Key Points
First-line or subsequent dasatinib or nilotinib can be safely stopped in CML patients with deep and long-lasting molecular responses.
A suboptimal response or resistance prior to dasatinib or nilotinib is associated with significantly worse treatment-free remission.
Abstract
STOP second generation (2G)–tyrosine kinase inhibitor (TKI) is a multicenter observational study designed to evaluate 2G-TKI discontinuation in chronic myeloid leukemia (CML). Patients receiving first-line or subsequent dasatinib or nilotinib who stopped therapy after at least 3 years of TKI treatment and in molecular response 4.5 (MR4.5) with undetectable BCR-ABL1 transcripts for the 2 preceding years at least were eligible for inclusion. This interim analysis reports outcomes of 60 patients with a minimum follow-up of 12 months (median 47, range: 12-65). Twenty-six patients (43.3%) experienced a molecular relapse defined as the loss of a major molecular response (MMR). Relapses occurred after a median time of 4 months (range: 1-38). Cumulative incidences of molecular relapse by 12 and 48 months were 35% (95% confidence interval [CI], 24.79% to 49.41%) and 44.76% (95% CI, 33.35% to 59.91%), respectively. Treatment-free remission (TFR) rates at 12 and 48 months were 63.33% (95% CI, 51.14% to 75.53%) and 53.57% (95% CI, 40.49% to 66.65%), respectively. In univariate analysis, prior suboptimal response or TKI resistance was the only baseline factor associated with significantly worse outcome. A landmark analysis demonstrated that loss of MR4.5 3 months after stopping TKI was predictive of failure to maintain MMR later on. During the treatment-free phase, no progression toward advanced phase CML occurred, and all relapsing patients regained MMR and MR4.5 after restarting therapy. In conclusion, discontinuation of first-line or subsequent 2G-TKI yields promising TFR rates without safety concerns. Further research is encouraged to better define conditions that will offer patients the highest chance to remain free from 2G-TKI therapy.
Introduction
In the early 2000s, the tyrosine kinase inhibitor (TKI) imatinib became the best standard of care for patients with chronic phase-chronic myeloid leukemia (CP-CML) on the basis of an unprecedented progression-free survival benefit leading to a near-to-normal life expectancy.1-4 Yet, drug resistance or inadequate tolerability affecting up to one third of patients led to the development of new generations of TKI with a greater potency against the oncogenic BCR-ABL1 kinase and different safety profiles.5 Two of these agents, the second- generation (2G)–TKI dasatinib and nilotinib, are efficient rescue therapies for patients with imatinib resistance or intolerance.6,7 Since 2010, they also became treatment options for newly diagnosed CP-CML, on the basis of higher rates of optimal responses and a lower risk of progression in comparison with imatinib.8,9 Despite these major advances, several studies established that TKI monotherapies were unable to eliminate quiescent BCR-ABL1+ hematopoietic stem cells.10-12 As a result, TKI were viewed as nondefinitively curative, and until recently, the recommendation was to pursue lifelong treatment.13 However, the notion that TKI may never be stopped was successfully challenged in imatinib-treated patients with deep and sustained molecular responses. In the pioneering STIM and TWISTER trials, patients with CP-CML on imatinib therapy for a minimum of 3 years who had achieved and maintained at least a 4.5-log reduction in residual disease with undetectable BCR-ABL1 transcripts for at least 2 years had a probability of maintaining such a deep molecular response level without any treatment of about 40%.14,15 These results were confirmed in subsequent studies performed independently.16,17 Then the loss of a major molecular response (MMR: BCR-ABL1 internationally standardized [IS] ratio ≤ 0.1%) rather than that of a deep molecular response was proposed as a new definition for molecular relapse and trigger for treatment resumption, allowing an increase in TKI cessation success rate of about 20%.18
Dasatinib and nilotinib induce significantly higher rates of deep molecular responses and a more rapid decline in BCR-ABL1 transcript levels than does imatinib at 400 mg daily in newly diagnosed CP-CML patients.8,9 In addition, patients in complete cytogenetic response (CCyR) who lack a deep molecular response on imatinib have a higher probability of achieving deep molecular responses after a switch to second-line nilotinib than do those who remain on imatinib.19 Altogether, these data suggest that the use of dasatinib or nilotinib may increase opportunities to attempt treatment cessation in comparison with imatinib. Experience with 2G-TKI discontinuation is currently limited. Few case reports or small series of patients who ceased therapy because of severe side effects, because of pregnancy, or against medical advice suggest that a treatment-free disease control may be achievable.20-24 The DADI trial recently showed that it was possible to stop dasatinib taken as a second or subsequent line of therapy in a series of 63 CP-CML patients with deep and stable molecular responses.25 In the STOP-2G TKI study, we aimed to evaluate outcomes of first-line or subsequent dasatinib or nilotinib discontinuation in CML patients with long-lasting and deep molecular responses, with treatment-free remission (TFR) as the primary endpoint.
Patients and methods
Patients
Adult patients meeting criteria for CP- or accelerated phase (AP)-CML at diagnosis and treated with dasatinib or nilotinib at any dose—either frontline or after imatinib intolerance, suboptimal response, or resistance as defined by the EuropeanLeukemiaNet (ELN)—were eligible to enter the study upon treatment discontinuation after 3 years or more of TKI therapy and 2 years or more of a molecular response 4.5 (MR4.5) with undetectable BCR-ABL1 transcripts (hereinafter named uMR4.5), namely under the same conditions as in the STIM study.14,26,27 Treatments with hydroxyurea, interferon-α (IFN-α) with or without cytarabine, or autologous hematopoietic stem cell transplantation (HSCT) prior to TKI were permitted. Patients who had undergone allogeneic HSCT were excluded, as well as were patients with nonmajor BCR-ABL1 transcripts and those with a history of progression to AP- or blast crisis (BC)-CML on-therapy. Patients who received chemotherapy or radiotherapy for other malignancies or immunosuppressive treatments during the past 12 months and those who failed a prior TKI discontinuation attempt for whatever reason were also excluded. The protocol for this observational multicenter study that included a total of 100 patients was approved by the institutional review board (IRB 00006477) of Hôpitaux Universitaires Nord Val de Seine, Paris Diderot University (Assistance Publique-Hôpitaux de Paris; registration no. 15-050) and conducted in accordance with applicable regulatory requirements. Data from patients fulfilling inclusion criteria without exclusion criteria were prospectively collected. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Monitoring and definition of molecular responses
Monitoring of molecular responses was performed in laboratories belonging to the French network of quality control and accreditation of molecular biology laboratories in the setting of hematological malignancies (Groupe des Biologistes Moléculaires des Hémopathies Maligne).28 Molecular response measurement followed the ELN recommendations for BCR-ABL1 messenger RNA quantification by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and scoring of deep molecular responses.29-31 MR4.5 was defined as detectable BCR-ABL1 IS ≤ 0.0032% or undetectable BCR-ABL1 in samples with ≥40,000 ABL1 transcripts until the end of 2012 and ≥32,000 ABL1 transcripts later on.30 After 2G-TKI discontinuation, BCR-ABL1 transcripts were quantified in the peripheral blood at the same frequency as in the STIM study, namely monthly during the first 12 months, every 2 to 3 months during the second year, and every 3 to 6 months for up to 5 years.14
Definition of molecular relapse and treatment resumption policy
Molecular relapse was defined by the loss of MMR on any single test and did not require confirmation by a second test, as in the A-STIM study.18 In case of MMR loss, it was advised to perform bone marrow cytogenetic analyses and BCR-ABL1 kinase domain mutation assessments in patients with BCR-ABL1 IS ≥ 1% and in those failing to regain MMR after therapy resumption. Molecular relapse triggered re-initiation of TKI therapy as prescribed prior to discontinuation, unless otherwise indicated. In case of relapse, it was recommended to measure BCR-ABL1 transcript levels every 3 months during the first 12 months following therapy resumption and every 3 to 6 months thereafter.
Endpoints
The primary endpoint was TFR at 12 months, with an interim analysis upon inclusion of at least 50 patients and a follow-up of at least 12 months. This interim analysis was planned in order to facilitate early detection of important safety signals such as disease progression or resistance during the treatment-free phase and upon treatment resumption. Secondary endpoints included longer-term TFR, safety (including progression to AP/BC-CML and sensitivity to treatment resumption), and search for factors associated with molecular relapse.
Statistical analyses
TFR was estimated by the method of Kaplan-Meier and defined as the time interval from 2G-TKI discontinuation to the date documenting the first occurrence of MMR loss or re-initiation of therapy (whichever came first), regardless of the reason. For all the other patients, data were censored at maximum molecular follow-up. Incidence of molecular relapse was calculated using the cumulative incidence function with re-initiation of therapy in the absence of a molecular relapse and death in MMR as competing events. For all the other patients, data were censored at maximum molecular follow-up. Evolution of BCR-ABL1 transcript levels between the date of identification of a molecular relapse and the date of treatment resumption was evaluated using the Wilcoxon matched-pairs signed-rank test. A Mann-Whitney U test was used for comparison of quantitative variables from 2 independent groups. Two-tailed P values of <0.05 were considered statistically significant. Molecular relapse was also analyzed on the basis of BCR-ABL1 transcript levels at 3 months with the cumulative incidence function. For this landmark analysis, patients with molecular assessments at 3 months and in MMR without treatment up to the 3-month time point were included. Distribution of molecular relapse between groups over time was considered after the landmark time and compared with the two-sided Gray’s test. Clinicobiological variables at baseline were assessed as potential prognostic factors for molecular relapse, with a P value of ≤.10 indicating eligibility for multivariate analysis. Quantitative factors were categorized into 2 groups with the cutoff set at median. All clinicobiological variables were assessed by univariate analysis using the Fine and Gray models and the two-sided Gray’s test. The NCSS 10 software and the GraphPad Prism 6 software were used for statistical analysis.
