in which management of cardiovascular risk factors was more intensive than in the ECLAP study. In multivariable analysis, previous arterial event (hazard ratio [HR], 1.7; 95% confidence interval [CI], 1.2-2.4) and hypertension (HR, 1.6; 95% CI, 1.2-2.2) were significantly associated with subsequent arterial events, whereas previous venous event (HR, 2.6; 95% CI, 1.5-4.4) and age >65 years (HR, 1.7; 95% CI, 1.2-2.5) were significant predictors of future venous thrombosis. Therefore, among the cardiovascular risk factors (active smoking, diabetes, and hypercholesterolemia), arterial hypertension had the most relevant prognostic role for the incidence of arterial thrombosis. Notably, hypertension was more frequent in PV than in a series of 891 patients with essential thrombocythemia (odds ratio [OR], 1.38; P = .022, adjusted for age and sex).5 This would indicate that in PV patients, hypertension is associated with the increased hematocrit (HCT) levels and is higher when comparing the highest and lowest hematocrit tertile.5 These findings underscore the notion that enhanced blood viscosity may lead to increased peripheral resistance to blood flow and, consequently, to an enhanced frequency of arterial hypertension. Therefore, the independent role of hypertension in the risk of arterial thrombosis suggests a closer evaluation of the relationship between factors that control blood pressure on one side and erythrocytosis on the other side.
A lesson in this regard may be derived from experience in nonclonal erythrocytosis. Erythrocytosis is a common complication of kidney transplantation, with a prevalence varying from 8% to 22%.6 The pathogenesis of erythrocytosis that follows renal transplantation is not fully understood and likely multifactorial. There is growing evidence that angiotensin II enhances erythropoietin-mediated proliferation of red cell precursors.7 In randomized controlled trials and cases series, it was documented that angiotensin-converting enzyme (ACE) inhibition or angiotensin II type 1 receptor AT1R antagonist drugs are safe and effective in the treatment of erythrocytosis that follows renal transplant8 and that ACE inhibitors are more effective than adenosine receptor antagonists in the management of posttransplant erythrocytosis.9,10 Accordingly, the European Best Practice guidelines on transplantation recommend ACE inhibitors or angiotensin receptor blockers as first-line therapy of erythrocytosis.11 Inhibition of the renin-angiotensin system has also proved effective in a randomized prospective trial in which patients with altitude hypoxia treated with low doses of enalapril had a control of blood pressure, while packed cell volume, hemoglobin levels, and proteinuria progressively decreased.12
Therefore, the question whether these findings may also apply in clonal erythrocytosis of PV may be of great practical interest. Recent data point to the participation of the renin-angiotensin system in the regulation of neoplastic erythropoiesis. Indeed, increased local bone marrow synthesis of ACE and other angiotensin components has been identified by demonstrating corresponding messenger RNAs in PV. Therefore, upregulation of the local bone marrow renin-angiotensin system in autonomous neoplastic clonal erythropoiesis of PV has been suggested.13 In keeping with this hypothesis, some evidence indicated that renin-angiotensin system inhibitors such as captopril and enalapril may have a role in the management of PV,14 leading to normalization of blood cell count and partial remission of heavy proteinuria.15 Overall, although available data for the control of neoplastic clonal erythrocytosis are limited, ACE inhibitors in PV may be safe and possibly efficacious.
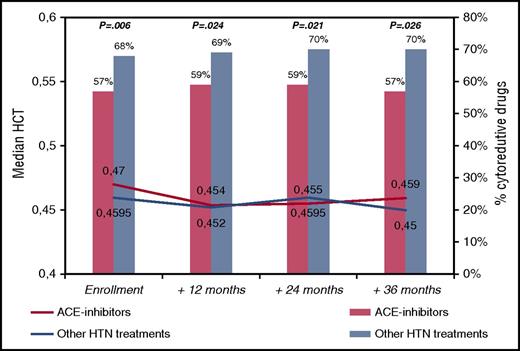
To tackle this issue, we reviewed the ECLAP database, looking at the group of patients with arterial hypertension. The ECLAP study included a cohort of 1638 PV patients who were screened for inclusion in the trial. To assure a representative sample of the spectrum of the disease burden of the participating centers, all patients (N = 1638) with new and old diagnoses of PV (made according to the criteria established by the Polycythemia Vera Study Group) were included in a prospective study with no exclusion criteria with respect to age, therapy, or duration of disease. Treatment strategies had to comply with the recommendation of maintaining the HCT value at <0.45 and the platelet count at <400 × 109/L. Cytoreductive therapy was used in 61% of cases and in the majority included hydroxyurea. Data regarding clinical outcomes, treatments, and laboratory values during the prospective follow-up were recorded at follow-up visits at 12, 24, 36, 48, and 60 months. At study entry, 647 patients were receiving antihypertensive drugs (ACE inhibitors [n = 285, 44%] or other drugs [n = 362, 56%]; in detail, calcium antagonists [n = 177, 49%], diuretics [n = 96, 27%] and beta-blockers [n = 103, 28%], alone or in combination). The group of patients taking ACE inhibitors did not significantly differ from patients receiving other antihypertensive drugs in terms of sex, age, conventional risk stratification, blood counts, and other than hypertension CV risk factors (data not shown). Instead, patients in the ACE group were treated with chemotherapy significantly less frequently than patients the other group (57% vs 68%, respectively; OR, 0.78, P = .006). This univariate finding was confirmed in multivariable model (OR, 0.62; 95% CI, 0.44-0.88; P = .008) adjusted for sex, age, previous thrombosis, blood counts, smoking, diabetes, and hypercholesterolemia. These findings remained constant during the follow-up visits of the study (Figure 1), where, in spite of less chemotherapy use, the median values of HCT were not different between the two groups compared. Of note, Kaplan-Meier curves of thrombosis-free survival were superimposable in the patients treated with or without ACE inhibitors (86% vs 87% at 36 months, respectively; log-rank test, P = .653; data not shown).
Median hematocrit values and need of cytoreduction by ACE inhibitors in PV patients treated for hypertension (n = 647). Hematocrit values are presented as medians. Cytoreductive treatments are expressed as percentages and the χ2 test was used to compare ACE inhibitors with other hypertension treatments at different time points (P values are quoted). HTN, hypertension.
Median hematocrit values and need of cytoreduction by ACE inhibitors in PV patients treated for hypertension (n = 647). Hematocrit values are presented as medians. Cytoreductive treatments are expressed as percentages and the χ2 test was used to compare ACE inhibitors with other hypertension treatments at different time points (P values are quoted). HTN, hypertension.
Therefore, from these data, it would seem that the most appropriate drugs for PV patients with hypertension are ACE inhibitors that could simultaneously control arterial blood pressure and abnormal erythropoiesis and consequently reduce the need of cytoreductive drugs. However, this remains a research question that should be confirmed in well-designed prospective clinical trials.
Authorship
Contribution: T.B. designed the research, interpreted data, and wrote the paper; A.M. collected data; and A.C. and A.G. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal