Key Points
HHV-8 MCD is associated with a decrease of iNKT and memory B cells.
iNKT decrease contributes to B-cell abnormalities in coculture experiments.
Abstract
Human herpesvirus 8 (HHV-8) is the causative agent of Kaposi sarcoma (KS) and multicentric Castleman disease (MCD), a life-threatening, virally induced B-cell lymphoproliferative disorder. HHV-8 is a B-lymphotropic γ-herpesvirus closely related to the Epstein-Barr virus (EBV). Invariant natural killer T (iNKT) cells are innate-like T cells that play a role in antiviral immunity, specifically in controlling viral replication in EBV-infected B cells. Decline of iNKT cells is associated with age or HIV infection, both situations associated with HHV-8–related diseases. We analyzed iNKT cells in both blood (n = 26) and spleen (n = 9) samples from 32 patients with HHV-8 MCD and compared them with patients with KS (n = 24) and healthy donors (n = 29). We determined that both circulating and splenic iNKT cell frequencies were markedly decreased in patients with HHV-8 MCD and were undetectable in 6 of them. Moreover, iNKT cells from patients with HHV-8 MCD displayed a proliferative defect after stimulation with α-galactosylceramide. These iNKT cell alterations were associated with an imbalance in B-cell subsets, including a significant decrease in memory B cells, particularly of marginal zone (MZ) B cells. Coculture experiments revealed that the decrease in iNKT cells contributed to the alterations in the B-cell subset distribution. These observations contribute to a better understanding of the complex interactions between HHV-8 and immune cells that cause HHV-8–related MCD.
Introduction
The B-lymphotropic γ-herpesvirus human herpes virus-8 (HHV-8), closely related to Epstein-Barr virus (EBV), establishes lifelong latency in B cells.1 Besides causing Kaposi sarcoma (KS), several lymphoproliferative disorders are associated with HHV-8 infection, such as HHV-8–related multicentric Castleman disease (HHV-8 MCD).2,3 HHV-8 MCD is a systemic lymphoproliferative disorder that occurs at higher rates in patients infected with HIV as well as in elderly patients who are not otherwise immunocompromised.2,3 It is characterized by recurrent flares of severe inflammatory symptoms that include fever, diffuse lymph nodes, and spleen enlargement.4 Clinical exacerbations of HHV-8 MCD are associated with a rapid expansion of HHV-8–infected plasmablasts as well as very high HHV-8 loads, suggesting an association of flares with uncontrolled virus replication.5
T-cell responses, particularly those of CD8+ T cells, play key roles in the control of chronic viral infections; however, the magnitude and functional profile of HHV-8–CD8+ T-cell responses are similar between asymptomatic HHV-8 carriers and patients with HHV-8 MCD.6 Further, HHV-8 MCD that generally occurs in patients with low CD4+ T-cell counts may occur despite CD4+ T-cell counts in normal ranges.3,7 Thus, HHV-8 MCD may not arise from a CD8+ T-cell qualitative defect or from a CD4+ T-cell quantitative defect.
The results accumulated over the past decade illuminate the critical role of innate immune responses that control herpesviruses, particularly those mediated by invariant natural killer T (iNKT) cells.8-12 iNKT cells represent a unique subpopulation of T cells with a highly restricted T-cell receptor (TCR) repertoire, which express rearranged genes encoding Vα24-Jα18 in humans, coupled with a restricted repertoire of TCR-β chains.13,14 After activation by glycolipid antigens presented by the major histocompatibility complex class 1–related molecule CD1d, iNKT cells acquire cytotoxic activity and rapidly secrete large quantities of T helper 1 and T helper 2 cytokines that stimulate the expansion and maturation of dendritic cells (DCs), natural killer (NK) cells, B cells, and conventional T cells.15,16
Accumulating evidence supports a role for iNKT cells in controlling herpesvirus infections. The absence of iNKT cells is a key immunological abnormality of X-linked lymphoproliferative (XLP-1) syndrome, characterized by fulminant infectious mononucleosis subsequent to primary EBV infection, dysgammaglobulinemia, and the presence of a high frequency of EBV-driven B-cell lymphoproliferative disorders.17 Defects in iNKT cells also occur in primary immunodeficiencies characterized by a high susceptibility to EBV, leading to EBV-associated pathologies.12,18 Further, in vitro studies reveal that EBV-infected B cells directly induce the production of interferon-γ (IFN-γ) and the cytotoxic activity of iNKT cells, whereas the depletion of iNKT cells increases viral titers and the number of EBV-infected B cells.19 Decline of iNKT cells is associated with age20 or HIV infection,21 which are both situations associated to HHV-8–related diseases.3,22
HHV-8 MCD resembles many aspects of EBV-induced B-cell polyclonal lymphoproliferative disorders such as XLP, and iNKT cells are essential for the immune control of EBV-infected B cells. Therefore, we postulated that iNKT cell responses are altered during HHV-8 MCD.
Patients, materials, and methods
Patients and controls
This study included 3 groups of subjects (Table 1). The first group comprised patients with HHV-8 MCD. Samples of their blood, spleen, or both were collected at Saint-Louis Hospital (E.O.) from 32 patients (24 HIV+ and 8 HIV−). Splenectomy was performed during active MCD; the recorded reasons were symptomatic splenomegaly, suspicion of lymphoma, or both. Blood samples were collected from 26 patients (18 HIV+ and 8 HIV–), spleen samples from 9 patients (8 HIV+ and 1 HIV−), and samples from both sources were available for 3 patients. The second group comprised 24 patients with KS from Saint-Louis (E.O.) and Cochin (N.D.) hospitals. The third group comprised 29 healthy donors (HDs) from the Etablissement Français du sang. Table 1 summarizes patients’ clinical and laboratory characteristics; supplemental Tables 1 and 2 (available on the Blood Web site) provide additional clinical details. Flare was defined as the presence of constitutional symptoms, progressive lymph node or spleen enlargement, and elevated levels of serum markers of inflammation. Remission was defined as the absence of these findings.