Results
Patient characteristics
At the time of analysis, 60 patients completed at least 12 months of follow-up after treatment cessation. Patient characteristics at baseline are detailed in Table 1. Median age was 60 years (range: 26-81), and 38 patients were females (63.3%). All patients were in CP at CML diagnosis, and the Sokal score was low in 32 patients (53.3%), intermediate in 16 patients (26.7%), high in 9 patients (15%) and unknown in 3 patients (5%). Prior therapies before TKI included IFN-α in 17 patients (28.3%). TKI type before discontinuation consisted of first-line nilotinib or dasatinib in 8 patients (13.3%), second-line dasatinib or nilotinib in 40 patients (66.7%), and third-line dasatinib or nilotinib in 12 patients (20%). A history of intolerance to imatinib, suboptimal response, or TKI resistance was reported in 39 (65%) and 13 (21.7%) patients, respectively. The median duration of TKI treatment was 76 months (range: 36-153). The median duration of 2G-TKI treatment prior to discontinuation was 39 months (range: 19-83), and the median duration of uMR4.5 was 29 months (range: 24-64). Thirty patients (50%) discontinued nilotinib, and 30 patients (50%) discontinued dasatinib. The median follow-up after treatment discontinuation was 47 months (range: 12-65). Four patients discontinued the study early because of lack of follow-up (n=2) or death from non-CML-related causes (n=2, including 1 patient who died of consequences of Alzheimer’s disease after treatment resumption owing to MMR loss, and 1 patient who died of heart failure in MMR without therapy).
Baseline characteristics of patients
| Parameters . | Results (n=60) . |
|---|---|
| Demographics | |
| Median age | 60 y (range: 26-81) |
| Female gender | 63.3% (n = 38) |
| CML at diagnosis | |
| Chronic phase | 100% (n = 60) |
| Sokal risk group | |
| Low | 53.3% (n = 32) |
| Intermediate | 26.7% (n = 16) |
| High | 15% (n = 9) |
| Unknown | 5% (n = 3) |
| Cytogenetics | |
| Ph1 without ACAs | 93.2% (n = 56) |
| Ph1 with ACAs | 3.4% (n = 2) |
| Unknown | 3.4% (n = 2) |
| M-bcr transcript type | 100% (n = 60) |
| Treatment history | |
| Prior IFN-α | 28.3% (n = 17) |
| 1st line dasatinib or nilotinib | 13.3% (n = 8) |
| 2nd line dasatinib or nilotinib | 66.7% (n = 40) |
| 3rd line dasatinib or nilotinib | 20% (n = 12) |
| Intolerance to imatinib | 65% (n = 39) |
| Suboptimal response or resistance to imatinib | 21.7% (n = 13) |
| Imatinib-resistant BCR-ABL1 mutation* | 30.8% (n = 4) |
| No mutation | 53.8% (n = 7) |
| Mutation status unknown | 15.4% (n = 2) |
| Median duration of TKI therapy | 76 mo (range: 36-153) |
| Median duration of 2G-TKI treatment | 39 mo (range: 19-83) |
| Median duration of uMR4.5 | 29 mo (range: 24-64) |
| Treatment discontinuation | |
| Dasatinib | 50% (n = 30) |
| Nilotinib | 50% (n = 30) |
| Parameters . | Results (n=60) . |
|---|---|
| Demographics | |
| Median age | 60 y (range: 26-81) |
| Female gender | 63.3% (n = 38) |
| CML at diagnosis | |
| Chronic phase | 100% (n = 60) |
| Sokal risk group | |
| Low | 53.3% (n = 32) |
| Intermediate | 26.7% (n = 16) |
| High | 15% (n = 9) |
| Unknown | 5% (n = 3) |
| Cytogenetics | |
| Ph1 without ACAs | 93.2% (n = 56) |
| Ph1 with ACAs | 3.4% (n = 2) |
| Unknown | 3.4% (n = 2) |
| M-bcr transcript type | 100% (n = 60) |
| Treatment history | |
| Prior IFN-α | 28.3% (n = 17) |
| 1st line dasatinib or nilotinib | 13.3% (n = 8) |
| 2nd line dasatinib or nilotinib | 66.7% (n = 40) |
| 3rd line dasatinib or nilotinib | 20% (n = 12) |
| Intolerance to imatinib | 65% (n = 39) |
| Suboptimal response or resistance to imatinib | 21.7% (n = 13) |
| Imatinib-resistant BCR-ABL1 mutation* | 30.8% (n = 4) |
| No mutation | 53.8% (n = 7) |
| Mutation status unknown | 15.4% (n = 2) |
| Median duration of TKI therapy | 76 mo (range: 36-153) |
| Median duration of 2G-TKI treatment | 39 mo (range: 19-83) |
| Median duration of uMR4.5 | 29 mo (range: 24-64) |
| Treatment discontinuation | |
| Dasatinib | 50% (n = 30) |
| Nilotinib | 50% (n = 30) |
BCR-ABL1 kinase domain mutations: A387P (n = 1), M244V (n = 2), and H396R + F359V (n = 1).
