Key Points
CCR5 blockade decreases peripheral T-cell activation, gut GVHD biomarkers, and acute GVHD incidence in allo-HSCT recipients.
CXCR3-mediated lymphocyte trafficking may represent an important resistance mechanism to CCR5 blockade in GVHD prophylaxis.
Abstract
Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Lymphocyte trafficking via chemokine receptors such as CCR5 plays a critical role in alloreactive responses, and previous data suggest that CCR5 blockade with maraviroc results in a low incidence of visceral GVHD. However, the full scope of clinical and immunologic effects of CCR5 blockade in HSCT has not been described. We compared a cohort of patients enrolled on a trial of reduced-intensity allo-HSCT with standard GVHD prophylaxis plus maraviroc to a contemporary control cohort receiving standard GVHD prophylaxis alone. Maraviroc treatment was associated with a lower incidence of acute GVHD without increased risk of disease relapse, as well as reduced levels of gut-specific markers. At day 30, maraviroc treatment increased CCR5 expression on T cells and dampened T-cell activation in peripheral blood without impairing early immune reconstitution or increasing risk for infections. Patients who developed acute GVHD despite maraviroc prophylaxis showed increased T-cell activation, naive T-cell skewing, and elevated serum CXCL9 and CXCL10 levels. Collectively, these data suggest that maraviroc effectively protects against GVHD by modulating alloreactive donor T-cell responses, and that CXCR3 signaling may be an important resistance mechanism to CCR5 blockade in GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative therapy for many hematologic malignancies and benign disorders. However, graft-versus-host disease (GVHD) remains a barrier to successful transplantation, despite considerable advances in our understanding of disease pathogenesis.1 GVHD is caused by alloreactive donor T cells that recognize recipient antigens as foreign and consequently mediate tissue destruction. Acute GVHD principally involves the gut, liver, and skin, complicating ∼30% to 50% of HLA-matched transplants from related donors and 50% to 70% from unrelated donors even with standard prophylaxis.2 Therefore, additional strategies to prevent GVHD are desperately needed.
One potential approach is to modulate lymphocyte trafficking, which plays a critical role in GVHD pathogenesis. Donor T cells must home to secondary lymphoid organs and then into target organs in order to recognize alloantigens presented by antigen-presenting cells and to cause tissue injury.3 This migration is carefully regulated by adhesion molecules and chemokine receptors expressed by lymphocytes, such as CCR5. For example, anti-CCR5 antibody prevented murine GVHD by blocking alloreactive CCR5+CD8+ T-cell homing to the gut and liver,4,5 and certain human CCR5 polymorphisms are associated with acute GVHD development, relapse, and overall survival (OS) following allo-HSCT.6-8 This suggests that CCR5 blockade may be an attractive strategy for GVHD prophylaxis.
We previously published a phase 1/2 trial of high-risk patients with hematologic malignancies undergoing reduced-intensity allo-HSCT with standard GVHD prophylaxis (tacrolimus, methotrexate) combined with maraviroc, a nonallosteric CCR5 antagonist.9 Maraviroc treatment resulted in an extremely low cumulative incidence of acute GVHD compared with historical rates and, importantly, 0% visceral GVHD before day 100.
Here, we present the full scope of clinical and immunologic effects of CCR5 blockade in allo-HSCT. As our previous publication presented single-arm outcomes with short follow-up, the goal of the current study was to put these data in perspective with longer follow-up by comparing these patients in a multivariable analysis to a contemporary cohort of 115 consecutive patients undergoing reduced-intensity allo-HSCT with standard GVHD prophylaxis alone. To understand the protective properties of CCR5 blockade, we monitored serum GVHD biomarkers and characterized immune reconstitution, CCR5 expression, and T-cell activation in control and maraviroc-treated cohorts. Because some patients developed GVHD despite maraviroc prophylaxis, we extended these studies using immunophenotyping and analysis of serum chemokine and cytokine levels to elucidate potential resistance mechanisms to CCR5 blockade.
Methods
Patient population
We performed a post hoc analysis of a previously reported phase 1/2 clinical trial (NCT00948753) assessing the safety and efficacy of adding maraviroc to standard GVHD prophylaxis in allo-HSCT recipients.9 In brief, 38 patients receiving allo-HSCT with a reduced-intensity conditioning regimen at the Hospital of the University of Pennsylvania were enrolled between June 2009 and March 2011. In addition to the uniform conditioning regimen of fludarabine and busulfan, patients received standard GVHD prophylaxis of tacrolimus (0.06 mg/kg per day) in 2 divided doses beginning 2 days before HSCT and IV methotrexate (15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11). They also received maraviroc (either 150 mg or 300 mg twice daily) beginning 2 days prior to transplantation until day 30. Prior to and after this time period as well as in between dose modifications during the phase 1 portion, 115 consecutive control patients meeting the same study criteria were allografted with the same conditioning regimen and standard GVHD prophylaxis (tacrolimus, methotrexate). Supplemental Figure 1 (available on the Blood Web site) illustrates the enrollment of the study and contemporary control cohorts.
Clinical outcomes
Time to disease relapse, acute GVHD, nonrelapse mortality (NRM), OS, and chronic GVHD were defined as the time from transplantation to the event. GVHD relapse-free survival (GRFS) was defined as the time from transplantation to acute GVHD grade 3 to 4, moderate to severe chronic GVHD, disease relapse, or death, whichever occurred first. Patients were censored at the time of last contact or a second transplantation for all outcomes, and at the time of donor lymphocyte infusion for GVHD outcomes. Disease relapse was defined as morphologic, cytogenetic, or radiologic evidence of disease demonstrating pretransplantation characteristics. Restaging evaluation, including bone marrow biopsies and appropriate imaging, was routinely performed at day 100 or earlier in patients with signs indicating early relapse. The Consensus Conference criteria and National Institutes of Health criteria were used for acute GVHD and chronic GVHD grading, respectively.10,11 Donor chimerism levels were measured in whole blood and after immunomagnetic positive selection of CD3+ cells from peripheral blood samples (STEMCELL Technologies, Vancouver, BC, Canada).