Patient and subject characteristics
| Patients/subjects and diagnoses . | n . | Male/female (n) . | Age, y (quartiles) . | CD4 cell count, cells/mm3 (quartiles) . | HHV-8 DNA, log10 copies/106 cells (quartiles) . | HIV RNA, log10 copies/mL (quartiles) . | |
|---|---|---|---|---|---|---|---|
| MCD patients | HIV− MCD | 8 | 7/1 | 68.5 (61-84)* | 562 (405-2117) | 5 (2.5-5.75) | NR |
| HIV+ MCD | 24 | 19/5 | 45.5 (33.5-51) | 307 (130-500)† | 5.7 (3.75-6.48) | 1.44 (<1.3-2.75) | |
| KS patients | HIV− KS | 11 | 10/1 | 62 (57-73)* | 576 (416-906.3) | <2 | NR |
| HIV+ KS | 13 | 11/2 | 40 (35.5-49) | 337 (120-449)† | <2 | 2.045 (<1.6-4.8) | |
| HDs | — | 29 | 14/15 | 28 (25-41) | — | NR | NR |
| Patients/subjects and diagnoses . | n . | Male/female (n) . | Age, y (quartiles) . | CD4 cell count, cells/mm3 (quartiles) . | HHV-8 DNA, log10 copies/106 cells (quartiles) . | HIV RNA, log10 copies/mL (quartiles) . | |
|---|---|---|---|---|---|---|---|
| MCD patients | HIV− MCD | 8 | 7/1 | 68.5 (61-84)* | 562 (405-2117) | 5 (2.5-5.75) | NR |
| HIV+ MCD | 24 | 19/5 | 45.5 (33.5-51) | 307 (130-500)† | 5.7 (3.75-6.48) | 1.44 (<1.3-2.75) | |
| KS patients | HIV− KS | 11 | 10/1 | 62 (57-73)* | 576 (416-906.3) | <2 | NR |
| HIV+ KS | 13 | 11/2 | 40 (35.5-49) | 337 (120-449)† | <2 | 2.045 (<1.6-4.8) | |
| HDs | — | 29 | 14/15 | 28 (25-41) | — | NR | NR |
Data are median (quartiles) values. No significant differences were found for ages between patients HIV− with MCD and with KS and for CD4 T-cell counts between patients HIV+ with MCD and with KS. Significant P < .05, Mann-Whitney test.
NR, not relevant.
P = .26.
P = .9.
Cell isolation
Blocks of spleen were mechanically dissociated and digested using type VII collagenase and DNase (Sigma-Aldrich) in RPMI 1640 supplemented with 2% fetal calf serum (Biowest). Cell aggregates were further dissociated using 10 mM EDTA (Sigma-Aldrich) with agitation. Spleen mononuclear cells (SMCs) were isolated from splenocyte suspensions, and peripheral blood mononuclear cells (PBMCs) were isolated from blood through Ficoll-Hypaque (Eurobio).
B cells were negatively isolated or depleted from PBMCs using magnetic beads; iNKT cells were depleted with anti-invariant chain Vα24-Jα18 of the TCR (iTCR)-coated magnetic beads (Miltenyi Biotec) (supplemental Figure 1).
Cell culture
To expand iNKT cells, PBMCs were cultured in RPMI 1640 (Eurobio), 2 mM l-glutamine, 1% pyruvate, 1% nonessential amino acids, 1% antibiotic (Life Technologies), 10% fetal calf serum, 200 IU/mL interleukin-2 (IL-2; Roche), and 100 ng/mL α-GalCer (Enzo Life Science). B cells depleted from PBMCs and negatively purified B cells were cultured in custom-made Yssel medium containing 1% human serum AB.23
Antibodies and flow cytometry
Dyes used were: Viability Dye efluor450, Viability Dye efluor786, and Viability Dye efluor506 (eBioscience), and Live/Dead Blue UV (Invitrogen). Antibodies used were: anti-CD3, CD4, CD8, CD19, CD27, immunoglobulin D (IgD), IgM, CD1d, CD23, CD21, CD14, CD16, CD3, CD56, HLA-DR, CD19, CD20, CD27, IgM (BD Biosciences, San Jose, CA), iTCR (Miltenyi Biotec), CD45 (Beckman Coulter), and CD1c (Ozyme).
We analyzed ≥5 × 105 cells. The limit of detection of iNKT cells was defined as <40 cells. The gating strategy of B-cell subset analysis is depicted in supplemental Figure 2. Data were acquired using a FACSCanto II or LSRFortessa (BD Biosciences), and the data were analyzed using FlowJo X (TreeStar).
Statistical analysis
The Mann-Whitney nonparametric 2-tailed test was used to compare 2 unpaired variables (percentages of iNKT, B cells, and B-cell subsets). The Kruskal-Wallis nonparametric 2-tailed test was used to compare more than 2 unpaired variables (percentages of iNKT subsets and mean fluorescence intensity [MFI] of CD1d). Comparisons of paired variables (percentages of iNKT cells between D0 and D12, % iNKT detected using iTCR or CD1d tetramers, and % iNKT detected using extracellular staining only or intra- and extracellular staining) were performed using the nonparametric 2-tailed Wilcoxon test. The Pearson correlation test was used to evaluate the correlations between individual indicators (percentage of iNKT D0/D12 and of iNKT/MZ B cells). All statistical analyses were performed using Prism 5 software (Graph Pad Software). P values < .05 indicate statistical significance.
Study approval
Blood samples were obtained after acquiring the patient’s informed consent, in accordance with Institutional Guidelines and the Declaration of Helsinki. The study complied with the human experimentation guidelines of Pitié-Salpêtrière Hospital.
Results
The frequencies of iNKT cells in blood and spleen are decreased in patients with HHV-8 MCD
iNKT cells are characterized by the coexpression of CD3 and iTCR. CD1d tetramers specifically identify iNKT cells, allowing us to confirm that in HDs, CD3+iTCR+ cells represented all iNKT cells (supplemental Figure 3A).
We analyzed the frequencies of iNKT cells in the PBMCs of 29 HDs, 21 patients infected with HHV-8 with KS, and 25 patients with HHV-8 MCD. Patients with HHV-8 MCD, irrespective of their HIV status, had similarly reduced frequencies of iNKTs in their PBMCs compared with those of patients with KS (P = .0006 and .0008, respectively) and HDs (P < .0001 and < .0001, respectively) (Figure 1A), and there was no difference between the frequencies of iNKT cells of patients with KS and HDs. Similarly, the frequencies of iNKT spleen cells from 7 patients with HHV-8 MCD were significantly lower compared with those of 8 HDs (P = .009) (Figure 1B). The frequencies of iNKT cells did not differ significantly after analyzing the expression of extracellular or intracellular iTCR of the 3 groups, indicating that the reduced frequencies of iNKT cells in patients with HHV-8 MCD were not explained by internalization of the iTCR (supplemental Figure 3B). The results of reverse transcriptase polymerase chain reaction assays of the levels of messenger RNAs encoding the Vα24 and Vβ11 chains of the iNKT TCR confirmed the flow cytometry findings (supplemental Figure 3C).
The frequencies of iNKT cells in blood and spleen are decreased in patients with HHV-8 MCD. PBMCs and SMCs from controls (HDs and patients with KS) or patients with HHV-8 MCD were stained ex vivo for surface expression of CD3, iTCR mAbs, CD4, and CD8. (A) Representative flow cytometry plot and cumulative data showing frequencies and median of iNKT cells in the PBMCs of 29 HDs, 21 KS patients, and 25 patients with HHV-8 MCD (17 HIV+ and 8 HIV−). Significant P < .05, Mann-Whitney test. (B) Representative flow cytometry plot and cumulative data showing frequencies and median of iNKT cells in the SMCs of 8 HDs and 7 patients with HHV-8 MCD. Significant P < .05, Mann-Whitney test. (C) Cumulative data showing frequencies and median of iNKT cell subsets (ie, CD4+, CD8+, CD4−CD8− (DN), and CD4+CD8+ (double positive [DP]) in the PBMCs of 27 HDs, 6 patients with HHV-8 MCD HIV−, and 10 patients with HHV-8 MCD HIV+. Significant P < .05, Mann-Whitney test.
The frequencies of iNKT cells in blood and spleen are decreased in patients with HHV-8 MCD. PBMCs and SMCs from controls (HDs and patients with KS) or patients with HHV-8 MCD were stained ex vivo for surface expression of CD3, iTCR mAbs, CD4, and CD8. (A) Representative flow cytometry plot and cumulative data showing frequencies and median of iNKT cells in the PBMCs of 29 HDs, 21 KS patients, and 25 patients with HHV-8 MCD (17 HIV+ and 8 HIV−). Significant P < .05, Mann-Whitney test. (B) Representative flow cytometry plot and cumulative data showing frequencies and median of iNKT cells in the SMCs of 8 HDs and 7 patients with HHV-8 MCD. Significant P < .05, Mann-Whitney test. (C) Cumulative data showing frequencies and median of iNKT cell subsets (ie, CD4+, CD8+, CD4−CD8− (DN), and CD4+CD8+ (double positive [DP]) in the PBMCs of 27 HDs, 6 patients with HHV-8 MCD HIV−, and 10 patients with HHV-8 MCD HIV+. Significant P < .05, Mann-Whitney test.
Despite the low but similar CD4+ T-cell counts in both populations (P = .9) (Table 1), the frequency of iNKT cells in patients with HIV+ MCD was significantly lower compared with that of patients with HIV+ KS (P = .002, supplemental Figure 4A). Further, despite the similar advanced ages of both groups (P = .3) (Table 1), the frequency of iNKT cells in patients with HIV− HHV-8 MCD patients was significantly lower compared with that of patients with HIV− KS (P = .003) (supplemental Figure 4B). Moreover, this decrease was not related to cell death during HHV-8 MCD flares, because it was also observed during remission (median, 23 months; range, 3-48 months) (supplemental Table 1) and was not associated with HHV-8 or HIV replication or with the administration of rituximab (supplemental Figure 5A-D).
Together, these findings confirm that patients with HHV-8 MCD harbor a quantitative defect in iNKT cells compared with patients with KS as well as HDs.
Decreased frequencies of iNKT cells in patients with HHV-8 MCD are associated with modifications of iNKT cell subsets
We analyzed the distributions of iNKT cell subsets (CD4+, CD8+, CD4−CD8− [double-negative, DN]), and CD4+CD8+ (double-positive) in patients with HHV-8 MCD compared with those of HDs (Figure 1C). We conducted separate analyses of these 2 subgroups of patients because HHV-8 MCD might occur during immunosuppression associated with HIV infection or in elderly HIV− patients. The iNKT cells of HDs were mainly DN with similar proportions of CD4+ and CD8+ iNKT cells. Compared with HDs, HIV− patients with HHV-8 MCD exhibited a significant decrease in DN-iNKT cells (P = .04) and higher proportions of CD4+ and CD8+ iNKT cells (Figure 1C). In contrast, HIV+ patients with HHV-8 MCD exhibited significant decreases in CD4+ (P = .04) and DN-iNKT cells (P = .003), whereas the CD8+ iNKT-cell subset was proportionally increased (P = .008).
Proliferative capacity of iNKT cells is impaired in patients with HHV-8 MCD
We compared the proliferative capacity of iNKT cells from patients and controls. The iNKT cells from HIV+ and HIV− patients with HHV-8 MCD failed to expand in vitro in response to α-GalCer and IL-2, in contrast to those from HDs and patients with KS (Figure 2A). The proportion of iNKT cells in HDs and patients with KS significantly increased (P = .001 and .004, respectively) between days 0 and 12. Further, there was no significant difference between the frequencies of iNKT cells from HDs and those of patients with KS on day 12 (P = .42). The results were similar for spleen cells (data not shown).
The proliferative capacity of iNKT cells is impaired in patients with HHV-8 MCD. PBMCs were cultured with α-GalCer and IL-2 for 12 days. Cells were stained with CD3 and iTCR mAbs. (A) Representative flow cytometry plots and bar chart show cumulative data from 13 HDs, 9 patients with KS, and 15 patients with HHV-8 MCD (HIV+, n = 10; HIV−, n = 5) showing frequencies of iNKT cells before and after α-GalCer and IL-2 stimulation. Bar chart shows cumulative data as frequencies and median ± interquartile ranges. Significant P < .05, Wilcoxon matched pairs test. (B) Correlation plots show the relationship between frequencies of iNKT cells at day 0 (D0) and D12 after α-GalCer and IL-2 stimulation. Significant P < .05, Spearman’s correlation. (C) Representative flow cytometry plots showing frequencies of iNKT cells at D0 and D12 in iNKT cell–depleted PBMCs from 8 HDs. Bar chart shows cumulative data as median ± interquartile ranges. Significant P < .05, Wilcoxon matched pairs test.