Molecular relapses
Twenty-six patients (43.3%) experienced a molecular relapse (hereinafter named relapsing patients), and the median time to molecular relapse was 4 months (range: 1-38). The median BCR-ABL1 IS value at MMR loss was 0.23% IS (range: 0.11-1.73). Twenty-one relapsing patients (80.8%) lost MMR within 12 months and only 5 (19.2%) after 12 months (between months 14 and 38). Individual evolutions in time of BCR-ABL1 transcripts from treatment cessation to MMR loss are shown in Figure 1A. One patient in whom BCR-ABL1 transcripts became detectable below the MMR threshold after 5 months was accidentally instructed to restart TKI therapy at month 6, and 1 patient died of heart failure in MMR after 55 months. Consequently, the cumulative incidence of molecular relapse was 35% (95% confidence interval [CI], 24.79% to 49.41%) by 12 months and 44.76% (95% CI, 33.35% to 59.91%) by 48 months (Figure 1B). Estimated TFR rates at 12 and 48 months were 63.33% (95% CI, 51.14% to 75.53%) and 53.57% (95% CI, 40.49% to 66.65%), respectively (Figure 1C).
Molecular relapses and TFR after 2G-TKI discontinuation. (A) Evolution of BCR-ABL1 transcripts over time as measured by RT-qPCR from 2G-TKI discontinuation until MMR loss in relapsing patients (n = 26). Red lines correspond to molecular relapses occurring within 12 months after treatment cessation, and blue lines to relapses occurring after 12 months. (B) Cumulative incidence of molecular relapses. (C) Treatment-free remission.
Molecular relapses and TFR after 2G-TKI discontinuation. (A) Evolution of BCR-ABL1 transcripts over time as measured by RT-qPCR from 2G-TKI discontinuation until MMR loss in relapsing patients (n = 26). Red lines correspond to molecular relapses occurring within 12 months after treatment cessation, and blue lines to relapses occurring after 12 months. (B) Cumulative incidence of molecular relapses. (C) Treatment-free remission.
Outcome after molecular relapse
Twenty-five out of the 26 relapsing patients resumed therapy and the median follow-up after therapy resumption was 36 months (range: 3-59). The median time frame from TKI discontinuation to resumption was 5.5 months (range: 2.5-38.3) and that between molecular relapse and treatment reintroduction was 1.1 months (range: 0.4-5.8). The 5.8-month delay in restarting therapy in one case with BCR-ABL1 IS of 0.13% at MMR loss was explained by patient reluctance to restart nilotinib because of the presence of cardiovascular comorbidities and a history of pleural effusion on dasatinib. After patient-physician discussion, bosutinib was finally chosen. Dasatinib instead of nilotinib was also chosen for another patient because of prior tolerance issues. The same TKI as prior to treatment cessation was restarted in the 23 other patients. One patient in whom MMR loss at 4 months appeared to be transient on subsequent analyses remained treatment-free upon his request. In 13 patients with a median elapsed time between molecular relapse and treatment resumption of 1.5 months (range: 0.7-5.8), BCR-ABL1 transcripts were measured once again on the day treatment was resumed. A significant and rapid increase between the 2 time points was observed with median BCR-ABL1 IS of 0.28% (range: 0.11-1.73) at first identification of molecular relapse and 1.1% (range: 0.21-2.81) (P = .0017) at treatment reintroduction (Figure 2A). Cytogenetic analyses were not performed, but the number of patients with BCR-ABL1 transcript levels above 1%, a level likely to correspond to a loss of CCyR, increased from 2 (15.3%) to 7 (53.8%) patients.32,33 Importantly, all patients remained in complete hematologic response; no progression toward advanced-phase CML was reported, and all patients remained sensitive to treatment rechallenge, except one elderly patient who could not be evaluated after dasatinib resumption because of death by complications from Alzheimer’s disease after 3 months. MMR, MR4.5, and uMR4.5 were regained after a median time of 2 (range: 1-6), 3 (range: 1-21), and 4 (range: 1-3) months, respectively (Figure 2B). Of note, 7 relapsing patients underwent a second treatment discontinuation attempt on the recommendation of their treating physician (n=3) or because of onset of medically important events (n=4) 31 months (range: 18-44) in median after therapy had been resumed. The median observation time after the second discontinuation attempt was 11 months (range: 6-24). The majority of patients (n=5) failed to remain treatment-free and lost MMR again after a median time of 3 months (range: 1-11), as has been previously described with imatinib.34
Evolution of BCR-ABL1 transcript levels from molecular relapse onward. (A) BCR-ABL1 transcript levels as measured by RT-qPCR at molecular relapse and at treatment resumption in patients with available evaluations at both time points (n=13). Horizontal bars represent median, minimum, and maximum values. (B) BCR-ABL1 transcript levels as measured by RT-qPCR at molecular relapse and every 3 months until 12 months after treatment resumption (n=25). One patient could not be evaluated after molecular relapse because of death unrelated to CML, and 1 patient had not yet been evaluated at 12 months. Solid dots correspond to detectable BCR-ABL1 transcripts. Open dots correspond to undetectable BCR-ABL1 transcripts.