PBMC isolation
Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll gradients and cryopreserved. All available PBMC samples at time of analysis for maraviroc-treated patients at day 30 and day 60 after transplantation were characterized by flow cytometry and compared with samples from a subset of the 115 control patients of similar age, disease, and donor type.
Immune phenotyping
Flow cytometry was performed using a FACSCanto cytometer and FACSDiva software (BD Biosciences). Cell surface molecule expression or intracellular FoxP3 expression was determined using the following antibodies: CD3 (SK7, UCHT1), CD4 (RPA-T4), CD8 (SK1), CD14 (MφP9), CD19 (4G7, HIB19), CD31 (WM59), CD27 (M-T271), CD38 (HB7), CD45RO (UCHL1), CD56 (NCAM 16.2), HLA-DR (L243), CD69 (FN50) (BD Biosciences); CCR5 (J418F1), CXCR3 (G025H7), FoxP3 (259D) (Biolegend); CCR7 (150503) (R&D Systems); CD25 (4E3) (Miltenyi Biotec). Absolute counts were determined by multiplying absolute lymphocyte count (ALC) by the percentage of the indicated population by flow cytometry. Mature CD4+ and CD8+ T cells were classified into CD45RO−CCR7+ (naive; TN), CD45RO+CCR7+ central memory (TCM), CD45RO+CCR7− effector memory (TEM), and CD45RO−CCR7− effector memory RA (TEMRA) cells.12,13
Cytokine measurement
Luminex multiplex assays were performed by the University of Pennsylvania Human Immunology Core. ST2 and Reg3a levels were measured by enzyme-linked immunosorbent assay using kits from R&D Systems (DST200) and MBL International (5323), respectively. Plates were read on a BioTek Synergy H1 Hybrid Reader and analyzed with Gen5 software (BioTek).
Statistics
The Student t test or Mann-Whitney U test were used to compare continuous variables. The Pearson correlation coefficient was used for correlation analysis. The cumulative incidence function was used to analyze acute GVHD, relapse, and NRM, taking into account death as a competing risk. OS and GRFS were assessed using the Kaplan-Meier method. Comparisons were conducted with the Gray test14 or Cox regression followed by multivariable modeling with backward elimination to adjust for important covariates. Analyses were conducted in STATA (version 13.1; College Station, TX).
Study approval
The study was approved by the institutional review board of the University of Pennsylvania, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Results
Maraviroc reduces the incidence of acute visceral GVHD
We performed a phase 1/2 trial of reduced-intensity allo-HSCT with standard GVHD prophylaxis (tacrolimus, methotrexate) combined with 33-day maraviroc treatment and previously reported single-arm results.9 To overcome inherent biases related to the use of historical controls or registry data, we prospectively collected data on contemporary controls from the same institution. In this report, maraviroc-treated patients were compared with a control cohort of 115 consecutive patients undergoing reduced-intensity allo-HSCT with standard GVHD prophylaxis alone. Maraviroc patients were enrolled from June 2009 to March 2011, with 3 patients excluded because of low drug exposure as in our previous report.9 Control patients were enrolled from January 2009 to May 2013, though enrollment was nonoverlapping (supplemental Figure 1). Table 1 presents the patient characteristics of the maraviroc and control cohorts, which had similar high-risk features including age, underlying hematologic disease, comorbidities, and donor-recipient HLA compatibility. There were no significant differences between the groups. Cumulative incidence analysis and multivariable regression analysis were used to assess the effect of maraviroc on clinical outcomes, using stepwise elimination to identify significant clinical covariates. The models for GVHD were adjusted for donor type and HLA mismatching, and models for relapse, NRM, and OS were adjusted for the Disease Risk Index, donor sex, and graft CD8+ T-cell dose.15 Median follow-up was 43.6 months at the time of analysis.
Patient characteristics
| . | Tac/MTX/MVC, N = 35 . | Tac/MTX, N = 115 . |
|---|---|---|
| Recipient age, mean (range), y | 60 (21-74) | 62 (27-76) |
| Recipient sex, M/F, % | 57/43 | 57/43 |
| Comorbidity Index, N (%) | ||
| Low | 15 (43) | 42 (38) |
| Intermediate | 13 (37) | 42 (38) |
| High | 7 (20) | 26 (24) |
| Diagnosis, N (%) | ||
| AML | 13 (37) | 54 (47) |
| MDS | 5 (14) | 28 (24) |
| NHL | 5 (14) | 9 (8) |
| MF | 4 (11) | 2 (2) |
| Other | 8 (23) | 22 (19) |
| Disease Risk Index, N (%) | ||
| Low | 2 (6) | 10 (9) |
| Intermediate | 22 (63) | 71 (62) |
| High/very high | 11 (31) | 34 (30) |
| Donor, N (%) | ||
| Matched related | 10 (29) | 48 (42) |
| Matched unrelated | 19 (54) | 49 (43) |
| Single-antigen mismatched unrelated | 6 (17) | 18 (16) |
| Donor age, mean (range), y | 39 (20-68) | 42 (18-73) |
| Donor sex, M/F, % | 57/43 | 55/45 |
| Cytomegalovirus status, N (%) | ||
| Recipient positive | 13 (37) | 51 (44) |
| Donor positive | 12 (34) | 46 (40) |
| Cell doses, mean (range) | ||
| CD34+, ×106 cells/kg | 6.8 (2.1-16.1) | 6.4 (1.4-21.4) |
| CD3+, ×108 cells/kg | 2.3 (0.6-5.4) | 2.4 (0.4-5.5) |
| CD4+, ×108 T cells/kg | 1.1 (0.3-2.7) | 1.5 (0.2-4.8) |
| CD8+, ×108 T cells/kg | 0.5 (0.1-1.3) | 0.6 (0.6-1.8) |
| . | Tac/MTX/MVC, N = 35 . | Tac/MTX, N = 115 . |
|---|---|---|
| Recipient age, mean (range), y | 60 (21-74) | 62 (27-76) |
| Recipient sex, M/F, % | 57/43 | 57/43 |
| Comorbidity Index, N (%) | ||
| Low | 15 (43) | 42 (38) |
| Intermediate | 13 (37) | 42 (38) |
| High | 7 (20) | 26 (24) |
| Diagnosis, N (%) | ||
| AML | 13 (37) | 54 (47) |
| MDS | 5 (14) | 28 (24) |
| NHL | 5 (14) | 9 (8) |
| MF | 4 (11) | 2 (2) |
| Other | 8 (23) | 22 (19) |
| Disease Risk Index, N (%) | ||
| Low | 2 (6) | 10 (9) |
| Intermediate | 22 (63) | 71 (62) |
| High/very high | 11 (31) | 34 (30) |
| Donor, N (%) | ||
| Matched related | 10 (29) | 48 (42) |
| Matched unrelated | 19 (54) | 49 (43) |
| Single-antigen mismatched unrelated | 6 (17) | 18 (16) |
| Donor age, mean (range), y | 39 (20-68) | 42 (18-73) |
| Donor sex, M/F, % | 57/43 | 55/45 |
| Cytomegalovirus status, N (%) | ||
| Recipient positive | 13 (37) | 51 (44) |
| Donor positive | 12 (34) | 46 (40) |
| Cell doses, mean (range) | ||
| CD34+, ×106 cells/kg | 6.8 (2.1-16.1) | 6.4 (1.4-21.4) |
| CD3+, ×108 cells/kg | 2.3 (0.6-5.4) | 2.4 (0.4-5.5) |
| CD4+, ×108 T cells/kg | 1.1 (0.3-2.7) | 1.5 (0.2-4.8) |
| CD8+, ×108 T cells/kg | 0.5 (0.1-1.3) | 0.6 (0.6-1.8) |
AML, acute myeloid leukemia; F, female; M, male; MDS, myelodysplastic syndrome; MF, myelofibrosis; MTX, methotrexate; MVC, maraviroc; NHL, non-Hodgkin lymphoma; Tac, tacrolimus.
Notably, maraviroc-treated patients had reduced risk of acute grade 2-4 GVHD (adjusted hazard ratio [aHR] = 0.42; 95% confidence interval [CI], 0.21-0.84; P = .015) and grade 3-4 GVHD (aHR = 0.43; 95% CI, 0.17-1.09; P = .075) (Figure 1A-B). This time-to-event analysis includes early and delayed acute GVHD occurring at any time after transplant. Median onset of acute GVHD was 2.8 months in the maraviroc group and 4.2 months in the control group (P = .36). Breakdown of GVHD organ involvement showed that maraviroc treatment significantly reduced visceral GVHD (aHR = 0.32; 95% CI, 0.14-0.73; P = .007) (Figure 1C), whereas the risk of skin GVHD was unchanged (Figure 1D). The decrease in visceral GVHD was primarily driven by a difference in acute gut GVHD (aHR = 0.20; 95% CI, 0.06-0.62; P = .006) and not liver GVHD (Figure 1E-F), although the incidence of liver GVHD may have been too small (26 cases total) to determine significant differences. Further supporting a role for CCR5 blockade in preventing visceral GVHD, maraviroc-treated patients showed decreased serum bilirubin concentrations with a reduced proportion of patients that had bilirubin levels >3.6 mg/dL (3 times the upper limit of normal) for >1 day (9.5% vs 2.9%) (Figure 1G). Importantly, all bilirubin elevations in the maraviroc cohort were attributed to medications (other than maraviroc) and resolved when the medications were discontinued. The numbers of GVHD-related deaths were 2 in the maraviroc cohort (5.7%) and 16 in the control cohort (13.9%). There was no difference in risk of chronic GVHD, relapse, NRM, GRFS, or OS (Figure 1H). Moreover, infection rates, neutrophil engraftment, platelet engraftment, and donor chimerism were similar (supplemental Tables 1-2; supplemental Figure 2). These data demonstrate that the protective effect of CCR5 blockade is driven by a reduced incidence of visceral GVHD.
CCR5 blockade with maraviroc reduces the incidence of acute GVHD. (A) Cumulative incidence of grade 2-4 acute GVHD in control or maraviroc-treated patients. (B) Cumulative incidence of grade 3-4 acute GVHD. (C) Cumulative incidence of visceral GVHD (gut or liver). (D-F) Cumulative incidence of organ-specific GVHD in the skin (D), gut (E), and liver (F). (G) Total bilirubin levels in control or maraviroc-treated patients up to 100 days after transplant. Each line represents an individual patient. The horizontal red line indicates 3 times upper limit of normal. (H) Forest plot of the indicated clinical parameters comparing control or maraviroc-treated patients. Adjusted hazard ratio (HR) and P values are indicated for each comparison.
CCR5 blockade with maraviroc reduces the incidence of acute GVHD. (A) Cumulative incidence of grade 2-4 acute GVHD in control or maraviroc-treated patients. (B) Cumulative incidence of grade 3-4 acute GVHD. (C) Cumulative incidence of visceral GVHD (gut or liver). (D-F) Cumulative incidence of organ-specific GVHD in the skin (D), gut (E), and liver (F). (G) Total bilirubin levels in control or maraviroc-treated patients up to 100 days after transplant. Each line represents an individual patient. The horizontal red line indicates 3 times upper limit of normal. (H) Forest plot of the indicated clinical parameters comparing control or maraviroc-treated patients. Adjusted hazard ratio (HR) and P values are indicated for each comparison.