The proliferative capacity of iNKT cells is impaired in patients with HHV-8 MCD. PBMCs were cultured with α-GalCer and IL-2 for 12 days. Cells were stained with CD3 and iTCR mAbs. (A) Representative flow cytometry plots and bar chart show cumulative data from 13 HDs, 9 patients with KS, and 15 patients with HHV-8 MCD (HIV+, n = 10; HIV−, n = 5) showing frequencies of iNKT cells before and after α-GalCer and IL-2 stimulation. Bar chart shows cumulative data as frequencies and median ± interquartile ranges. Significant P < .05, Wilcoxon matched pairs test. (B) Correlation plots show the relationship between frequencies of iNKT cells at day 0 (D0) and D12 after α-GalCer and IL-2 stimulation. Significant P < .05, Spearman’s correlation. (C) Representative flow cytometry plots showing frequencies of iNKT cells at D0 and D12 in iNKT cell–depleted PBMCs from 8 HDs. Bar chart shows cumulative data as median ± interquartile ranges. Significant P < .05, Wilcoxon matched pairs test.
The frequencies of iNKT cells obtained on day 12 of culture did not correlate with their frequencies on day 0 in patients with KS and HDs (r = 0.24, P = .28) or with HHV-8 MCD (r = −0.09, P = .75) (Figure 2B), demonstrating that the failure of iNKT cells to proliferate in patients with HHV-8 MCD was not associated with the decreased number of cells. To go further, we depleted iNKT cells from healthy PBMCs, and the frequencies of iNKT cells were similar to those of patients with HHV-8 MCD. On culture day 12 of these iNKT-depleted PBMCs, there was a significant increase in iNKT cells (P = .0078), similar to that of healthy unmanipulated PBMCs (Figure 2C).
IL-15 is a key factor that contributes to iNKT cell activation and proliferation24 ; therefore, we found that in vitro IL-15 did not expand or rescue the selective defect in iNKT cells (data not shown). Together, these results show a proliferative defect in vitro of iNKT cells from patients with HHV-8 MCD that was independent of their decreased frequencies in vivo.
iNKT cell defects are not associated with an altered expression of CD1d on the surface of APCs from patients with HHV-8 MCD
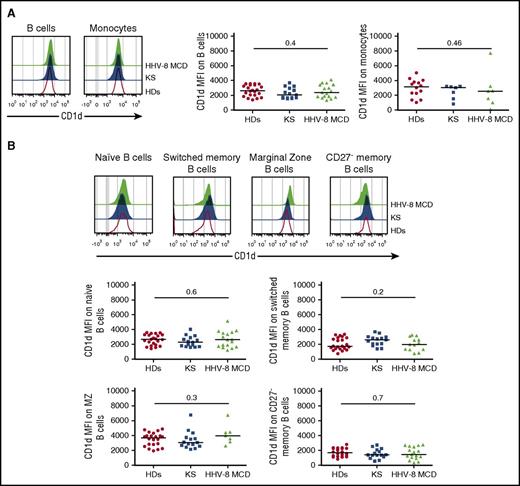
The proliferation and homeostasis of iNKT cells depend on the expression of CD1d by antigen-presenting cells (APCs),25,26 and the lytic HHV-8 replication cycle may promote downregulation of CD1d on the surface of infected cells.27 Therefore, we analyzed CD1d expression on B cells and monocytes from HDs and from patients with KS or HHV-8 MCD. The MFI values of CD1d on B cells and classical monocytes were similar among groups (Figure 3A).
iNKT cell defects are not associated with an alteration of the expression of CD1d on the surface of APCs from patients with HHV-8 MCD. PBMCs from controls (HDs and patients with KS) or patients with HHV-8 MCD were surface stained with CD19 to identify B cells, CD14 and HLA-DR to identify monocytes, and CD27, IgD, IgM, CD21, CD23, CD1d, and CD1c to identify B-cell subsets. (A) Representative histograms showing surface expression of CD1d on CD19+ B cells and CD14+ monocytes from HDs, patients with KS, and patients with HHV-8 MCD. Scatter plots showing MFI and median of CD1d on B cells from 22 HDs, 15 patients with KS, and 17 patients with HHV-8 MCD, and monocytes from 14 HDs, 7 patients with KS, and 7 patients with HHV-8 MCD. Significant P < .05, Kruskal-Wallis test. (B) Representative histograms displaying CD1d expression on naïve, switched memory, marginal zone, and CD27− memory CD19+ B cells in the PBMCs of HDs, patients with KS, and patients with HHV-8 MCD. Scatter plots showing MFI and median of CD1d in each subpopulation of B cells in 22 HDs, 15 patients with KS, and 17 patients with HHV-8 MCD. Significant P < .05, Kruskal-Wallis test.
iNKT cell defects are not associated with an alteration of the expression of CD1d on the surface of APCs from patients with HHV-8 MCD. PBMCs from controls (HDs and patients with KS) or patients with HHV-8 MCD were surface stained with CD19 to identify B cells, CD14 and HLA-DR to identify monocytes, and CD27, IgD, IgM, CD21, CD23, CD1d, and CD1c to identify B-cell subsets. (A) Representative histograms showing surface expression of CD1d on CD19+ B cells and CD14+ monocytes from HDs, patients with KS, and patients with HHV-8 MCD. Scatter plots showing MFI and median of CD1d on B cells from 22 HDs, 15 patients with KS, and 17 patients with HHV-8 MCD, and monocytes from 14 HDs, 7 patients with KS, and 7 patients with HHV-8 MCD. Significant P < .05, Kruskal-Wallis test. (B) Representative histograms displaying CD1d expression on naïve, switched memory, marginal zone, and CD27− memory CD19+ B cells in the PBMCs of HDs, patients with KS, and patients with HHV-8 MCD. Scatter plots showing MFI and median of CD1d in each subpopulation of B cells in 22 HDs, 15 patients with KS, and 17 patients with HHV-8 MCD. Significant P < .05, Kruskal-Wallis test.
B cells play a pivotal role in maintaining iNKT cell homeostasis with a differential expression of CD1d on B-cell subsets and MZ B cells that express the highest levels of CD1d25,28 ; therefore, we determined the levels of CD1d in the blood and splenic B-cell subsets. There was no significant difference in CD1d expression between all B-cell subsets from HDs and patients with KS or HHV-8 MCD (Figure 3B). Similarly, there was no significant difference in the expression of CD1d by SMCs of each patient group, except for CD27− memory B cells (supplemental Figure 6).