Evolution of BCR-ABL1 transcript levels from molecular relapse onward. (A) BCR-ABL1 transcript levels as measured by RT-qPCR at molecular relapse and at treatment resumption in patients with available evaluations at both time points (n=13). Horizontal bars represent median, minimum, and maximum values. (B) BCR-ABL1 transcript levels as measured by RT-qPCR at molecular relapse and every 3 months until 12 months after treatment resumption (n=25). One patient could not be evaluated after molecular relapse because of death unrelated to CML, and 1 patient had not yet been evaluated at 12 months. Solid dots correspond to detectable BCR-ABL1 transcripts. Open dots correspond to undetectable BCR-ABL1 transcripts.
Patterns of BCR-ABL1 transcript levels in nonrelapsing patients
Several imatinib discontinuation studies in patients with durable uMR4.5 showed that peripheral blood BCR-ABL1 transcripts could be intermittently detected at low amounts in a substantial proportion of patients who successfully stopped the drug.14,15,18 Here, 33 patients did not experience a molecular relapse after dasatinib or nilotinib cessation (hereinafter named nonrelapsing patients), and 32 of them remained treatment-free during a median observation time of 47 months (range: 12-65). Only 12 nonrelapsing patients (36.3%) maintained an uMR4.5 throughout the entire follow-up. Among the 21 remaining patients (63.7%), 15 had detectable BCR-ABL1 transcripts in single samples and 6 in at least 2 consecutive samples on one or more occasions at any time during follow-up, always below the MMR threshold. Overall, the cumulative incidence of MR4.5 loss on at least one occasion in nonrelapsing patients was 26.47% by 12 months (95% CI, 15.12-46.35) and 34.09% by 48 months (95% CI, 20.91-55.59).
BCR-ABL1 transcripts at early time points and molecular relapse
Our observation that BCR-ABL1 transcripts could become intermittently detectable in a substantial proportion of patients even in the absence of molecular relapse and that most molecular relapses occurred during the first year led us to ask whether molecular response categories early after 2G-TKI discontinuation were indicative of outcome. To this purpose, we evaluated the incidence of molecular relapse by a landmark analysis at 3 months in the 49 patients still in MMR without treatment at this time point. At 3 months, 38 of these were in MR4.5 (including 31 in uMR4.5), and 11 had less than a MR4.5 (0.0032% < BCR-ABL1 IS ≤ 0.1%). Results showed that the cumulative incidence of relapse was lowest among patients maintaining deepest molecular response levels (overall P ≤ .00001) (Figure 3A). The cumulative incidence of molecular relapse by 48 months was 17.59% (95% CI, 8.34% to 37.12%) for patients maintaining a MR4.5 at 3 months and 81.82% (95% CI, 61.92% to 100%) for those with less than a MR4.5 but in MMR at 3 months. Corresponding TFR rates at 48 months were 79.78% (95% CI, 66.03-93.53) in the former and 18.18% (95% CI, 0% to 40.97%) in the latter (Figure 3B).
Landmark analysis according to molecular response categories in patients with MMR 3 months after 2G-TKI discontinuation. (A) Cumulative incidence of relapse and overall P value (Gray’s test) are shown. (B) TFR and overall P value (log rank) are shown.
Landmark analysis according to molecular response categories in patients with MMR 3 months after 2G-TKI discontinuation. (A) Cumulative incidence of relapse and overall P value (Gray’s test) are shown. (B) TFR and overall P value (log rank) are shown.