CCR5 blockade decreases levels of the gut-specific biomarker Reg3a
Several biomarkers are associated with systemic or organ-specific acute GVHD,16,17 and so we compared plasma concentrations of select biomarkers from control and maraviroc-treated patients. Regenerating islet-derived 3α (Reg3a) is an antimicrobial protein expressed by Paneth cells and is a validated biomarker of GVHD involving the gastrointestinal tract.18 Consistent with reduced gut GVHD with CCR5 blockade, maraviroc-treated patients demonstrated significantly decreased Reg3a levels at day 30 (Figure 2A). We observed reduced Reg3a levels even after excluding patients who developed GVHD by day 30 or day 100, suggesting that the decrease in Reg3a levels is a drug effect and not a consequence of GVHD development or treatment (supplemental Figure 3). In contrast, levels of tumor necrosis factor receptor-1 (TNF-RI), which has been associated with acute GVHD incidence,19 and suppression of tumorigenicity (ST2), which has been associated with treatment-resistant GVHD (all sites) and mortality,20 showed no difference between cohorts (Figure 2B-C). Reg3a levels were also significantly reduced at earlier time points (day 14) in maraviroc-treated patients compared with controls (Figure 2D), and levels of Reg3a but not ST2 progressively declined between day 7 and day 21 with maraviroc (Figure 2E-F). Thus, biomarker analysis further supports a role for CCR5 blockade in preventing gut GVHD.
CCR5 blockade decreases plasma levels of the gut GVHD biomarker Reg3a. (A-C) Serum concentrations of Reg3a (A), ST2 (B), or TNF-RI (C) from control (CON) and maraviroc-treated (MVC) patients at day 30 posttransplant. (D) Reg3a levels from control and maraviroc-treated patients at day 14 posttransplant. (E-F) Serum concentrations of Reg3a (E) or ST2 (F) from maraviroc-treated patients at the indicated time points. (G-I) Day 30 serum concentrations of Reg3a (G), ST2 (H), or TNF-RI (I) from maraviroc-treated patients who developed or did not develop grade 2-4 GVHD by day 180. Mean ± standard error (SE); *P < .05, Student t test for panels A-D and G-I; Mann-Whitney U test for panels E-F. ns, not significant.
CCR5 blockade decreases plasma levels of the gut GVHD biomarker Reg3a. (A-C) Serum concentrations of Reg3a (A), ST2 (B), or TNF-RI (C) from control (CON) and maraviroc-treated (MVC) patients at day 30 posttransplant. (D) Reg3a levels from control and maraviroc-treated patients at day 14 posttransplant. (E-F) Serum concentrations of Reg3a (E) or ST2 (F) from maraviroc-treated patients at the indicated time points. (G-I) Day 30 serum concentrations of Reg3a (G), ST2 (H), or TNF-RI (I) from maraviroc-treated patients who developed or did not develop grade 2-4 GVHD by day 180. Mean ± standard error (SE); *P < .05, Student t test for panels A-D and G-I; Mann-Whitney U test for panels E-F. ns, not significant.
As some patients developed GVHD despite maraviroc prophylaxis, we compared day 30 biomarker levels between maraviroc responders and nonresponders (ie, patients who developed grade 2-4 GVHD by day 180). Interestingly, we observed lower Reg3a concentrations in maraviroc responders (Figure 2G) but no difference in ST2 and TNF-RI levels (Figure 2H-I), consistent with the predominant effect of CCR5 blockade in the gut.
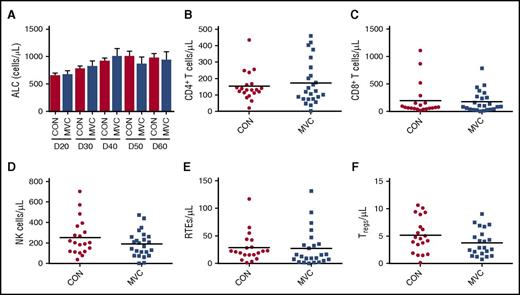
No effect of maraviroc on early immune cell reconstitution
We next investigated whether maraviroc modulates immune cell recovery after allo-HSCT. There were no significant differences in ALC, CD4+ T-cell, CD8+ T-cell or natural killer cell counts between cohorts (Figure 3A-D). To assess thymic output, we examined CD31+ naive CD4+ T cells, which represent the youngest T lymphocytes in the periphery known as recent thymic emigrants (RTEs).21 We observed comparable RTE numbers in control and maraviroc-treated patients (Figure 3E). Together, these data suggest that maraviroc does not impair early lymphocyte recovery after HSCT.
No effect of maraviroc on early immune cell reconstitution. (A) ALC from peripheral blood of control and maraviroc-treated patients at the indicated time points. (B) CD4+ T-cell count from control and maraviroc-treated patients at day 30 (P = .79, Mann-Whitney U test). (C) CD8+ T-cell count at day 30 (P = .93). (D) NK-cell count at day 30 (P = .28). (E) Absolute RTE count as represented by CD31+CD45RO−CCR7+CD4+ T cells at day 30 (P = .32). (F) Numbers of CD25+Foxp3+CD4+ Tregs at day 30 (P = .15).
No effect of maraviroc on early immune cell reconstitution. (A) ALC from peripheral blood of control and maraviroc-treated patients at the indicated time points. (B) CD4+ T-cell count from control and maraviroc-treated patients at day 30 (P = .79, Mann-Whitney U test). (C) CD8+ T-cell count at day 30 (P = .93). (D) NK-cell count at day 30 (P = .28). (E) Absolute RTE count as represented by CD31+CD45RO−CCR7+CD4+ T cells at day 30 (P = .32). (F) Numbers of CD25+Foxp3+CD4+ Tregs at day 30 (P = .15).
