Key Points
Bispecific antibodies binding CD3 and CLL-1 deplete CLL-1+ target cells in animal models.
An appropriately engineered CLL-1/CD3 bispecific antibody could be effective in treating AML.
Abstract
Acute myeloid leukemia (AML) is a major unmet medical need. Most patients have poor long-term survival, and treatment has not significantly changed in 40 years. Recently, bispecific antibodies that redirect the cytotoxic activity of effector T cells by binding to CD3, the signaling component of the T-cell receptor, and a tumor target have shown clinical activity. Notably, blinatumomab is approved to treat relapsed/refractory acute lymphoid leukemia. Here we describe the design, discovery, pharmacologic activity, pharmacokinetics, and safety of a CD3 T cell–dependent bispecific (TDB) full-length human IgG1 therapeutic antibody targeting CLL-1 that could potentially be used in humans to treat AML. CLL-1 is prevalent in AML and, unlike other targets such as CD33 and CD123, is not expressed on hematopoietic stem cells providing potential hematopoietic recovery. We selected a high-affinity monkey cross-reactive anti–CLL-1 arm and tested several anti-CD3 arms that varied in affinity, and determined that the high-affinity CD3 arms were up to 100-fold more potent in vitro. However, in mouse models, the efficacy differences were less pronounced, probably because of prolonged exposure to TDB found with lower-affinity CD3 TDBs. In monkeys, assessment of safety and target cell depletion by the high- and low-affinity TDBs revealed that only the low-affinity CD3/CLL1 TDB was well tolerated and able to deplete target cells. Our data suggest that an appropriately engineered CLL-1 TDB could be effective in the treatment of AML.
Introduction
The standard of care for acute myeloid leukemia (AML) has not significantly changed in 40 years, and patients with relapsed/refractory disease or poor prognostic factors continue to have inadequate survival.1 Although some targeted therapies such as FLT3 inhibitors have demonstrated encouraging results in early clinical trials,2 the clinical benefit of such agents is restricted to a small portion of patients. Recently, clinical activity of bispecific antibodies that redirect the cytotoxic activity of effector T cells by binding to CD3, the signaling component of the T-cell receptor, and a tumor-associated antigen has been demonstrated by the approval of blinatumomab, a bispecific T-cell engager (BiTE) targeting human CD3 and CD19 for relapsed/refractory acute lymphoid leukemia (ALL).3,4 A similar approach for AML, a disease with limited treatment options, could transform the clinical outcome.
Because T cell–directed killing using the CD3/tumor antigen bispecific does not differentially kill cancer cells over normal cells, tumor antigen selection is crucial to achieve acceptable safety. Hematologic cancers have the advantage of lineage markers that are broadly expressed in tumors and whose expression on normal cells is tolerable because normal cells can be replaced through hematopoiesis. For example, blinatumomab and rituximab (anti-CD20) both deplete normal B cells, but levels generally recover, and with modern supportive care, measures such as IV immune globulin, the safety risk is minimized for B-cell depletion. Target selection for AML is a larger challenge. As a disease of myeloid lineage precursors, the best-characterized and most prevalent surface antigens of AML, CD33, and CD123 are also expressed on hematopoietic stem cells (HSCs).5-8 Preservation of HSCs is paramount in the ability to restore normal immune functions. With these restrictions in mind, an alternative target for AML is C-type lectin-like molecule-1 (CLL-1), present on the surface of committed myeloid cells and overexpressed in AML, but absent on megakaryocytic progenitor cells and CD34+/CD38– HSCs.9,10 Furthermore, CLL-1 is associated with a very low-frequency subpopulation of CD34+/CD38–, chemoresistant leukemic stems cells (LSCs), which are associated with rapid disease relapse.11,12 This expression pattern suggests that CLL-1 would be a preferable CD3 bispecific target to CD33 or CD123.
Beyond target selection, development of the optimal therapeutic needs to consider pharmacokinetic (PK) properties. Blinatumomab and other similar BiTE and dual-affinity retargeting (DART) molecules have short half-lives because they lack the Fc domain function that imparts extended circulation. This necessitates constant infusion to maintain exposure.13 A full-length human IgG1 bispecific antibody engineered for improved PK and altered Fc-mediated functions could address many of these shortfalls.
In this report, we describe the design, discovery, pharmacologic activity, and safety of a CD3 T cell–dependent bispecific (TDB) full-length humanized IgG1 therapeutic antibody targeting CLL-1 that could potentially be used in humans to treat AML. Preclinical studies in mice and cynomolgus monkeys indicate the importance of selecting a CD3 affinity resulting in the desired balance between potency, PK, and safety for optimizing the performance of a T cell–recruiting bispecific antibody.
Materials and methods
Cell lines
Human AML cell lines (Molm-13, ML-2, THP-1, EOL-1, Nomo-1, U937, HL-60, and PL-21) were from the Genentech Cell Line Repository. Cells were maintained in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, and 1% penicillin-streptomycin at 37°C in 5% CO2.
Antibody production
Antibodies were produced targeting human CLL-1 and CD3 followed by humanization and pharmacologic optimization.14 The lead antibody for CLL-1, and one of 3 anti-human CD3ε molecules with different affinities to CD3ε, as determined by Biacore (CD3εL [low, KD = 50.0 nM], CD3εH [high, KD = 0.5 nM], and CD3εVH [very high, KD = 0.05 nM]), were assembled as a full-length bispecific humanized IgG1 in a knob-and-hole format that faithfully resembles the native antibody structure.15 To minimize antibody-mediated effector functions, mutations (CH3 N297G) were introduced in the Fc domain to reduce interactions with Fcγ receptors.