Baseline factors associated with molecular relapse
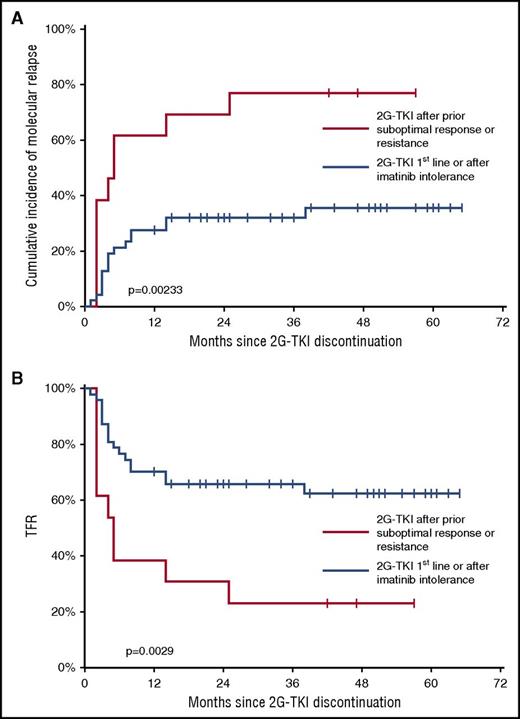
In some imatinib discontinuation trials, the Sokal score and imatinib treatment duration appear to have a prognostic value with respect to the risk of molecular relapse.14,17,35 To analyze the relevance of clinical and biological variables at baseline in our study, we assessed the following parameters as potential prognostic factors of molecular relapse using univariate analysis: age, sex, Sokal risk group, prior IFN-α therapy, duration of TKI treatment, duration of uMR4.5, type of 2G-TKI, and prior history of suboptimal response or TKI resistance. Results showed that patients who were prescribed dasatinib or nilotinib as first-line therapy or because of intolerance to imatinib, but who were optimal responders to imatinib, had significantly fewer molecular relapses than did those who received these drugs because of prior suboptimal response or resistance (overall P = .00233) (Table 2). The incidence of molecular relapse in the former and latter groups were 35.51% (95% CI, 23.73% to 53.15%) and 76.92% (95% CI, 57.11% to 100%) by 48 months, respectively (Figure 4A). Corresponding TFR rates at 48 months in the former and latter groups were 62.36% (95% CI, 47.88-76.84) and 23.08% (95% CI, 0.17-45.98), respectively (Figure 4B). No significant association between the other parameters and molecular relapse was found (Table 2). Of note, it was not possible to analyze the impact of the type of imatinib treatment failure (suboptimal response, primary or secondary resistance, and mutation status) on the incidence of molecular relapse upon dasatinib or nilotinib discontinuation because of the small number of patients in each subgroup. These results led us to ask whether molecular relapses had more aggressive characteristics in patients with former suboptimal response or TKI resistance. The median time to molecular relapse was 3 months (range: 2-25) in the 10 patients with prior suboptimal response or resistance and 4 months (range: 1-28) in the 16 patients who lacked such treatment history (P = .365). Median BCR-ABL1 IS percentage at MMR loss in the former and latter groups were 0.19 (range: 0.11-1.73) and 0.24 (range: 0.11-1.25) (P = .785), respectively, and no efficacy issues were encountered in either group upon treatment resumption (data not shown).
Potential prognostic factors for molecular relapse: univariate analysis
| Variable . | Cumulative incidence of relapse by 48 mo (95% CI) . | Overall P value . |
|---|---|---|
| Age | 0.154 | |
| >60 y (n = 28) | 54.78% (38.85-77.24) | |
| ≤60 y (n = 32) | 36.72% (22.49-59.95) | |
| Sex | 0.577 | |
| Male (n = 22) | 36.36% (20.92-63.2) | |
| Female (n = 38) | 49.66% (35.45-69.55) | |
| Sokal score | 0.623 | |
| Low (n = 32) | 42.71% (27.88-65.3) | |
| Intermediate + high (n = 25) | 49.1% (33.07-74.74) | |
| Prior IFN-α | 0.637 | |
| Yes (n = 17) | 41.76% (23.67-73.7) | |
| No (n = 43) | 45.94% (32.65-64.64) | |
| TKI treatment duration | 0.643 | |
| >76 mo (n = 30) | 47.11% (32.13-69.08) | |
| ≤76 mo (n = 30) | 42.29% (27.10-66) | |
| 2G-TKI treatment duration | 0.772 | |
| >39 mo (n = 29) | 44.83% (29.94-67.13) | |
| ≤39 mo (n = 31) | 44.31% (29.22-67.19) | |
| uMR4.5 duration | 0.597 | |
| >29 mo (n = 29) | 37.93% (23.81-60.42) | |
| ≤29 mo (n = 31) | 50.52% (35.13-72.66) | |
| 2G-TKI type | 0.331 | |
| Dasatinib (n = 30) | 50.83% (35.52-72.76) | |
| Nilotinib (n = 30) | 38.62% (29.99-62.15) | |
| Prior intolerance or resistance to TKI | 0.00233 | |
| Yes (n = 13) | 35.51% (23.73-53.15) | |
| No (n = 47) | 76.92% (57.11-100) |
| Variable . | Cumulative incidence of relapse by 48 mo (95% CI) . | Overall P value . |
|---|---|---|
| Age | 0.154 | |
| >60 y (n = 28) | 54.78% (38.85-77.24) | |
| ≤60 y (n = 32) | 36.72% (22.49-59.95) | |
| Sex | 0.577 | |
| Male (n = 22) | 36.36% (20.92-63.2) | |
| Female (n = 38) | 49.66% (35.45-69.55) | |
| Sokal score | 0.623 | |
| Low (n = 32) | 42.71% (27.88-65.3) | |
| Intermediate + high (n = 25) | 49.1% (33.07-74.74) | |
| Prior IFN-α | 0.637 | |
| Yes (n = 17) | 41.76% (23.67-73.7) | |
| No (n = 43) | 45.94% (32.65-64.64) | |
| TKI treatment duration | 0.643 | |
| >76 mo (n = 30) | 47.11% (32.13-69.08) | |
| ≤76 mo (n = 30) | 42.29% (27.10-66) | |
| 2G-TKI treatment duration | 0.772 | |
| >39 mo (n = 29) | 44.83% (29.94-67.13) | |
| ≤39 mo (n = 31) | 44.31% (29.22-67.19) | |
| uMR4.5 duration | 0.597 | |
| >29 mo (n = 29) | 37.93% (23.81-60.42) | |
| ≤29 mo (n = 31) | 50.52% (35.13-72.66) | |
| 2G-TKI type | 0.331 | |
| Dasatinib (n = 30) | 50.83% (35.52-72.76) | |
| Nilotinib (n = 30) | 38.62% (29.99-62.15) | |
| Prior intolerance or resistance to TKI | 0.00233 | |
| Yes (n = 13) | 35.51% (23.73-53.15) | |
| No (n = 47) | 76.92% (57.11-100) |
Quantitative variables were categorized into 2 groups with cutoffs set at median. P < .05 was considered statistically significant.