iNKT cell defects in patients with HHV-8 MCD are associated with a decrease in circulating and splenic MZ B cells
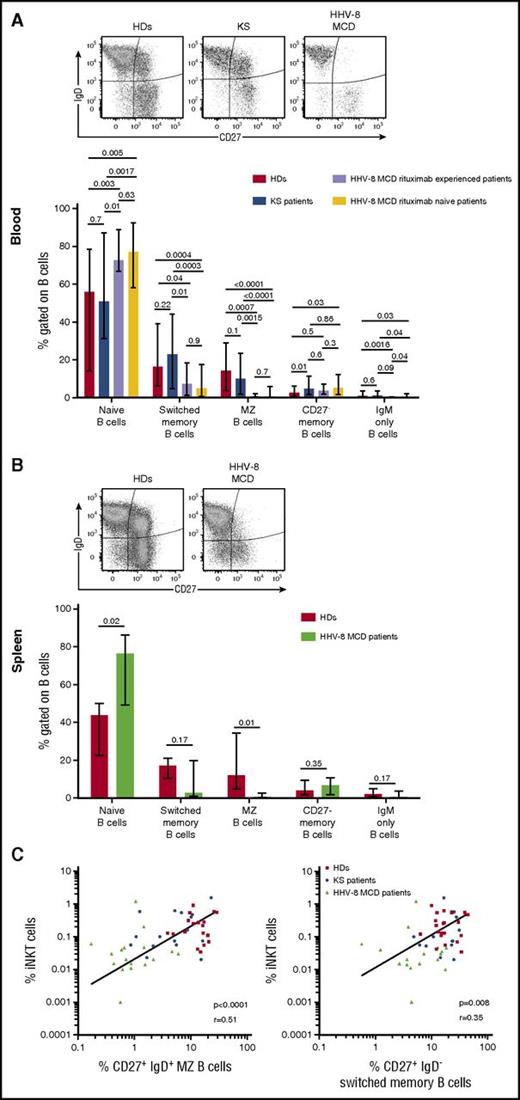
The analysis of B-cell subsets in the PBMCs from 22 HDs, 18 patients with KS, and 17 patients with HHV-8 MCD (Figure 4A) revealed a decrease in the frequency of circulating MZ B cells from patients with HHV-8 MCD, treated with rituximab or not, compared with patients with KS (P = .0015 and <.0001, respectively) and HDs (P = .0007 and <.0001, respectively). This defect was associated with a significant decline of switched memory peripheral B cells of patients with HHV-8 MCD, independent of rituximab treatment, compared with patients with KS (P = .01 and .0003, respectively) and HDs (P = .04 and .0004, respectively). Note that rituximab treatment was distant to evaluation in most cases (median >1 year).
iNKT cells defects in patients with HHV-8 MCD are associated with a profound decrease in circulating and splenic MZ B cells. PBMCs and SMCs from controls (HDs and patients with KS) or patients with HHV-8 MCD were surface stained with CD19, CD27, IgD, IgM, CD21, CD23, CD1d, and CD1c to identify B-cell subsets. (A) Representative flow cytometry plot showing surface expression of CD27 and IgD on CD19+ B cells from blood samples of HDs, patients with KS, and patients with HHV-8 MCD. Bar chart showing frequencies of naïve (CD27− IgD+ IgM+), switched memory (CD27+ IgD− IgM−), MZ (CD27+ IgDlow IgM+ CD21hi CD23− CD1chi), CD27− memory (CD27− IgD− IgM−), and IgM only (CD27+ IgD− IgM+) CD19+ B cells in 22 HDs, 18 patients with KS, and 17 patients with HHV-8 MCD. Bar chart shows cumulative data as frequencies and median ± ranges. Significant P < .05, Kruskal-Wallis and Mann-Whitney tests. (B) Representative flow cytometry plot showing surface expression of CD27 and IgD on CD19+ B cells from spleen samples from HDs and patients with HHV-8 MCD. Bar chart showing frequencies of naïve, switched memory, MZ, CD27− memory, and IgM only CD19+ B cells as defined in PBMCs from 6 HDs and 4 patients with HHV-8 MCD. Bar chart shows cumulative data as frequencies and median ± ranges. Significant P < .05, Mann-Whitney tests. (C) Correlation plots show the relationship between frequencies of iNKT with MZ B cells (CD27+ IgD+) or switched memory B cells (CD27+ IgD−) in the PBMCs of HDs, patients with KS, and patients with HHV-8 MCD. Spearman’s correlation test.
iNKT cells defects in patients with HHV-8 MCD are associated with a profound decrease in circulating and splenic MZ B cells. PBMCs and SMCs from controls (HDs and patients with KS) or patients with HHV-8 MCD were surface stained with CD19, CD27, IgD, IgM, CD21, CD23, CD1d, and CD1c to identify B-cell subsets. (A) Representative flow cytometry plot showing surface expression of CD27 and IgD on CD19+ B cells from blood samples of HDs, patients with KS, and patients with HHV-8 MCD. Bar chart showing frequencies of naïve (CD27− IgD+ IgM+), switched memory (CD27+ IgD− IgM−), MZ (CD27+ IgDlow IgM+ CD21hi CD23− CD1chi), CD27− memory (CD27− IgD− IgM−), and IgM only (CD27+ IgD− IgM+) CD19+ B cells in 22 HDs, 18 patients with KS, and 17 patients with HHV-8 MCD. Bar chart shows cumulative data as frequencies and median ± ranges. Significant P < .05, Kruskal-Wallis and Mann-Whitney tests. (B) Representative flow cytometry plot showing surface expression of CD27 and IgD on CD19+ B cells from spleen samples from HDs and patients with HHV-8 MCD. Bar chart showing frequencies of naïve, switched memory, MZ, CD27− memory, and IgM only CD19+ B cells as defined in PBMCs from 6 HDs and 4 patients with HHV-8 MCD. Bar chart shows cumulative data as frequencies and median ± ranges. Significant P < .05, Mann-Whitney tests. (C) Correlation plots show the relationship between frequencies of iNKT with MZ B cells (CD27+ IgD+) or switched memory B cells (CD27+ IgD−) in the PBMCs of HDs, patients with KS, and patients with HHV-8 MCD. Spearman’s correlation test.
Further, the frequency of splenic MZ B cells from patients with HHV-8 MCD was significantly decreased compared with that of HDs (P = .009) (Figure 4B), demonstrating that this defect was not caused by sequestration of MZ B cells in the spleen. Moreover, there was a significant positive correlation between the frequencies of iNKT and MZ B cells (r = 0.51, P < .001) and between those of iNKT and switched B cells (r = 0.35, P = .008) (Figure 4C) in the blood of HDs and patients with KS and HHV-8 MCD. Together, these findings indicate that HHV-8 MCD is associated with a combined deficit in the populations of iNKT and MZ B cells in blood and spleen.