Flow cytometry
Whole blood and tissues were stained directly with antibody reagents or stained after treatment with ACK lysis and preincubation with 0.5 mg/mL human IgG for 20 minutes on ice. Samples were incubated with primary antibody for 40 minutes in the dark, and constant time-based data acquisition was performed on a 50-µL acquisition sample volume using a BD LSR Fortessa flow cytometer (BD Biosciences, San Jose, CA) and data analysis with the software, Flowjo (Flowjo LLC, Ashland, OR). A list of fluorochrome-labeled antibodies is provided in the supplemental Methods, available on the Blood Web site.
In vitro killing and T-cell activation
In all experiments, the target cells were preincubated for 1 to 2 hours in RPMI 1640 medium with ∼0.5 mg/mL low endotoxin human IgG (Molecular Innovations, Novi, MI) to reduce nonspecific binding to cell surface FcγRs by TDBs. Peripheral blood mononuclear cells were isolated from normal human and cynomolgus donors by Ficoll separation, and autologous CD8+ or pan–T cells were enriched using kits from Miltenyi Biotech (San Diego, CA). Assays were performed with 60 000 cells per well of effector CD8+ T cells and 20 000 cells per well of hIgG-blocked target cells; (E:T) of 3:1, or pan–T cells at 5:1. TDBs were tested in the range of 0 to 10 µg/mL and incubated between 20 and 40 hours. Quantitation of human CLL-1+ (hCLL-1+) target cells and T-cell activation was determined by flow cytometry.
Dual targeting BAC transgenic mice
A genetically engineered mouse model (GEMM), double BAC transgenic (2xBAC-Tg) C57BL/6 mice expressing hCLL-1 and human CD3ε (hCD3ε) was generated at Genentech and maintained in accordance with the American Association of Laboratory Animal Care guidelines. All in vivo experimental procedures conformed to the guiding principles of the American Physiology Society and were approved by Genentech’s Institutional Animal Care and Use Committee. Animals were confirmed to express both human antigens and shown ex vivo to have functional CD8+ T cells by flow cytometry. Complete details are described in the supplemental Methods.
Rodent in vivo efficacy and PK
We tested the PK of the TDBs in target-deficient (SCID-beige) and target-expressing (2xBAC-Tg) animals. SCID-beige mice (6-8 weeks old) were randomly assigned to 3 groups (n = 9 per group, 3 animals per time point): Group 1 (CLL1/CD3L), Group 2 (CLL1/CD3H), and Group 3 (CLL1/CD3VH) and PK were evaluated after a single IV dose of 0.5 mg/kg. The study using the 2xBAC-Tg mice was conducted as described above except animals were randomly placed into groups of 12. Blood and/or tissues were collected at the following time points: Day −7 (predose), and postdose at 15 minutes; 2 hours; 6 hours; and days 1, 7, and 14. In the case of the 2xBAC-Tg mice, 3 animals per group were removed from the study at 15 minutes and days 1, 7, and 14 to enumerate the number of hCLL-1+ target cells and T-cell activation in blood and tissues by flow cytometry. Antibody and cytokine serum concentrations were measured at all time points by Luminex multiplex assays.
Cynomolgus monkey pharmacodynamics and toxicity study
The toxicity, toxicokinetics, and pharmacodynamics (PD) of anti–CLL-1 TDBs were assessed in naïve, Mauritian Macaca fascicularis, with confirmed CLL-1 expression by flow cytometry. Four groups of male cynomolgus monkey (n = 3; except for 0.2 mg/kg dose group where n = 6) were administered a single 1-hour IV infusion of vehicle, CLL1/CD3H (0.5 mg/kg), or CLL1/CD3L (0.2 or 0.5 mg/kg), monitored for 8 to 29 days for toxicity and pharmacologic effect (target cell depletion and recovery), and given complete gross and microscopic necropsies. Serum test-article concentrations at multiple time points were determined by enzyme-linked immunosorbent assay and concentration-time profiles were used to estimate PK parameters using WinNonlin software (Pharsight, Mountain View, CA). PD effects were determined by differential leukocyte counts, and flow cytometry to quantify myeloid cell depletion and T-cell activation markers (CD69/25) in the peripheral blood and bone marrow. A list of antibodies and description of cytokine quantitation are described in the supplemental Methods.
Results
Development of an anti–hCLL-1/anti-hCD3 T cell–dependent bispecific antibody
We developed antibodies to both human and cynomolgus CLL-1 and CD3ε to test the safety and efficacy of TDB candidates to treat AML. A panel of antibodies was interrogated for binding specificity and biological function to these antigens. Five of 36 mouse hybridoma antibodies recognized both human and cynomolgus CLL-1 proteins. Subsequently, one antibody was humanized and engineered for improved chemical stability based on its monovalent high affinity (KD = 3.1 nM) to both human and cynomolgus CLL-1. Because a wide range of affinities to CD3ε has been reported for CD3 T-cell bispecific therapeutics (1-270 nM), we investigated the relationship between CD3 affinity to CD3 and activity, PK, and safety. Three humanized antibodies with differential affinities to human CD3ε were developed: low (50 nM), high (0.5 nM), and very high (0.05 nM). The knob-and-hole technology was used to produce TDBs with a single peak in gel filtration (>97%-99% monomer) with <0.3% to 0.7% aggregates, no detection of homodimers by mass spectroscopy, and low LAL values (supplemental Figure 1). The nomenclature for the TDBs comprising an anti–CLL-1 arm paired with 1 of 3 different anti-CD3ε affinity arms are CLL1/CD3L, CLL1/CD3H, and CLL1/CD3VH. Similarly, the anti-gD, nontargeting CLL-1 arm (NT-TDB), follows the same nomenclature.