CD4+CD25+Foxp3+ regulatory T cells (Tregs) play critical roles in maintaining immune tolerance, and dysregulation of Tregs has been associated with GVHD.22 As some Tregs express CCR5,23 we examined whether Treg recovery is affected by maraviroc treatment. There was no significant difference in Treg counts at day 30 (Figure 3F), suggesting that maraviroc does not disrupt early Treg homeostasis.
Maraviroc increases CCR5 expression on immune cells
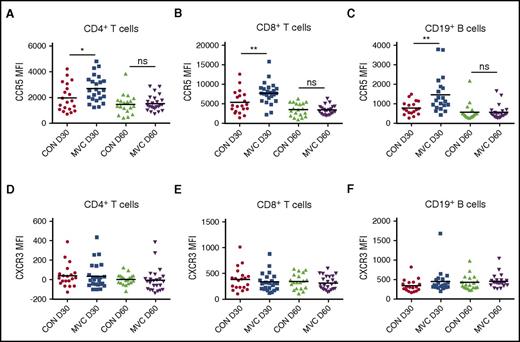
Surface CCR5 receptor-ligand complexes undergo internalization, which is inhibited by maraviroc.9 Previous in vitro studies demonstrated that maraviroc increases CCR5 expression on T cells, but its in vivo effect on chemokine receptor levels in allo-HSCT patients has not been studied.24 Therefore, we examined CCR5 expression at day 30 and day 60 posttransplant.
Compared with T cells from control patients at day 30, T cells from maraviroc-treated patients showed increased surface CCR5 levels by mean fluorescence intensity (MFI) (1989 vs 2684 for control vs maraviroc CD4+ T cells, P = .03; 5322 vs 7710 for control vs maraviroc CD8+ T cells, P = .009) (Figure 4A-B). Increased CCR5 expression with maraviroc was observed across TCM and TEM subsets (supplemental Figure 4). We also monitored CCR5 expression on CD19+ B cells, which generally have low CCR5 expression limited to the CD27+ memory subset.25 Interestingly, CD19+ B cells from maraviroc-treated patients also showed increased CCR5 expression at day 30 (Figure 4C). Differences in CCR5 expression on all cell types were attenuated between cohorts by day 60 (Figure 4A-C). Collectively, these data demonstrate that the effect of maraviroc on CCR5 expression dissipates after the drug is discontinued, suggesting the potential of longer maraviroc exposure in future trials.
Maraviroc increases CCR5 expression on immune cells. (A-C) Expression of CCR5 by MFI at day 30 and day 60 in control and maraviroc-treated patients on CD4+ T cells (A), CD8+ T cells (B), and CD19+ B cells (C). (D-F) Expression of CXCR3 by MFI at day 30 and day 60 in control patients and maraviroc-treated patients on CD4+ T cells (D), CD8+ T cells (E), and CD19+ B cells (F). *P < .05, **P < .01, Student t test.
Maraviroc increases CCR5 expression on immune cells. (A-C) Expression of CCR5 by MFI at day 30 and day 60 in control and maraviroc-treated patients on CD4+ T cells (A), CD8+ T cells (B), and CD19+ B cells (C). (D-F) Expression of CXCR3 by MFI at day 30 and day 60 in control patients and maraviroc-treated patients on CD4+ T cells (D), CD8+ T cells (E), and CD19+ B cells (F). *P < .05, **P < .01, Student t test.
To evaluate whether the modulation of CCR5 expression is specific, we examined surface expression of the related chemokine receptor CXCR3, which also controls T-lymphocyte migration and has been implicated in GVHD pathogenesis.26-28 CXCR3 expression on T cells and B cells was equivalent between cohorts, suggesting that maraviroc selectively regulates CCR5 expression and does not increase other trafficking molecules associated with effector T-cell responses (Figure 4D-F). Percentages of T cells coexpressing CCR5 and CXCR3 were also similar (supplemental Figure 5).
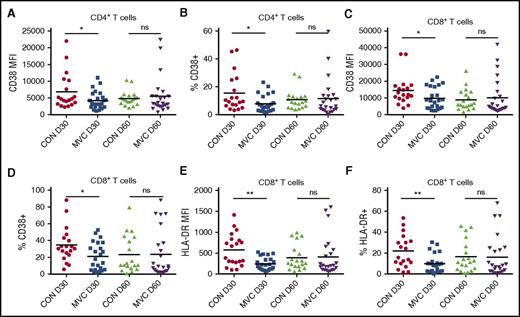
Maraviroc treatment dampens peripheral T-cell activation
CCR5 blockade reduced the incidence of acute GVHD, but the specific immunologic effects that inhibit alloreactive immune responses are not defined. To determine whether maraviroc modulates lymphocyte activation, we monitored the expression of activation markers CD38, HLA-DR, CD69, and CD25 on T cells. At day 30, CD38 expression was lower on CD4+ and CD8+ T cells from maraviroc-treated patients compared with controls (Figure 5A-D). Moreover, maraviroc-treated patients showed a significant decrease in HLA-DR+CD8+ T cells by MFI and percentage (Figure 5E-F). Similar to CCR5 expression, however, the effects of maraviroc on CD38 and HLA-DR expression waned by day 60 (Figure 5A-F). After excluding patients who developed GVHD before day 30 (data not shown) or day 100 (supplemental Figure 6A-B), we still found that maraviroc patients had decreased percentages of CD38+CD4+ T cells and HLA-DR+CD8+ T cells, indicating that the reduction in T-cell activation is not simply an effect of reduced GVHD at this time point or influenced by GVHD treatment. In contrast, we observed no difference in CD69 and CD25 expression (supplemental Figure 6C-H). This was not unexpected as CD69 and CD25 are transient activation markers, and PBMCs did not undergo in vitro stimulation. These data suggest that by impairing alloreactive T-cell trafficking, maraviroc dampens peripheral T-cell activation.