Normal B cells do not rescue the impaired proliferation of iNKT cells in patients with HHV-8 MCD
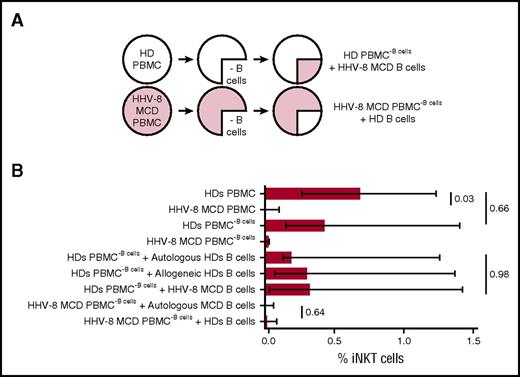
iNKT and B cells cooperate to their respective development and homeostasis.25,29-33 We analyzed the capacity of B cells isolated from patients with HHV-8 MCD to cause the dysfunction of iNKT cells, because HHV-8 MCD is characterized by severe abnormalities of B cells. We assessed the proliferative response of iNKT cells present in PBMCs from HDs to α-GalCer and IL-2. The B cells in these PBMC cultures were replaced with negatively purified allogeneic B cells isolated from patients with HHV-8 MCD (healthy PBMC−B cells + MCD B cells). Conversely, B cell–depleted PBMCs from patients with HHV-8 MCD and reconstituted with allogeneic B cells isolated from HDs were treated with α-GalCer and IL-2 to determine the proliferative response of iNKT cells (Figure 5A).
Normal B cells do not rescue the impaired proliferation of iNKT cells in patients with HHV-8 MCD. (A) Experimental design. B cells from HDs were isolated by negative selection, cocultured with allogeneic B cell–depleted PBMCs from patients with HHV-8 MCD, and stimulated for 7 days with α-GalCer and IL-2. Alternatively, negative isolated B cells from patients with HHV-8 MCD were cultured with allogeneic B cell–depleted PBMCs from HDs. As controls, B cells from HDs were cultured with allogeneic and autologous B cell–depleted PBMCs from HDs; B cells from patients with HHV-8 MCD were cultured with autologous B cell–depleted PBMCs derived from patients with HHV-8 MCD. Cells were stained with CD3 and iTCR mAbs to identify iNKT cells. (B) Bar chart shows cumulative data as frequencies and median ± interquartile ranges of iNKT cells at day 7 in the PBMCs of 6 HDs and 3 patients with HHV-8 MCD. Significant P < .05, Kruskal-Wallis and Mann-Whitney tests.
Normal B cells do not rescue the impaired proliferation of iNKT cells in patients with HHV-8 MCD. (A) Experimental design. B cells from HDs were isolated by negative selection, cocultured with allogeneic B cell–depleted PBMCs from patients with HHV-8 MCD, and stimulated for 7 days with α-GalCer and IL-2. Alternatively, negative isolated B cells from patients with HHV-8 MCD were cultured with allogeneic B cell–depleted PBMCs from HDs. As controls, B cells from HDs were cultured with allogeneic and autologous B cell–depleted PBMCs from HDs; B cells from patients with HHV-8 MCD were cultured with autologous B cell–depleted PBMCs derived from patients with HHV-8 MCD. Cells were stained with CD3 and iTCR mAbs to identify iNKT cells. (B) Bar chart shows cumulative data as frequencies and median ± interquartile ranges of iNKT cells at day 7 in the PBMCs of 6 HDs and 3 patients with HHV-8 MCD. Significant P < .05, Kruskal-Wallis and Mann-Whitney tests.
Replacing the B cells in cultures of PBMCs isolated from HDs with HHV-8 MCD B cells did not reduce the expansion of the iNKT cells (Figure 5B). Similarly, replacing the B cells in cultures of PBMCs from patients with HHV-8 MCD with healthy B cells did not rescue the diminished proliferation of iNKT cells (Figure 5B). Moreover, depletion of B cells from PBMCs from HDs did not affect the proliferation of iNKT cells treated with α-GalCer and IL-2. Together, these results indicate that B cells do not mediate iNKT cell dysfunction in patients with HHV-8 MCD.
iNKT cells are required to maintain CD27+IgD+/− memory B cells in culture
We used the same experimental protocol to assess the effects of proliferation-defective iNKT cells on the abnormalities of B cells that occur in patients with HHV-8 MCD. For this purpose, we determined the capacity of iNKT cells treated with α-GalCer and IL-2 to sustain the expansion of the populations of CD27+IgD+ and CD27+IgD− memory B cells in vitro.
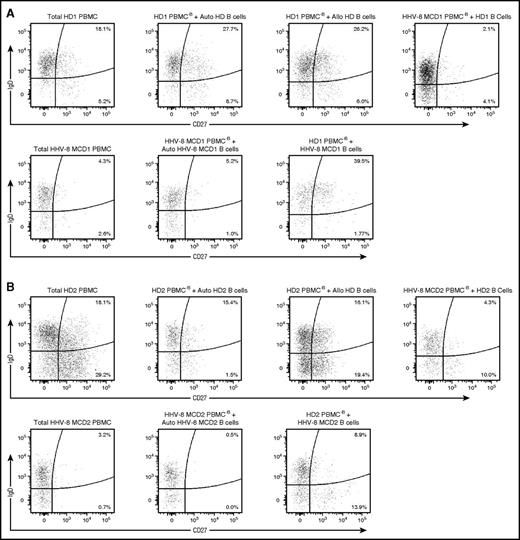
Cultures of unmanipulated PBMCs from HDs or those depleted of B cells and reconstituted with autologous or allogeneic B cells maintained their populations of CD27+IgD+ and CD27+IgD− memory B cells (frequencies between 15.4%-27.7% and 1.5%-29.2%, respectively) (Figure 6). In contrast, in B cell–depleted cultures of PBMCs from patients with HHV-8 MCD and reconstituted with B cells from HDs and treatment with α-GalCer and IL-2 did not maintain the population of memory B cells; thus, the frequencies of CD27+IgD+ and CD27+IgD− memory B cells at the end of the cultures were <5% and <10%, respectively. Similarly, treatment of PBMCs from patients with HHV-8 MCD did not sustain the expansion of CD27+IgD+ and CD27+IgD− memory B cells (0.5%-5.2% and 0%-1.5%, respectively).