CLL-1 TDBs are highly active against CLL-1–expressing AML tumor cell lines
We tested the CLL1/CD3L and CLL1/CD3H TDBs for in vitro cell killing using purified human CD8 T cells. The TDBs reduced the number of target cells in a dose-dependent manner, with potency in the low ng/mL range (Table 1; Figure 1A). Notably, the sensitivity of the cell lines to the TDB did not correlate with the amount of target surface expression and cell lines with low CLL-1 expression were sensitive to the TDBs. The EC50s were higher for the CLL1/CD3L TDB by four- to 100-fold, most likely because of decreased activation of CD8+ T cells (Figure 1B). There was no tumor cell killing by nontargeting (NT) TDBs. Furthermore, CLL1/CD3H TDB was effective at lysing AML CD11b+/CD33+ and CD11b–/CD33+ target cells from 2 confirmed cases of AML in the presence of purified allogeneic CD8 T cells with an EC50 of 0.45 to 3 ng/mL (Figure 1C-D). CD11b–/CD33+ cells were significantly reduced, whereas the CD11b+/CD33+ cells were nearly ablated by CLL1/CD3H from donor 1. Together, these results demonstrate that using a bispecific antibody targeting CLL-1 and CD3ε to redirect the human immune system is effective against a variety of AML subtypes and clinical AML samples.
AML tumor cell growth suppression is dependent on affinity to CD3ε
| AML cell lines . | MFI ratio CLL-1 . | FAB subtype . | CLL1/CD3H EC50 (ng/mL) . | CLL1/CD3H % depletion . | CLL1/CD3L EC50 (ng/mL) . | CLL1/CD3L % depletion . |
|---|---|---|---|---|---|---|
| Molm-13 | 1.1 | M5 | 19.9 | 51 | 147 | 57 |
| ML-2 | 2.4 | M4 | 2.8 | 100 | 304 | 81 |
| THP-1 | 4 | M5 | 2.5 | 92 | 131 | 90 |
| EOL-1 | 4.7 | M4-EOS | 2 | 82 | 172 | 78 |
| Nomo-1 | 4.9 | M5 | 11.8 | 76 | 263 | 84 |
| HL-60 | 12.7 | M5 | 2.2 | 100 | 119 | 47 |
| U937 | 21 | M2 | 2.7 | 100 | 70 | 89 |
| PL-21 | 21.4 | M3 | 5.8 | 99 | 129 | 98 |
| AML cell lines . | MFI ratio CLL-1 . | FAB subtype . | CLL1/CD3H EC50 (ng/mL) . | CLL1/CD3H % depletion . | CLL1/CD3L EC50 (ng/mL) . | CLL1/CD3L % depletion . |
|---|---|---|---|---|---|---|
| Molm-13 | 1.1 | M5 | 19.9 | 51 | 147 | 57 |
| ML-2 | 2.4 | M4 | 2.8 | 100 | 304 | 81 |
| THP-1 | 4 | M5 | 2.5 | 92 | 131 | 90 |
| EOL-1 | 4.7 | M4-EOS | 2 | 82 | 172 | 78 |
| Nomo-1 | 4.9 | M5 | 11.8 | 76 | 263 | 84 |
| HL-60 | 12.7 | M5 | 2.2 | 100 | 119 | 47 |
| U937 | 21 | M2 | 2.7 | 100 | 70 | 89 |
| PL-21 | 21.4 | M3 | 5.8 | 99 | 129 | 98 |
CLL-1 expression for each AML cell line is expressed as the MFI Ratio between CLL-1 MFI divided by Isotype MFI; (Burkitt lymphoma cell line, Namalwa, was used as a negative control and had a MFI ratio of 0.9). EC50 is the concentration of TDB, CLL1/CD3H, or CLL1/CD3L, which was required to inhibit cell growth of AML tumor cell lines in the presence of purified effector human CD8+ T cells and TDB. Nonspecific targeting by CLL1/CD3H was performed on two B cell lines, WSU (non-Hodgkin lymphoma) and BJAB (Burkitt lymphoma), and were shown to have no effect on cell growth compared with a positive control anti-CD20/CD3H TDB and a negative control nontargeting (NT)/CD3H TDB (data not shown). Percent depletion measures the depth of the response or amount of tumor cell lyses as determined by fluorescence-activated cell sorting.
AML tumor cell growth suppression is dependent on affinity to CD3ε and effects of CLL1/CD3H TDB on AML BMMNCs. (A) Dose-dependent survival curves determined by Prism-6 using a nonlinear regression with sigmoidal dose response where X is the log [ ]. Percent survival is calculated by dividing the treatment live events by its untreated control replicate live events, and multiplying by 100. HL60 was performed in a separate experiment from the other cell lines (data not shown for CLL1/CD3H). (B) EOL-1 dose-dependent survival and percent of CD8+CD69+CD25+ effector cells. One of 3 experiments, each using a different blood donor. Similar results were observed for other AML cell lines. (C-D) CD8+ T cells were purified from peripheral blood of a healthy human donor (ALLCells, Alameda, CA) for donor 1 and a Genentech donor for donor 2 by Ficoll density gradient centrifugation and a human CD8+ T cell isolation kit from Miltenyi (130-096-495). Patient AML bone marrow was obtained from the Stanford Cancer Center and purified by Ficoll centrifugation. The AML blasts were preincubated for ∼6 hours with hIgG (EU = 0.07/mg) to reduce nonspecific binding to FcγRs. The E:T ratio was 3:1 (150 000 CD8 T cells to 50 000 blasts). Medium used for the assay was RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, Pen-Strep, 1X cytokine bullet (IL-3, IL-6, SCF, Flt3; StemCell Technologies, Vancouver, Canada), 0.1 μg/mL GM-CSF and 0.1 μg/mL G-CSF. The test articles were NT/CD3H and CLL1/CD3H diluted serially in threefold steps. The assay was set up in triplicate with either a 20-hour (donor 2; blue lines) or 40-hour (donor 1; red lines) exposure to test article. (C) Dot plot shows SSC/CD45 profile of AML patient donor 1 and donor 2. Histogram shows positive staining of CD11b–/CD34+ AML blasts for human CD33 (red) and CLL-1 (blue) compared with isotype control antibody. (D) Allogeneic CD8+ effector T cells in the presence of threefold serially diluted CLL1/CD3H shows concentration-dependent killing of AML blasts with an EC50 ∼0.45 ng/mL for donor 1 compared with the NT/CD3H TDB. Dotted line (NT/CD3H) and solid line (CLL1/CD3H). Donor 1 phenotype was consistent with AML LSCs—CD45+/CD34+/CD33+/CLL-1+/CD38– (99%) with <1% CD38+. Donor 2 phenotype was consistent with AML progenitors—CD45+/CD34+/CD33+/CLL-1+/CD38+ (∼97%).