Maraviroc treatment dampens peripheral T-cell activation. (A) CD38 expression by MFI on CD4+ T cells at day 30 and day 60 in control and maraviroc-treated patients. (B) Percentage of CD38+ cells among CD4+ T cells as measured by flow cytometry. (C) CD38 expression by MFI on CD8+ T cells at day 30 and day 60 in control and maraviroc-treated patients. (D) Percentage of CD38+ cells among CD8+ T cells. (E) HLA-DR expression by MFI on CD8+ T cells at day 30 and day 60. (F) Percentage of HLA-DR+ cells among CD8+ T cells at day 30 and day 60. *P < .05, **P < .01, Student t test.
Maraviroc treatment dampens peripheral T-cell activation. (A) CD38 expression by MFI on CD4+ T cells at day 30 and day 60 in control and maraviroc-treated patients. (B) Percentage of CD38+ cells among CD4+ T cells as measured by flow cytometry. (C) CD38 expression by MFI on CD8+ T cells at day 30 and day 60 in control and maraviroc-treated patients. (D) Percentage of CD38+ cells among CD8+ T cells. (E) HLA-DR expression by MFI on CD8+ T cells at day 30 and day 60. (F) Percentage of HLA-DR+ cells among CD8+ T cells at day 30 and day 60. *P < .05, **P < .01, Student t test.
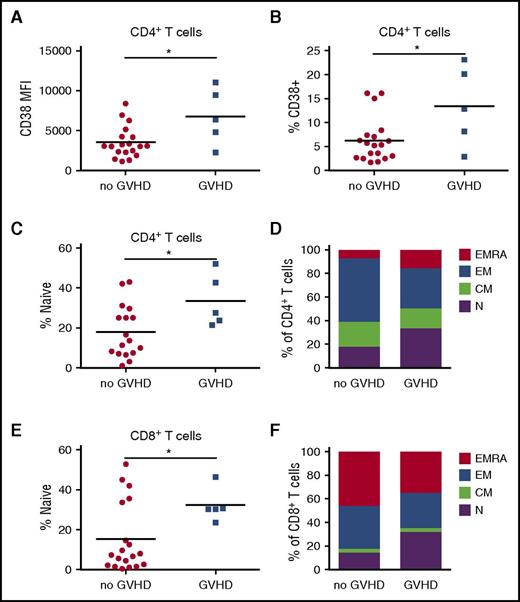
Maraviroc nonresponders show increased T-cell activation and naive T-cell bias
Some patients developed acute GVHD despite maraviroc treatment, and so we evaluated differences in immune parameters between maraviroc responders and nonresponders to define biomarkers for improved outcomes and to identify possible resistance mechanisms. To determine whether maraviroc responsiveness is associated with the degree of immune activation, we compared expression of T-cell activation markers. Maraviroc-treated patients who developed GVHD showed increased CD38 expression on CD4+ T cells at day 30 (Figure 6A-B). These data suggest that peripheral CD4+ T-cell activation is associated with the failure of maraviroc to prevent GVHD.
Maraviroc nonresponders show increased peripheral T-cell activation and naive T-cell skewing. (A) CD38 expression by MFI on CD4+ T cells at day 30 from maraviroc-treated patients who developed or did not develop grade 2-4 GVHD by day 100. (B) Percentage of CD38+ cells among CD4+ T cells at day 30 from maraviroc-treated patients who developed or did not develop grade 2-4 GVHD by day 100. (C) Day 30 percentage of naive CD4+ T cells from patients who developed or did not develop grade 2-4 GVHD by day 100. (D) Percentage of naive (N), central memory (CM), effector memory (EM), and effector memory RA (EMRA) among CD4+ T cells at day 30. (E) Day 30 percentage of CD8+ T cells from patients who developed or did not develop grade 2-4 GVHD by day 100. (F) Percentage of indicated T-cell subsets among CD8+ T cells at day 30. *P < .05, Student t test.
Maraviroc nonresponders show increased peripheral T-cell activation and naive T-cell skewing. (A) CD38 expression by MFI on CD4+ T cells at day 30 from maraviroc-treated patients who developed or did not develop grade 2-4 GVHD by day 100. (B) Percentage of CD38+ cells among CD4+ T cells at day 30 from maraviroc-treated patients who developed or did not develop grade 2-4 GVHD by day 100. (C) Day 30 percentage of naive CD4+ T cells from patients who developed or did not develop grade 2-4 GVHD by day 100. (D) Percentage of naive (N), central memory (CM), effector memory (EM), and effector memory RA (EMRA) among CD4+ T cells at day 30. (E) Day 30 percentage of CD8+ T cells from patients who developed or did not develop grade 2-4 GVHD by day 100. (F) Percentage of indicated T-cell subsets among CD8+ T cells at day 30. *P < .05, Student t test.
It has been hypothesized that the cells responsible for GVHD reside primarily in the TN cell compartment, which is supported by mouse models in which naive T cells cause more severe GVHD than memory cells.29 In addition, there are fivefold to 20-fold higher frequencies of minor H antigen-specific human naive CD8+ T cells than memory CD8+ T cells.30 At day 30, maraviroc nonresponders demonstrated a significant increase in the percentage of CD4+ TN cells compared with maraviroc responders (Figure 6C), with a concomitant trend toward decreased CD4+ TEM cells (Figure 6D) and no difference in Tregs (supplemental Figure 7A-B). We observed a similar significant increase in CD8+ TN cells in maraviroc nonresponders with a trend toward reduced CD8+ TEM and TEMRA cells (Figure 6E-F).
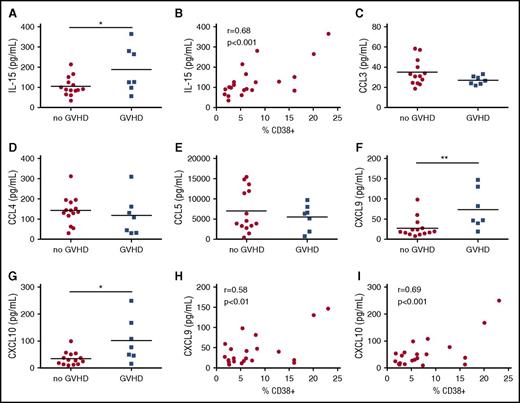
Maraviroc nonresponders show increased CXCR3 ligands and IL-15
To complement our flow cytometric analysis, we monitored day 30 serum levels of chemokines and cytokines that have been associated with lymphocyte proliferation and activation, comparing maraviroc responders and nonresponders. Interestingly, maraviroc nonresponders had increased interleukin-15 (IL-15) levels compared with maraviroc responders (Figure 7A). Previous studies suggest that IL-15 is an important mediator of GVHD,31,32 although we found no difference in day 30 IL-15 levels in control patients with or without GVHD in our study (supplemental Figure 8A). Intriguingly, we observed a positive correlation between the percentage of CD38+CD4+ T cells and IL-15 (Pearson correlation coefficient, r = 0.68, P = .0007), suggesting that elevated IL-15 is associated with resistance to CCR5 blockade (Figure 7B).
Maraviroc nonresponders show increased CXCR3 chemokines and IL-15. (A) Day 30 serum levels of IL-15 from maraviroc-treated patients who developed or did not develop grade 2-4 acute GVHD by day 180. (B) Correlation of IL-15 concentration and percentage of CD38+CD4+ T cells at day 30 (r, Pearson’s correlation coefficient). (C-G) Day 30 serum levels of CCL3 (C), CCL4 (D), CCL5 (E), CXCL9 (F), CXCL10 (G) from maraviroc-treated patients who developed or did not develop grade 2-4 GVHD by day 180. (H) Correlation of CXCL9 concentration and percentage of CD38+CD4+ T cells at day 30. (I) Correlation of CXCL10 concentration and percentage of CD38+CD4+ T cells at day 30. *P < .05, **P < .01, Student t test.
Maraviroc nonresponders show increased CXCR3 chemokines and IL-15. (A) Day 30 serum levels of IL-15 from maraviroc-treated patients who developed or did not develop grade 2-4 acute GVHD by day 180. (B) Correlation of IL-15 concentration and percentage of CD38+CD4+ T cells at day 30 (r, Pearson’s correlation coefficient). (C-G) Day 30 serum levels of CCL3 (C), CCL4 (D), CCL5 (E), CXCL9 (F), CXCL10 (G) from maraviroc-treated patients who developed or did not develop grade 2-4 GVHD by day 180. (H) Correlation of CXCL9 concentration and percentage of CD38+CD4+ T cells at day 30. (I) Correlation of CXCL10 concentration and percentage of CD38+CD4+ T cells at day 30. *P < .05, **P < .01, Student t test.
CCR5 recognizes 3 ligands (CCL3, CCL4, and CCL5),33 and in HIV patients, maraviroc increased plasma CCL4 levels.34 Although there was no difference in CCR5 ligand levels between cohorts in our study (data not shown), we wanted to exclude the possibility that CCR5 ligands were increased in select patients to explain escape from the protective effect of CCR5 blockade. However, there was no difference in CCL3, CCL4, and CCL5 levels between maraviroc responders and nonresponders (Figure 7C-E).
We next examined levels of CXCL9 and CXCL10, chemokines that signal through CXCR3, which has been linked to T-cell trafficking to GVHD target organs in mice and humans.26-28,35 Although there was no significant difference in CXCL9 or CXCL10 levels in control patients with or without GVHD (supplemental Figure 8B-C), maraviroc nonresponders demonstrated increased levels of CXCL9 and CXCL10 compared with maraviroc responders (Figure 7F-G). Moreover, we observed a positive correlation between CD38+CD4+ T-cell percentages and serum levels of CXCL9 (r = 0.58 and P = .006) and CXCL10 (r = 0.69 and P = .0005) (Figure 7H-I). There was no difference in CXCR3 expression on T cells from maraviroc responders and nonresponders (supplemental Figure 9). Taken together, these data suggest that CXCR3-mediated lymphocyte migration secondary to enhanced CXCL9 and CXCL10 levels may circumvent CCR5 blockade and promote GVHD in maraviroc-treated patients.
Discussion
Lymphocyte chemotaxis plays a fundamental role in acute GVHD, and we hypothesize that blockade of lymphocyte migration may be a viable strategy for improved therapeutics, quite distinguishable from typical efforts to use immunosuppressive medications. Indeed, our phase 1/2 trial demonstrated that brief 33-day treatment with the CCR5 antagonist maraviroc reduced the incidence of acute GVHD involving the gut and liver compared with historical rates.9 In the present study, we expand on these results by comparing maraviroc-treated patients to a contemporary control cohort receiving standard GVHD prophylaxis alone. Longer follow-up reveals a sustained reduction in acute GVHD incidence in maraviroc-treated patients compared with the control cohort, with a stronger effect on visceral vs skin GVHD and importantly no adverse impact on disease relapse, infections, or immune recovery. Thus, these data add further support that CCR5 blockade protects against GVHD.
We found that maraviroc diminished peripheral T-cell activation as there were fewer CD38+ T cells and HLA-DR+CD8+ T cells in the maraviroc cohort, independent of GVHD occurrence. The mechanism for reducing T-cell activation remains to be elucidated, but perhaps CCR5 blockade decreases alloreactive T-cell trafficking to sites of GVHD initiation as seen in preclinical models,5 consequently reducing systemic inflammation. Because the reduction in T-cell activation faded by day 60 and GVHD continues to emerge even at later time points beyond 6 months, a longer maraviroc course may better suppress GVHD initiation, a hypothesis that is currently being examined in a phase 2 clinical trial (NCT01785810).
Further supporting the activity of CCR5 blockade in preventing gut GVHD, maraviroc patients had decreased plasma levels of the gut-specific GVHD biomarker Reg3a at days 14 and 30, consistent with a role for maraviroc in blocking T-cell migration to target organs. This difference was maintained even after excluding patients who developed GVHD by the time of analysis or day 100. Interestingly, within the maraviroc cohort, we observed higher Reg3a levels at day 30 in patients who went on to develop GVHD, suggesting that biomarker levels at early time points may predict response to CCR5 blockade even prior to GVHD onset. Indeed, other groups have performed biomarker analyses incorporating Reg3a at fixed early time points and have validated their role in predicting outcome.36 To the best of our knowledge, our study is the first prospective analysis that shows the impact of a novel prophylaxis regimen on GVHD biomarker levels in humans.
Importantly, although CCR5 deficiency affects lymphocyte trafficking to target tissues, T cells would still be able to recognize pathogen-derived antigens.5 Furthermore, humans with CCR5 deficiency are not grossly susceptible to infections, and in fact, we observed no increase in infection rate with maraviroc in our study. This suggests that maraviroc can dampen alloreactive T-cell responses while not impairing immunity against infections.
To identify additional biomarkers that predict clinical response and elucidate potential resistance mechanisms to CCR5 blockade, we examined differences between patients who responded to maraviroc and those who did not. Maraviroc nonresponders demonstrated increased T-cell activation, as well as increased TN cells at day 30. This skewing may be clinically relevant, as preclinical models showed that reconstitution with donor naive but not memory T cells causes severe GVHD.29 Recently, a clinical trial in which TN cells were depleted from donor grafts demonstrated improved steroid-responsiveness in acute GVHD.37 Further suggesting that altered reconstitution of T-cell subsets can impact GVHD outcomes, high TN cell counts at day 28 are associated with acute GVHD development,38 and early skewing of T cells toward relatively immature subsets (naive and central memory) is associated with chronic GVHD.39 Thus, elevated proportions of TN cells may serve as a biomarker for maraviroc resistance.
The chemokine receptor CXCR3 has also been linked to GVHD pathogenesis. In mice, CXCR3 deficiency in donor T cells or CXCR3 blockade inhibited GVHD.26,35 In humans, serum CXCL10 levels were elevated in allo-HSCT recipients who developed acute GVHD.27,40 Furthermore, blocking CXCL10 in mice receiving CCR5−/− donor cells attenuated GVHD and improved survival.41 We found that CXCL9 and CXCL10 were upregulated in maraviroc-treated but not control patients who developed GVHD. Furthermore, in the maraviroc group, CXCL9 and CXCL10 levels were positively correlated with T-cell activation. This supports a model in which elevated CXCR3 ligands may be an important resistance mechanism to CCR5 blockade in GVHD prophylaxis and not just a general effect of GVHD.
Although this study better characterizes the clinical and immunologic effects of maraviroc in allo-HSCT, there are some limitations. First, this study compared a single-group study population to a contemporary cohort. Ongoing randomized trials will more directly test the efficacy of maraviroc in GVHD prophylaxis. In addition, although there was a reduced incidence of acute GVHD with maraviroc treatment without a significant increase in infection or disease relapse, there was no difference in NRM or OS. It is possible that NRM and OS were primarily affected by the proportion of patients in our trial who had a very high comorbidity score or very high-risk diseases, including several patients with refractory T-cell lymphoma and refractory leukemia. Despite a twofold difference in the incidence of GVHD-related death, this study is underpowered to detect it in a statistically significant manner. Therefore, these outcomes are being examined in a multicenter randomized study (BMT-CTN 1203; NCT02208037).
Taken together, our analysis reveals a distinct immunologic effect for CCR5 blockade in allo-HSCT recipients and suggests a novel resistance mechanism. These studies bolster CCR5 antagonism as an effective strategy for GVHD prevention that preserves immunity against tumor and pathogens while providing support for extended maraviroc treatment duration and investigation into CXCR3 as a therapeutic target.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Megan Sykes for helpful discussions.
This work was supported by a Career Development Award from the Conquer Cancer Foundation (R.R.) and an Amy Strelzer Manasevit Award from the National Marrow Donor Program (R.R.). Additional support was provided from the National Institutes of Health, National Heart, Lung, and Blood Institute grant U01-HL069286 (D.L.P.) and National Cancer Institute grants K23-CA178202 (R.R.) and P30-CA016520 (R.H.V.). The clinical trial described in this manuscript was partially funded by an investigator-initiated research grant from Pfizer Inc.
Authorship
Contribution: R.R. and R.H.M. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; A.P.H., L.P.R., and X.K.W. performed experiments and analyzed data; L.C. collected and analyzed clinical information; R.M. consulted on statistical analysis; Y.Z., S.G.E., J.A.H., D.L.P., and R.H.V. designed experiments and reviewed the manuscript; and all authors reviewed the final manuscript.
Conflict-of-interest disclosure: The authors disclose off-label use of maraviroc for the prevention of GVHD and research support from Pfizer to R.R., D.L.P., and R.H.V.
Correspondence: Ran Reshef, Division of Hematology/Oncology and the Columbia Center for Translational Immunology, Department of Medicine, Columbia University Medical Center, 630 W 168th St, Mailbox 127, New York, NY 10032; e-mail: ran.reshef@columbia.edu.