iNKT cells are required to maintain CD27+ IgD+/− memory B cells in culture. B cells from HDs were isolated by negative selection, cocultured with allogeneic B cell–depleted PBMCs from patients with HHV-8 MCD, and stimulated for 7 days with α-GalCer and IL-2. Alternatively, negative isolated B cells from patients with HHV-8 MCD were cultured with allogeneic B cell–depleted PBMCs from HDs. As controls, B cells from HDs were cultured with allogeneic and autologous B cell–depleted PBMCs from HDs and B cells from patients with HHV-8 MCD were cultured with autologous B cell–depleted PBMCs derived from patients with HHV-8 MCD. Cells were stained with CD19, CD20, CD27, IgD, and IgM to identify B-cell subsets. Representative flow cytometry plots of B-cell analysis showing the frequencies of CD27+ IgD+ and CD27+ IgD− B cells at day 7 from HDs and patients with HHV-8 MCD in 2 independent experiments, HD1/HHV-8 MCD1 (A) and HD2/HHV-8 MCD2 (B).
iNKT cells are required to maintain CD27+ IgD+/− memory B cells in culture. B cells from HDs were isolated by negative selection, cocultured with allogeneic B cell–depleted PBMCs from patients with HHV-8 MCD, and stimulated for 7 days with α-GalCer and IL-2. Alternatively, negative isolated B cells from patients with HHV-8 MCD were cultured with allogeneic B cell–depleted PBMCs from HDs. As controls, B cells from HDs were cultured with allogeneic and autologous B cell–depleted PBMCs from HDs and B cells from patients with HHV-8 MCD were cultured with autologous B cell–depleted PBMCs derived from patients with HHV-8 MCD. Cells were stained with CD19, CD20, CD27, IgD, and IgM to identify B-cell subsets. Representative flow cytometry plots of B-cell analysis showing the frequencies of CD27+ IgD+ and CD27+ IgD− B cells at day 7 from HDs and patients with HHV-8 MCD in 2 independent experiments, HD1/HHV-8 MCD1 (A) and HD2/HHV-8 MCD2 (B).
However, in B cell–depleted cultures of PBMCs from HDs, which were reconstituted with B cells from patients with HHV-8 MCD, treatment with α-GalCer and IL-2 increased the populations of CD27+IgD+ and CD27+IgD− memory B cells (39.5% and 8.9% and 1.77% and 13.9%, respectively) compared with cultures of PBMCs from patients with HHV-8 MCD. Residual normal B cells did not cause this increase, because B cells were not detected in B cell–depleted cultures of PBMCs isolated from HDs (supplemental Figure 7). Together, these results suggest that iNKT cells are required to maintain and expand the population of memory B cells.
Discussion
The population of iNKT cells is an important component of the immune response to herpesviruses, particularly in patients with primary immunodeficiencies.17,19,34 Moreover, primary infection by HHV-8 of an EBV− child with an XLP-1 phenotype and a complete absence of iNKT cells was associated with the development of an HHV-8–driven lymphoproliferative disorder resembling HHV-8 MCD.35 These observations prompted us to analyze the frequency and function of iNKT cells in patients with HHV-8 MCD. Our results convincingly support the conclusion that these patients, regardless of HIV status, are characterized by a profound decrease in the frequencies and functional activities of iNKT cells.
The heterogeneous distribution of iNKT cells and their decreased number in the circulation might reflect altered tissue redistribution; however, the percentage of iNKT cells was significantly decreased in SMCs from patients with HHV-8 MCD. Moreover, deficiencies in iNKT cells occur during flares and remission, which likely reflect a persistent abnormality of iNKT cell homeostasis, in contrast to the migration if iNKT cells to sites of inflammation during flares.
This significant quantitative deficit was accompanied by a significant defective proliferation of the residual iNKT cells after treatment with α-GalCer and IL-2, which was not reversible by exogenous IL-15, a key contributor to iNKT activation and proliferation.24
Although mature iNKT cells do not require continued interactions with CD1d in the periphery to maintain homeostatic proliferation,36,37 B cells of patients with systemic lupus erythematosus support iNKT cell homeostasis through the presentation of lipid antigens.25 The HHV-8 MIR1/2 proteins downregulate CD1d expression at the surface of infected cells, leading to their lack of recognition by iNKT cells and subsequent escape from immune surveillance.27 We hypothesized that the defects of iNKT cells may be explained by altered interactions with APCs; therefore, we analyzed the CD1d expression by APCs, specifically B cells and monocytes, from patients with HHV-8 MCD and did not detect a significant difference. However, the number of cells available was insufficient to determine the expression of CD1d on DCs and nonclassical monocytes. HHV-8–infected B cells likely represent a very small proportion of circulating B cells, and we therefore cannot exclude the possibility that CD1d expression may be decreased in HHV-8–infected B cells.
We reveal a profound alteration in the distribution of B-cell subsets in patients with HHV-8 MCD, reflected by a significant decrease in memory B cells, particularly MZ B cells. It is important to consider critical observations required to interpret these results. First, the pathogenesis of HHV-8 MCD is associated only during flares, with IL-6 overproduction.4 Second, the therapeutic monoclonal antibody (mAb) rituximab, which specifically targets B cells and is widely used to treat HHV-8 MCD, induces a complete, albeit transient, depletion of B cells. Evidence indicates that after B-cell reconstitution, the distribution of B-cell subsets may vary depending on the disease.38-41 However, the deficit in memory B cells that occurs in patients with HHV-8 MCD is evident during flares and remission, before rituximab treatment as well as after B-cell reconstitution. Thus, the pronounced abnormalities of B-cell subsets in HHV-8 MCD are not caused by IL-6 overproduction or treatment with rituximab.
There is a significant positive correlation between the decreased frequencies of iNKT and MZ B cells observed in patients and HDs; these cells cooperate to mediate their respective development.25,29-33 Therefore, we analyzed the relationship between these cell types in coculture experiments. We observed that normal B cells did not rescue iNKT cell proliferation in PBMCs from patients with HHV-8 MCD, supporting the conclusion that they likely are not directly involved in the events leading to the deficiency of iNKT cells. In contrast, we show that iNKT cells were required to maintain CD27+IgD+/− memory B cells in culture. These results indicate that the functional defect of iNKT cells is not explained by abnormalities of the B-cell subset but instead may contribute to the anomalous decrease in memory and MZ B cells in patients with HHV-8 MCD. Although the possibility that iNKT and B-cell defects involve a third partner, particularly DCs, cannot be excluded, our results are consistent with recent evidence that iNKT cells induce the expansion of unswitched memory CD27+IgD+ B cells in vitro.42
HHV-8 MCD and KS occur most frequently in HIV-immunocompromised patients7,43 or in not otherwise immunocompromised elderly people who are not infected with HIV.3 Age and HIV infection are associated with a decline in iNKT cells and likely contribute to the defects observed in the present study. Moreover, the overall modifications of iNKT cell subsets observed in patients with HHV-8 MCD are consistent with those associated with advanced age or HIV infection of patients without MCD.20,21 CD8+ iNKT cells secrete high levels of IFN-γ, but the function of these cells during viral infection is not clearly characterized.44 It cannot be ruled out, however, that the increase of the proportion of CD8+ iNKT cells reflect their antiviral activities in these HHV-8–infected patients.