AML tumor cell growth suppression is dependent on affinity to CD3ε and effects of CLL1/CD3H TDB on AML BMMNCs. (A) Dose-dependent survival curves determined by Prism-6 using a nonlinear regression with sigmoidal dose response where X is the log [ ]. Percent survival is calculated by dividing the treatment live events by its untreated control replicate live events, and multiplying by 100. HL60 was performed in a separate experiment from the other cell lines (data not shown for CLL1/CD3H). (B) EOL-1 dose-dependent survival and percent of CD8+CD69+CD25+ effector cells. One of 3 experiments, each using a different blood donor. Similar results were observed for other AML cell lines. (C-D) CD8+ T cells were purified from peripheral blood of a healthy human donor (ALLCells, Alameda, CA) for donor 1 and a Genentech donor for donor 2 by Ficoll density gradient centrifugation and a human CD8+ T cell isolation kit from Miltenyi (130-096-495). Patient AML bone marrow was obtained from the Stanford Cancer Center and purified by Ficoll centrifugation. The AML blasts were preincubated for ∼6 hours with hIgG (EU = 0.07/mg) to reduce nonspecific binding to FcγRs. The E:T ratio was 3:1 (150 000 CD8 T cells to 50 000 blasts). Medium used for the assay was RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, Pen-Strep, 1X cytokine bullet (IL-3, IL-6, SCF, Flt3; StemCell Technologies, Vancouver, Canada), 0.1 μg/mL GM-CSF and 0.1 μg/mL G-CSF. The test articles were NT/CD3H and CLL1/CD3H diluted serially in threefold steps. The assay was set up in triplicate with either a 20-hour (donor 2; blue lines) or 40-hour (donor 1; red lines) exposure to test article. (C) Dot plot shows SSC/CD45 profile of AML patient donor 1 and donor 2. Histogram shows positive staining of CD11b–/CD34+ AML blasts for human CD33 (red) and CLL-1 (blue) compared with isotype control antibody. (D) Allogeneic CD8+ effector T cells in the presence of threefold serially diluted CLL1/CD3H shows concentration-dependent killing of AML blasts with an EC50 ∼0.45 ng/mL for donor 1 compared with the NT/CD3H TDB. Dotted line (NT/CD3H) and solid line (CLL1/CD3H). Donor 1 phenotype was consistent with AML LSCs—CD45+/CD34+/CD33+/CLL-1+/CD38– (99%) with <1% CD38+. Donor 2 phenotype was consistent with AML progenitors—CD45+/CD34+/CD33+/CLL-1+/CD38+ (∼97%).
A dual-targeting BAC-transgenic mouse model for preclinical evaluation of αCLL-1 TDBs
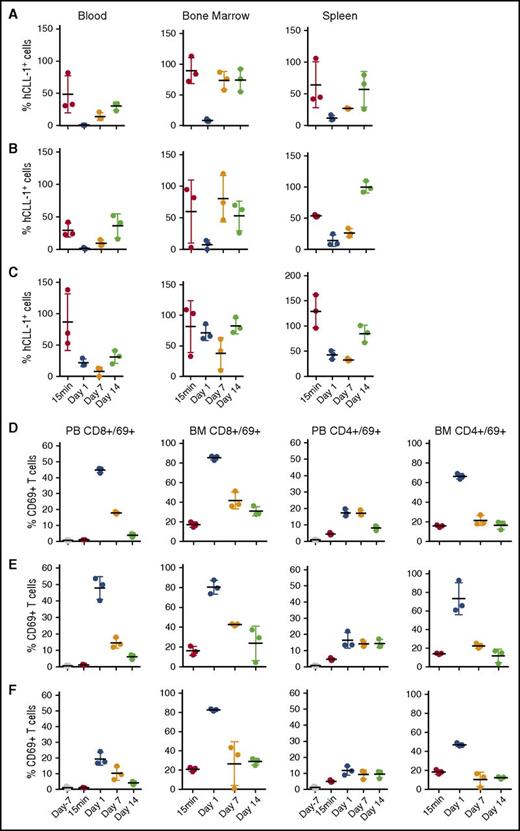
A 2xBAC-Tg GEMM with an intact immune system was developed as a tool to rank order the in vivo potency of our CLL1/CD3 TDBs. We generated mice that coexpress hCD3ε and hCLL-1 antigens. Human-CD3ε was expressed on CD4+ and CD8+ T cells, whereas hCLL-1 was expressed on a subset of myeloid cells (monocytes and eosinophils but not neutrophils) (supplemental Figure 2A-B). Normally, hCLL-1 is uniformly expressed on human monocytes and neutrophils.9,10 Dose-dependent upregulation of CD69 on CD3+CD8+ T cells from 2xBAC-Tg was confirmed upon exposure to CLL1/CD3 TDBs ex vivo; however, ∼tenfold more CLL1/CD3L TDB was required for maximal T-cell activation (supplemental Figure 2C). Finally, we investigated whether the GEMM could be used to monitor target cell depletion beyond the normal recovery period of blood cells (days 10-14). Sca1+/c-kit+ HSCs did not express hCLL-1 on their surface (supplemental Figure 2D), and therefore it is expected that interpretation of efficacy past days 10 to 14 might be confounded by normal hematopoiesis. Overall, these results validate the use of the 2xBAC-Tg dual target–mediated model for screening in vivo potency and behavior of CLL1/CD3 TDBs.
In vivo potencies of CLL-1 TDBs are determined by the anti-CD3ε arm affinity
The 2xBAC-Tg GEMM was designed to demonstrate that an CLL1/CD3 TDB has the capacity to activate T cells to kill autologous transgenic human CLL-1+ cells without manipulation of the E:T in vivo. First we investigated how manipulating the affinity of the anti-CD3ε arm would affect overall potency in vivo using CLL1/CD3 TDBs. In a PK/PD study, 2xBAC-Tg mice that were administered CLL1/CD3 TDBs, regardless of anti-CD3ε affinity (L/H/VH), exhibited rapid depletion of human CLL-1+ monocytes/eosinophils in blood, bone marrow, and spleen (Figure 2A-C), with concurrent marked CD4+ and CD8+ T-cell activation (elevation in surface CD69) and expansion, compared with vehicle and NT-TDB controls (Figure 2D-F). This result suggests that loss of target cells from the circulation was probably not a result of margination. Interestingly, a trend toward a delayed rebound of target cells through day 7 was observed in mice given CLL1/CD3L. Assessment beyond day 10 is probably confounded by normal hematopoiesis, but this trend was also observed in another study, presented in supplemental Figure 3A-B. Further evidence for T cell–mediated target cell killing was exemplified by the accompanied elevation of proinflammatory cytokines, such as interleukin-2 (IL-2)/IL-6 2 to 6 hours post-dose (supplemental Figure 4). Although delayed T-cell margination cannot be excluded as a cause for the increased potency of CLL1/CD3L, all CLL-1–specific TDBs showed similar numbers of CD8 T cells in spleen and lymph nodes at day 1, equivalent to predose levels but declined by two- to threefold by day 7, and CD4 counts in spleen tracked with the naïve controls but were reduced in lymph nodes (data not shown). Subsequently, the relationship between PK and in vivo potency was addressed for the TDBs.
Depletion of human CLL-1+ 2xBAC-Tg mouse cells from blood and bone marrow after single IV dose administration of 0.5 mg/kg CLL-1 TDB. (A) CLL1/CD3VH, (B) CLL1/CD3H, and (C) CLL1/CD3L. Percent of hCLL-1+ cells were derived from the average naïve untreated mice hCLL-1+ cells; blood (n = 39), bone marrow and spleen (n = 4). Upregulation of CD69 on CD8+ and CD4+ T cells by (D) CLL1/CD3VH, (E) CLL1/CD3H, and (F) CLL1/CD3L. Time points are terminal bleeds. The mean and standard deviation are represented by horizontal and vertical lines, respectively.
Depletion of human CLL-1+ 2xBAC-Tg mouse cells from blood and bone marrow after single IV dose administration of 0.5 mg/kg CLL-1 TDB. (A) CLL1/CD3VH, (B) CLL1/CD3H, and (C) CLL1/CD3L. Percent of hCLL-1+ cells were derived from the average naïve untreated mice hCLL-1+ cells; blood (n = 39), bone marrow and spleen (n = 4). Upregulation of CD69 on CD8+ and CD4+ T cells by (D) CLL1/CD3VH, (E) CLL1/CD3H, and (F) CLL1/CD3L. Time points are terminal bleeds. The mean and standard deviation are represented by horizontal and vertical lines, respectively.
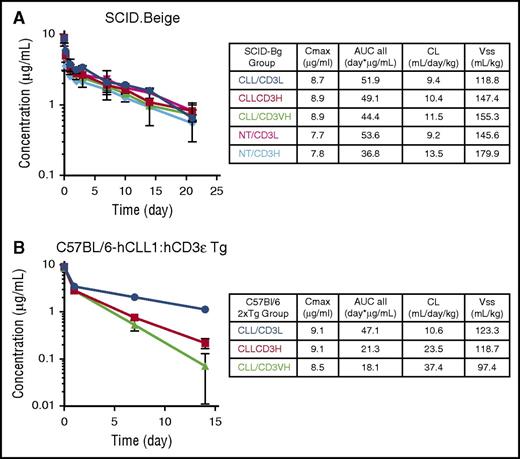
In target-deficient SCID mice, PK profiles of CLL1/CD3 TDBs were similar and comparable with the NT-TDBs (Figure 3A). However, in 2xBAC-Tg mice, CD3ε arm affinity-dependent differences in PK were apparent (Figure 3B); CLL1/CD3H or VH TDBs showed two to four times faster clearance, with a proportional decrease in exposure compared with the CLL1/CD3L TDB. The significant reduction of hCLL-1+ cells at day 1 suggests the presence of sufficient drug concentrations for all CLL1/CD3 TDBs, whereas the delay of target-cell rebound by CLL1/CD3L TDB can be partially explained by the higher exposure at day 7 compared with the TDBs with higher CD3ε affinities. Therefore, CD3 affinity is likely a driving factor in determining the potency of the CLL1/CD3 TDB in vitro, but in vivo PK and effector T-cell biology play important roles in overall TDB activity.
PK profiles for CLL-1/CD3 TDBs in target-deficient and target-expressing mice. Animals were administered a single IV dose of 0.5 mg/kg of either nontargeting (NT) TDBs or CLL-1–specific TDBs over a period of 14 to 21 days. (A) Target-deficient SCID.Beige mice PK values, (B) target-expressing 2xBACT-Tg (human CLL-1 and human CD3ε) PK values.
PK profiles for CLL-1/CD3 TDBs in target-deficient and target-expressing mice. Animals were administered a single IV dose of 0.5 mg/kg of either nontargeting (NT) TDBs or CLL-1–specific TDBs over a period of 14 to 21 days. (A) Target-deficient SCID.Beige mice PK values, (B) target-expressing 2xBACT-Tg (human CLL-1 and human CD3ε) PK values.
Preclinical PK, PD, and toxicity profile of anti–CLL-1/low affinity anti-CD3ε TDB in cynomolgus monkeys
Significant differences in CLL-1 expression on myeloid cells between our GEMM and humans/cynomolgus monkeys, and the concern of unnatural expression of hCD3ε expression in mice, prompted us to test our CLL1/CD3 TDBs in cynomolgus, which express CLL-1 on the surface of monocytes and granulocytes, albeit at lower levels than humans (supplemental Figure 5A). However, unlike human CD34+CD38– HSCs, which are negative for cell surface CLL-1, cynomolgus CD34+CD38– cells express very low levels of CLL-1 (supplemental Figure 5A).9,10 The former is further supported by our assessment of human bone marrow mononuclear cells (BMMNCs) exposed to CLL1/CD3H TDB and measuring the hematopoietic potential of HSCs using a colony-forming unit (CFU) assay. In supplemental Figure 5B, the number of CFU-GM/GEMM colonies was ∼25% of the untreated or NT/CD3H controls, whereas the number of BFU-E/CFU-E were similar for all groups. The lower CFU-GM/GEMM for CLL1/CD3H-treated BMMNCs is most likely caused by the depletion of the majority of progenitor and mature CLL-1+ myeloid cells compared with untreated and NT-TDB controls before plating of the CFU assay. In addition, potency of CLL1/CD3H TDB to activate cynomolgus T cells and kill autologous CD14+ cells or EOL-1 cells was demonstrated in vitro (supplemental Figure 5C).
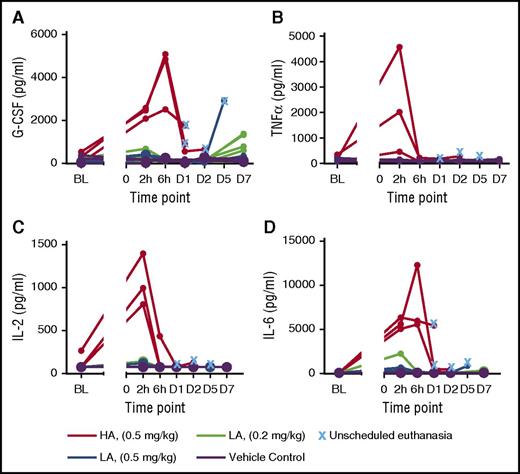
Initially, animals were given a single 0.5 mg/kg IV infusion of TDBs. All animals receiving the CLL1/CD3H developed vascular shock with fever and were euthanized within 6 to 30 hours. Histopathologic examination indicated congestion and edema in multiple organs, and hepatocellular dissociation consistent with circulatory collapse. This was likely because of marked elevation of serum cytokines and proinflammatory mediators 2 to 6 hours after dosing, including tumor necrosis factor-α, IL-2, granulocyte colony-stimulating factor (G-CSF), IL-6, IL-1ra, IL-5, and MCP-1. Animals administered CLL1/CD3L did not exhibit toxicity within 48 hours and had variable increases in systemic cytokines 2 to 6 hours after dosing that were generally much smaller and sustained for a shorter period of time than those in the CLL1/CD3H group (Figure 4; supplemental Table 1). However, these animals developed febrile neutropenia on day 4, and were successfully treated with antibiotics and nonsteroidal anti-inflammatory medications with the exception of one animal that was euthanized on day 6. Cytokine increases were present at euthanasia, but were smaller than with CLL1/CD3H and were without histologic correlates. A second cohort (N = 6) was administered a 0.2-mg/kg dose of CLL1/CD3L without adverse clinical signs and reached study end at myeloid nadir (day 8; n = 3) or recovery (day 22; n = 3). The PK profile for cynomolgus was consistent with the results of the GEMM study (supplemental Figure 6) in that it depended on CD3ε affinity. TDBs dosed at 0.5 mg/kg had comparable Cmax with faster clearance of CLL1/CD3H. Clearance of CLL1/CD3L was nearly dose proportional.
Cytokine levels in cynomolgus monkeys receiving CLL1/CD3H (HA) and CLL1/CD3L (LA). (A) G-CSF, (B) tumor necrosis factor-α (TNFα), (C) IL-2, and (D) IL-6. Cytokine release typically occurred within 2 to 6 hours of administration of either molecule and was markedly increased in those animals that received 0.5 mg/kg CLL1/CD3H compared with those that received CLL1/CD3L. All animals that required unscheduled euthanasia had comparatively elevated cytokine levels compared with those that survived to scheduled necropsy. The one animal that received 0.5 mg/kg CLL1/CD3L and required unscheduled necropsy had perimortem increases in G-CSF, IL-6, and other innate cytokines such as MCP-1 (data not shown).
Cytokine levels in cynomolgus monkeys receiving CLL1/CD3H (HA) and CLL1/CD3L (LA). (A) G-CSF, (B) tumor necrosis factor-α (TNFα), (C) IL-2, and (D) IL-6. Cytokine release typically occurred within 2 to 6 hours of administration of either molecule and was markedly increased in those animals that received 0.5 mg/kg CLL1/CD3H compared with those that received CLL1/CD3L. All animals that required unscheduled euthanasia had comparatively elevated cytokine levels compared with those that survived to scheduled necropsy. The one animal that received 0.5 mg/kg CLL1/CD3L and required unscheduled necropsy had perimortem increases in G-CSF, IL-6, and other innate cytokines such as MCP-1 (data not shown).