Outcome after 2G-TKI discontinuation according to prior suboptimal response or TKI resistance. (A) Cumulative incidence of relapse and overall P value (Gray’s test) are shown. (B) TFR and overall P value (log rank) are shown.
Outcome after 2G-TKI discontinuation according to prior suboptimal response or TKI resistance. (A) Cumulative incidence of relapse and overall P value (Gray’s test) are shown. (B) TFR and overall P value (log rank) are shown.
Discussion
Evidence that imatinib cessation is feasible in CML patients with deep and durable molecular responses has challenged the idea of a lifelong dependency on TKI. Here, we demonstrate that TFR is not restricted to imatinib-treated patients and that first-line or subsequent dasatinib or nilotinib may also be successfully and safely stopped. Under stringent treatment-cessation conditions and loss of MMR as a definition for molecular relapse and trigger for treatment resumption, we report a TFR rate of 63.33% (95% CI, 51.14% to 75.53%) at 12 months. In the A-STIM study in which imatinib was discontinued under comparable circumstances with respect to minimal treatment length and deep molecular response level and with the same molecular relapse definition, the estimated TFR rate at 12 months was 64% (95% CI, 54% to 75%).18 Although we admit that no direct comparison can be made between the 2 studies, we can reasonably suggest that outcome after stopping dasatinib or nilotinib may be close to that obtained after stopping imatinib.
In our study, the majority of molecular relapses occurred within 12 months after 2G-TKI cessation, and few relapses were observed at later time points. This time distribution pattern of molecular relapses was also described in several imatinib discontinuation studies.15,18,35 All together, these results and ours emphasize the need for a regular monitoring of the residual disease even in the long term, regardless of which TKI was previously used. Another reason for considering a prolonged survey as an essential component of care is our observation that BCR-ABL1 transcripts become occasionally detectable at low levels in a substantial proportion of patients who could remain treatment-free, a phenomenon that was also observed after stopping imatinib or IFN-α.15,18,36 Of course, one might argue that these findings may simply correspond to false-positive results or to variations in RT-qPCR sensitivity from one test to another. Nevertheless, this observation and evidence of persisting primitive leukemic cells in most if not all TKI-treated patients regardless of treatment discontinuation outcome disfavor a definitive cure.37-39 Thus in spite of recent reassuring long-term follow-up data from the STIM trial, very-long-term relapses cannot be ruled out, as it is the case after allogeneic HSCT.35,40,41
The identification of patients who are less likely to succeed with the discontinuation of dasatinib or nilotinib is a key issue, because they may be offered novel strategies in the near future.42-44 Here, analysis of baseline parameters allowed us to demonstrate that patients with a history of suboptimal response or TKI resistance had a significantly higher incidence of molecular relapse than did newly diagnosed patients and those with prior intolerance to imatinib. This result is corroborated by research from the DADI trial, in which discontinuation of second-line or subsequent dasatinib was proposed for CP-CML patients with deep molecular response (BCR-ABL1 IS < 0.0069%) for at least 1 year.25 An explanation for this common finding remains elusive, but if we admit that all study patients harbored a reservoir of BCR-ABL1+ hematopoietic stem cells, it may be elucidated, because we are gaining a better understanding of their biological heterogeneity.45 Fortunately, in our study, relapses in patients with former suboptimal response or TKI resistance failed to show any specific aggressive features in comparison with those occurring in patients who received 2G-TKI first-line therapy or who received 2G-TKI after intolerance to prior therapy. As with the DADI trial, our study did not reveal any significant association between molecular relapse and age, sex, Sokal score, past IFN-α exposure, duration of TKI treatment, or uMR4.5 duration. These results are in apparent contradiction to those of the STIM study, in which the Sokal score and duration of imatinib administration had an impact on TFR.35 Whether optimal timing for 2G-TKI discontinuation may be guided by treatment or by deep molecular response duration will need to be discovered by dedicated studies in patients with less-heterogeneous treatment histories. Another finding of our work consisted of the lack of prognostic value of the 2G-TKI type on the relapse rate. Finally, in addition to baseline parameters, assessment of the clinical significance of BCR-ABL1 transcript levels in a landmark analysis allowed us to demonstrate the value of early molecular response categories in predicting treatment discontinuation outcomes. A rise at 3 months above the MR4.5 level was associated with a significantly higher risk of relapse at later time points than was the maintenance of a MR4.5, underlying the importance of a frequent molecular monitoring soon after treatment cessation.