Although the age-related decline of iNKT cell counts is likely not systematic, because HDs >75 years of age have similar median levels of iNKT cells compared with younger persons (D. Sauce, Sorbonne Université, Université Pierre et Marie Curie, Centre d'Immunologie et des Maladies Infectieuses, INSERM U1135, oral communication, 28 June 2016), the analysis of larger number of patients report a global decline of iNKT cell with age.45 The mechanism of this decline observed in the elderly is unknown and may reflect exhaustion of thymic output or increased sensitivity of iNKT cells to apoptosis.
In HIV-infected patients, the number of iNKT cells is decreased, particularly the number of CD4+ iNKT cells. This deficit is variably restored after antiretroviral therapy,46 which is consistent with findings that HHV-8 MCD may relapse in HIV+ patients despite apparently effective immunovirological control under antiretroviral therapy.7 During the natural course of the disease, persistent antigen exposure, microbial translocation, and increasing immune activation may lead to “immune exhaustion,” which leads to the progressive loss of the functions of lymphocyte subsets. In this regard, iNKT cells from HIV-infected patients express high levels of programmed death-1 (PD-1).46 Similarly, in patients with systemic lupus erythematosus, iNKT cells that fail to proliferate express increased levels of PD-1, which is characteristic of an anergic or exhausted phenotype.47,48
However, in the present study, the decrease in iNKT cells in patients with HHV-8 MCD was independent of age, HIV status, CD4 T-cell count, exposure to rituximab, or the time of sample acquisition (flare or remission). Whether age, HIV status, or both contribute to the iNKT cell defects observed here, our results demonstrate that they are constantly and particularly profound only in the context of HHV-8 MCD. These findings therefore support the hypothesis that a deficiency in iNKT cells contributes to the pathogenesis of HHV-8 MCD.
Nonetheless, several patients with KS presented very low numbers of circulating iNKT cells, consistent with the wide variations of circulating iNKT frequencies,49 although they did not exhibit any clinical signs of HHV-8 MCD. Further, the numbers of iNKT cells in patients with HHV-8 MCD in remission were near the limit of detection, suggesting that an iNKT cell defect alone may be insufficient to account for the pathogenesis of HHV-8 MCD.
These observations notwithstanding, our results demonstrate that iNKT cells are important contributors to the complex interaction between HHV-8 and immune cells and that their dysfunction may contribute to the development of HHV-8–related B-cell malignancies in HHV-8–infected patients.
Presented in abstract form at the 49th annual meeting of the French Society of Immunology, Lille, France, 4 November 2014.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Flow Cytometry Platerforme CyPs for their support in flow cytometry, Mathilde Ban for technical assistance, Jehane Fadlallah and Laurence Gerard for patient information, Morgane Griesbeck for technical advice, Hans Yssel for providing Yssel medium and for critical and constructive reading, Rima Zorob and Véronique Morin for technical assistance with reverse transcriptase polymerase chain reaction analysis and comments, and Enago (www.enago.com) for the English language review. The authors also thank the patients who participated in the study.
This work was supported by grants from the French National Agency for Research on AIDS and Viral Hepatitis by a scholarship from the Association pour la Recherche contre le Cancer (A.D.).
Authorship
Contribution: Z.S., A.D., E.O., and G.C. designed the study; Z.S., A.D., B.A., and G.C. wrote the manuscript; G.C., A.D., E.O., L.G., D.B., C.F., and N.D. selected patients; Z.S., A.E., and B.H. performed immunological assays; F.A. and A.-G.M. supervised the virological assays; Z.S., C.P., and G.C. supervised the cytometric analysis; Z.S. and G.C. performed the statistical analysis; and Z.S. and A.O. performed reverse transcriptase polymerase chain reaction analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guislaine Carcelain, Département d’immunologie, Hôpital Pitié-Salpêtrière, 83 bd de l’Hôpital, 75013 Paris, France; e-mail: guislaine.carcelain@aphp.fr.
References
Author notes
Z.S. and A.D. contributed equally to this work.

![Figure 1. The frequencies of iNKT cells in blood and spleen are decreased in patients with HHV-8 MCD. PBMCs and SMCs from controls (HDs and patients with KS) or patients with HHV-8 MCD were stained ex vivo for surface expression of CD3, iTCR mAbs, CD4, and CD8. (A) Representative flow cytometry plot and cumulative data showing frequencies and median of iNKT cells in the PBMCs of 29 HDs, 21 KS patients, and 25 patients with HHV-8 MCD (17 HIV+ and 8 HIV−). Significant P < .05, Mann-Whitney test. (B) Representative flow cytometry plot and cumulative data showing frequencies and median of iNKT cells in the SMCs of 8 HDs and 7 patients with HHV-8 MCD. Significant P < .05, Mann-Whitney test. (C) Cumulative data showing frequencies and median of iNKT cell subsets (ie, CD4+, CD8+, CD4−CD8− (DN), and CD4+CD8+ (double positive [DP]) in the PBMCs of 27 HDs, 6 patients with HHV-8 MCD HIV−, and 10 patients with HHV-8 MCD HIV+. Significant P < .05, Mann-Whitney test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/7/10.1182_blood-2016-06-719716/4/m_blood719716f1.jpeg?Expires=1769102768&Signature=iex6hrNORlaobSuJu4J2fvEcempMYJWTfRVhgicZIGceBizezLAVxkc148jwv~~fKJWDdmsXm6jDtGwT3nrm6GUM1ZaliJEQc8ccCv12pCCexSv5wK2-RIfb8Sy2i9x9~CaQtYBswnc8zK76qD0U2EAssQKw9reXRJoLydoHoec1ZimE4altcmE7M4kS5xBgqLNWrwy-QgNW1~VUQt76hF~7pxpzFrdXKvotyy-oUL6Ec6rYz9zr-VeDudXyLu4bvds-CsldanriLuVhl2dk5rXvwptnRPOnO2Oco6B8QdrQm2EBTJuv36lWv6ZaoPhuuu9U-JdkQaUtCco~0SQkHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal