In this issue of Blood, Harder et al demonstrate that breakthrough hemolysis in patients with paroxysmal nocturnal hemoglobinuria (PNH) treated with eculizumab is due to strong activation of the complement cascade, leading to higher C3b density, which may cause a conformation change limiting the ability of eculizumab to block terminal complement.1
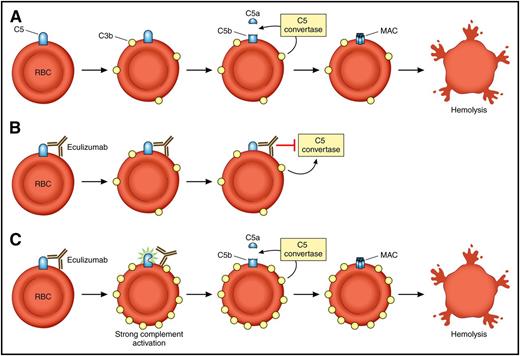
Model of eculizumab function and breakthrough hemolysis in the setting of strong complement activation. (A) In PNH, loss of CD59 and CD55 results in C3b priming of C5 on red cells and allows for cleavage of C5 into C5a and C5b. This leads to formation of the MAC on PNH red cells and results in intravascular hemolysis. (B) The patient with PNH on eculizumab. Eculizumab binds to C5, and through steric hindrance, inhibits the ability of the C5 convertase to cleave C5 into C5a and C5b. The MAC does not form, and the red cell is protected. (C) The patient with PNH on eculizumab in the setting of strong complement activation (infection, surgery, etc) experiences accumulation of C3b on the cell surface (strong activation). This results in a conformation change in C5 that disrupts the steric hindrance induced by eculizumab and leads to breakthrough intravascular hemolysis. RBC, red blood cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Model of eculizumab function and breakthrough hemolysis in the setting of strong complement activation. (A) In PNH, loss of CD59 and CD55 results in C3b priming of C5 on red cells and allows for cleavage of C5 into C5a and C5b. This leads to formation of the MAC on PNH red cells and results in intravascular hemolysis. (B) The patient with PNH on eculizumab. Eculizumab binds to C5, and through steric hindrance, inhibits the ability of the C5 convertase to cleave C5 into C5a and C5b. The MAC does not form, and the red cell is protected. (C) The patient with PNH on eculizumab in the setting of strong complement activation (infection, surgery, etc) experiences accumulation of C3b on the cell surface (strong activation). This results in a conformation change in C5 that disrupts the steric hindrance induced by eculizumab and leads to breakthrough intravascular hemolysis. RBC, red blood cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
PNH is a clonal hematopoietic stem cell disease associated with a complement-dependent hemolytic anemia.2 The disease manifests after the expansion of a multipotent hematopoietic stem cell that acquires a mutation of the PIGA gene. The PIGA gene product is required for the biosynthesis of glycophosphatidylinositol anchors, a glycolipid moiety that attaches dozens of proteins to the plasma membrane of cells. Thus, all blood cells derived from the PIGA mutant stem cell, including mature red blood cells, are missing glycophosphatidylinositol–anchor proteins. Two of these proteins, CD55 and CD59, are complement regulatory proteins. CD55 inhibits C3 convertases, and CD59 blocks formation of the membrane attack complex (MAC) by inhibiting incorporation of C9 into the MAC. The loss of CD55 and CD59 renders PNH erythrocytes susceptible to intravascular hemolysis. Eculizumab is a humanized monoclonal antibody that blocks terminal complement by binding to C5 and is the treatment of choice for PNH.3 Eculizumab virtually eliminates the intravascular hemolysis in PNH patients as evidenced by the rapid fall in lactate dehydrogenase levels after administration of the drug. Through steric hindrance, the antibody protects C5 from cleavage by the C5 convertase and thus inhibits terminal complement activity by decreasing the generation of C5a and C5b. It therefore inhibits terminal complement and compensates for the CD59 deficiency. Eculizumab is a first-in-class, breakthrough drug for PNH that prevents thrombosis (the leading cause of death in PNH), improves quality of life, and often eliminates the need for blood transfusions; however, most patients continue to have low level hemolysis and mild to moderate anemia. Until now, most of residual hemolysis in patients with PNH on eculizumab was attributed to extravascular hemolysis. Previous work has shown that PNH red cells in patients on eculizumab often accumulate C3 fragments because eculizumab blocks complement at C5 (upstream of CD59 but downstream of CD55).4 CD55 (also known as decay accelerating factor) shortens the half-life of C3 by acting on the C3 convertases. Because PNH red cells are missing CD55, these C3 fragments accumulate and lead to opsonization and destruction of the PNH red cells in the spleen. Importantly, when patients with PNH on eculizumab acquire bacterial or viral infections, they frequently develop “breakthrough” intravascular hemolysis accompanied by worsening anemia, a significant rise in lactate dehydrogenase levels, hemoglobinuria, and a return of their PNH symptoms. The intravascular hemolysis typically resolves with clearance of the infection. The mechanism of breakthrough hemolysis, presumed to be complement mediated, was not entirely clear.
Harder et al performed a series of elegant ex vivo experiments to model breakthrough hemolysis of PNH red cells in the setting of C5 inhibition and to improve our understanding of the mechanism of action of eculizumab. The authors demonstrate that residual lytic activity of the terminal pathway of complement depends on the strength of the complement activator and the resulting surface density of C3b, which amplifies the alternative pathway through its potent amplification loop (see figure). They also show that at high densities of C3b on red cells, C5 inhibitors such as eculizumab and the tick-derived C5 inhibitor OmcI (Coversin)5 reduce but do not abolish terminal complement activity. Thus, C5 inhibitors stabilize the unprimed C5 conformation, making it much less susceptible to cleavage by convertases. C5 is more susceptible to cleavage under conditions of more intense priming due to excess C3b on the red cells, even in the presence of a C5 inhibitor. Interestingly, a combination of eculizumab and Coversin, which bind to different epitopes of C5, has additive effects and together can abolish residual lytic activity even in the presence of strong activation. Similarly, combining eculizumab with drugs or conditions that inhibit the amplification loop of the alternative pathway of complement was also able to abolish residual lytic activity.
These findings give insight into the mechanism of action of eculizumab and improve our understanding of the eculizumab breakthrough hemolysis that may occur in patients with PNH following infections, surgery, pregnancy, and other conditions that increase complement activation over baseline.6 These mechanisms may also be relevant to atypical hemolytic uremic syndrome, which is also treated with C5 inhibition. A number of new complement inhibitors are being developed, and it is conceivable that the combination of different C5 inhibitors or a C5 inhibitor and an inhibitor of the alternative pathway of complement may be able to reduce or eliminate the residual hemolysis and/or breakthrough intravascular hemolysis that occurs with eculizumab alone.7,8
A limitation of this study is that the experiments were performed ex vivo and used rabbit, sheep, or human erythrocytes as a readout for terminal complement activity. Thus, these findings may not be as relevant in vivo and may not directly apply to atypical hemolytic uremic syndrome where terminal complement-mediated attack targets nucleated cells that have the ability to repair their membrane. It is also important to point out that PNH patients do extremely well on eculizumab, and only a minority experience severe breakthrough hemolysis. In some, but not all, instances this can be overcome by increasing the dose of eculizumab. Nevertheless, the work by Harder et al lends valuable insight into our understanding of complement activation and may inform future clinical trials of novel complement inhibitors in human disease.
Conflict-of-interest disclosure: R.A.B. is on the Scientific Advisory Board for Alexion Pharmaceutical, Achillion Pharmaceutical, and Apellis Pharmaceutical and has received research funding from Alexion Pharmaceutical and Apellis Pharmaceutical.