Importantly, we did not encounter any safety issues after discontinuation of dasatinib or nilotinib, although we recognize that our study was not designed to detect a potential “TKI withdrawal syndrome,” such as that described after imatinib discontinuation.46 All relapsing patients who resumed therapy regained MMR and deep molecular responses. However, the rapid increase in BCR-ABL1 transcripts during the time window between MMR loss and treatment resumption highlights the necessity of prompt treatment reintroduction once a molecular relapse is identified, because we cannot exclude the possibility that a long delay may lead to the accumulation of additional genetic events and the loss of disease control.47
In conclusion, our study represents a substantial step toward a better knowledge of patient outcome after dasatinib or nilotinib discontinuation. Of course, the definition of optimal conditions for 2G-TKI cessation, namely those offering the best chances of remaining treatment-free, will need further clarification before widespread practice of TFR in routine patient care, especially in the first-line setting. Several other discontinuation trials of dasatinib or nilotinib given in first- or second-line therapy are already underway, but to what extent these drugs will offer TFR opportunities in comparison with imatinib will require dedicated trials and real-life studies. Research efforts are also required to uncover mechanisms preventing residual leukemic cells from expanding and causing molecular relapse, with the goal of developing clinically relevant strategies for expanding TFR probabilities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and the Groupe des Biologistes Moléculaires des Hémopathies Maligne network of molecular biologists.
Authorship
Contribution: D.R., F.E.N., F.G., P.R., and F.-X.M. designed the study; D.R. collected data and wrote the manuscript; D.R. and F.-X.M. interpreted the results; D.R. and J.G. performed statistical analyses; D.R., F.E.N., M.T., F.G., A.G.-B., M.G., V.C., G.G., L.L., G.E., J.-M.P., B.V., M.E.-B., J.-C.I., A.C., H.J.-A., M.-P.N., P.R., and F.-X.M. took care of patients and critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: D.R. has received honoraria from Ariad, Bristol Myers Squibb, Novartis, and Pfizer. F.E.N. has received research grants from Novartis and honoraria from Novartis, Ariad/Incyte, and Bristol Myers Squibb. F.G. has consulted for Celgene and Pfizer and has received honoraria from Celgene, Novartis, and Pfizer. A.G.-B. has received honoraria from Ariad, Bristol Myers Squibb, Novartis, and Pfizer and has consulted for Ariad, Bristol Myers Squibb, and Pfizer. M.G. has received honoraria from Ariad, Bristol Myers Squibb, and Novartis. V.C. has received honoraria from Ariad, Bristol Myers Squibb, Novartis, and Pfizer. L.L. has received research grants from Novartis and Pfizer and honoraria from Ariad. G.E. has consulted for Bristol Myers Squibb and Novartis and is a member on an entity's board of directors or advisory committee for Ariad, Bristol Myers Squibb, Novartis, and Pfizer. The remaining authors declare no competing financial interests.
A complete list of the members of the France Intergroupe des Leucémies Myéloïdes Chroniques appears in “Appendix.”
Correspondence: Delphine Rea, Service d’Hématologie Adulte, Hôpital Saint-Louis, 1, Ave Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: delphine.rea@aphp.fr.
Appendix: study group members
The members of the France Intergroupe des Leucémies Myéloïdes Chroniques are: Shanti Ame, Jean-Noel Bastie, Frédéric Bauduer, Samantha Benghiat, Marc Berger, Nathalie Cambier, Lydia Campos, Jean-Michel Cayuela, Aude Charbonnier, Rémi Chapelon, Jean-Claude Chomel, Valérie Coiteux, Pascale Cony-Makhoul, Houria Debarri, Viviane Dubruille, Stéphanie Dulucq, Janine Dumont, Martine Escoffre-Barbe, Gabriel Etienne, Madeleine Etienne, Pascale Flandrin-Gresta, Martine Gardembas, Stéphane Girault, Sylvie Glaisner, François Guilhot, Joelle Guilhot, Denis Guyotat, Violaine Havelange, Sandrine Hayette, Zehaira Hebibi, Eric Hermet, Françoise Huguet, Jean-Christophe Ianotto, Hyacinthe Johnson-Ansah, Simona Lapusan, Laurence Legros, Katell Le Du, Eric Lippert, Véronique Maguer-Satta, François-Xavier Mahon, Gérald Marit, Iuliana Martiniuc, Mauricette Michallet, Frédéric Millot, Monfray Jeremy, Marie-Joelle Mozziconacci, Franck-Emmanuel Nicolini, Marie-Pierre Noel, Amélie Penot, Giangiacomo Pica, Isabelle Plantier, Stéphane Prost, Philippe Quittet, Delphine Rea, Catherine Roche-Lestienne, Philippe Rodon, Philippe Rousselot, Lydia Roy, Nathalie Sorel, Laure Stalnikiewicz, Antoine Thyss, Isabelle Tigaud, Fabienne Vacheret, and Madalina Uzunov.