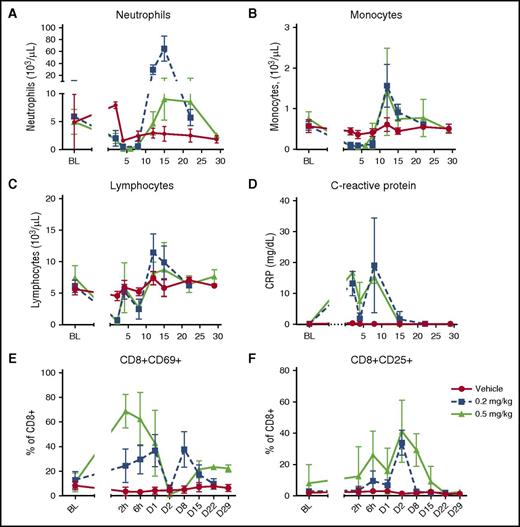
In general, clinical pathology showed similar changes in all cohorts consisting of expected pharmacologic reduction of leukocytes associated with a transient acute phase response. The magnitude of acute phase inflammation was proportional to cytokine elevation, with those animals exhibiting morbidity having higher serum cytokine levels. Leukocyte reduction/margination was apparent 24 hours post dose, with a greater decrease in neutrophils in animals that received CLL1/CD3H (range, 170-680/μL) compared with those that received the CLL1/CD3L (range, 1080-2890/μL). In surviving animals that received CLL1/CD3L, the nadir of monocyte and neutrophil reduction was similar between the 0.2 mg/kg and 0.5 mg/kg dose groups and occurred between days 2 and 8 and 4 and 8, respectively (Figure 5A-B). Rebound neutrophilia (days 12-15) was followed by return to baseline of all myeloid cells by days 22 to 29. Myeloid depletion correlated with an early decrease of circulating lymphocytes, activation of T cells (CD69+ or CD25+), and increases in C-reactive protein (Figure 5C-F). Lymphocyte margination is commonly seen with TDBs and is thought to be caused by lymphocyte activation.16,17 Furthermore, bone marrow CD11b+ and CLL-1+ cells were attenuated on day 8 compared with day 22, consistent with the marked reduction of late-stage myeloid cells/neutrophils observed in hematoxylin and eosin–stained sections of bone marrow and spleen (Figure 6). Early myeloid progenitor cells in bone marrow were increased on day 8, consistent with clinical recovery. No other clinical signs, including neurotoxicity, were noted and CLL1/CD3L-related histopathologic lesions at necropsy on day 8 were limited to transient germinal center B-cell loss, mild adrenocortical hyperplasia, and minimal thymic reduction—all exhibited reversibility.
Clinical pathology and peripheral blood flow cytometry from cynomolgus monkeys receiving CLL1/CD3L TDB. Target cell depletion of neutrophils (A) and monocytes (B) is similar between both dose groups, with nadirs between day 4 and 8 and 2 and 8 post dose, respectively. Rebound neutrophilia between days 10 and 15 is marked and greater in the 0.2 mg/kg dose group. Note, the mild decrease in neutrophil count on day 2 in the vehicle group is likely secondary to repeat blood sampling. Reduction of lymphocytes (C) occurred in 2 waves (days 2-8) concurrent to target cell depletion, elevation of acute phase proteins such as C-reactive protein (D), and increased activation of CD8+ cytotoxic T cells as measured by CD69 (E) and CD25 (F) expression. The immediate increase in percentage of CD69+ CD8+ T cells was greater (peak 71.3%-80.7% vs 25.2%-57.0%), and the percentage of CD25+ T cells was elevated for longer (return to baseline D15-D22 vs D8-D15) in the 0.5 mg/kg group.
Clinical pathology and peripheral blood flow cytometry from cynomolgus monkeys receiving CLL1/CD3L TDB. Target cell depletion of neutrophils (A) and monocytes (B) is similar between both dose groups, with nadirs between day 4 and 8 and 2 and 8 post dose, respectively. Rebound neutrophilia between days 10 and 15 is marked and greater in the 0.2 mg/kg dose group. Note, the mild decrease in neutrophil count on day 2 in the vehicle group is likely secondary to repeat blood sampling. Reduction of lymphocytes (C) occurred in 2 waves (days 2-8) concurrent to target cell depletion, elevation of acute phase proteins such as C-reactive protein (D), and increased activation of CD8+ cytotoxic T cells as measured by CD69 (E) and CD25 (F) expression. The immediate increase in percentage of CD69+ CD8+ T cells was greater (peak 71.3%-80.7% vs 25.2%-57.0%), and the percentage of CD25+ T cells was elevated for longer (return to baseline D15-D22 vs D8-D15) in the 0.5 mg/kg group.
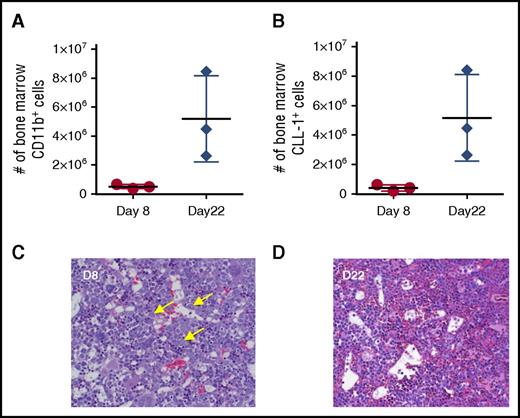
Myeloid depletion in the bone marrow of cynomolgus monkeys receiving 0.2 mg/kg CLL1-CD3L. Myeloid cell number, assessed by either CD11b reactivity (A) or CLL-1 reactivity (B), were markedly attenuated on day 8 (nadir of peripheral neutrophils) compared with day 22 (peripheral neutrophil recovery). Horizontal and vertical lines represent mean and standard deviation, respectively. (C) Bone marrow histology at day 8 revealed marked depletion of late-stage myeloid cells with a relative increase in early myeloid progenitors (arrows), suggesting that hematopoietic stem cells were not affected at this dose. (D) By day 22, late-stage myeloid cells were recovered and minimally increased in relative abundance (asterisks). Images of FFPE 5-µm sections stained with hematoxylin and eosin were acquired on an Olympus BX53 (Olympus, Burlingame, CA) camera, objective UPlanSApo 40x/0.95 at 20-23°C, and photographed with an Infinity 2 camera and analyzed with Infinity Analyze software (Lumenera, Ottawa, Ontario, Canada).
Myeloid depletion in the bone marrow of cynomolgus monkeys receiving 0.2 mg/kg CLL1-CD3L. Myeloid cell number, assessed by either CD11b reactivity (A) or CLL-1 reactivity (B), were markedly attenuated on day 8 (nadir of peripheral neutrophils) compared with day 22 (peripheral neutrophil recovery). Horizontal and vertical lines represent mean and standard deviation, respectively. (C) Bone marrow histology at day 8 revealed marked depletion of late-stage myeloid cells with a relative increase in early myeloid progenitors (arrows), suggesting that hematopoietic stem cells were not affected at this dose. (D) By day 22, late-stage myeloid cells were recovered and minimally increased in relative abundance (asterisks). Images of FFPE 5-µm sections stained with hematoxylin and eosin were acquired on an Olympus BX53 (Olympus, Burlingame, CA) camera, objective UPlanSApo 40x/0.95 at 20-23°C, and photographed with an Infinity 2 camera and analyzed with Infinity Analyze software (Lumenera, Ottawa, Ontario, Canada).
Discussion
The present work describes the development of a T cell–dependent bispecific antibody (TDB) to treat AML. CLL-1 as a target makes sense based on its prevalence in AML and our FACS data that show no CLL-1 expression on HSCs compared with reports of significant expression for CD33 and low or uncertain expression for CD123.7,8 Our in vitro HSC/progenitor CFU assay suggests minimal impact on hematopoiesis by a CLL-1 TDB, whereas it is ambiguous for a CD123-directed molecule given the reports of marked impairment of human hematopoiesis by a CAR-T-CD123, but none with a CD123-DART.18-20 Rather than using an antibody-fragment–based format like the CD33 BiTE21 or CD123 DART,19 a full-length IgG was used for improving PK and potentially lowering immunogenicity. We hypothesized that CD3 affinity would be a key driver of both efficacy and safety. Interestingly, our high-affinity CD3 TDBs were more potent than the low affinity CD3 TDB in vitro, but all CLL-1 TDBs had comparable potency in vivo. A major reason for this is most likely a result of PK differences among the TDBs. In a GEMM and cynomolgus monkey, PK data were consistent with the fact that CLL-1 TDBs behaved in a CD3-affinity–dependent manner—low-affinity CD3 arm (Kd = 50 nM) exhibited slower clearance and higher drug exposure compared with CLL-1 TDBs with higher affinity CD3 arms (Kd = 0.05-0.5 nM). In the absence of either human-CLL-1 or -CD3 in mice, PK profiles were similar for all CLL-1/CD3 TDBs and equivalent to nontargeting TDBs. Although higher affinity CD3 TDBs targeting CLL-1 resulted in more complete target cell depletion in the GEMM, the duration of target-cell rebound attributed to hematopoiesis was slower for the CLL1/CD3L TDB, resulting in a trend of more sustained target depletion—CLL1/CD3L has a more desirable PK profile compared with CLL1/CD3H/-VH TDBs. In addition to PK, other factors may be responsible, including variations in effector cell composition, number, and E:T ratio in vivo. Alternatively, the low-affinity CD3ε arm could have induced slower T-cell activation, thereby allowing accumulation of preactivated effector T cells. In support of this notion, it has been reported that an immunologic synapse is not required for T-cell activation because stimulation through TCR precedes formation of a mature immunologic synapse.22 Furthermore, the larger differential affinity between the CLL-1 and CD3L arms compared with CD3H/VH may have favored more preferential binding to human CLL-1 compared to human CD3, and enabled serial recruitment of CD3+ T cells and destruction of human CLL-1+ cells, as has been described for blinatumomab.23
Safety of the high- and low-affinity CD3ε variants was also examined in the cynomolgus monkey. The high-affinity variant was poorly tolerated, most likely because of extensive cytokine release. However, this is contrary to the minimal cytokine release and less severe neutropenia reported for a CD123-DART19 ; CD123 expression in humans is generally not expressed on circulating neutrophils and mature monocytes.24 It is possible that the significantly higher cytokine release with CLL-1 TDB in our monkey study is caused more by complete myeloid cell lyses, including neutrophils, and release of cytokines like IL-6. Early clinical experience with blinatumumab showed significant toxicities related to cytokine release syndrome (CRS) and generalized immune activation that were mitigated by incorporating an initial stepwise increase in dose level.13 We were able to circumvent toxicities related to CRS by using a low-affinity CD3 arm and identifying a dose level that provided acceptable safety while maintaining robust activity. Finally, CLL1/CD3L TDB was otherwise well tolerated without clinical or histopathologic signs of neurotoxicity, an adverse event that has been reported for CD19 T-cell therapies, including blinatumumab.25 However, it should be noted that central nervous system adverse events such as headache and aphasia cannot be assessed in preclinical species.
Although CLL-1 TDB was effective in vitro at killing primary AML cells, multiple factors may affect the translation of our preclinical data to AML patients. Similar to CD123-DART, CLL-1 expression is lower in monkeys than humans and this may affect precise translation of CRS risk to humans. Also, the expression of CLL-1 on AML cells is slightly lower than CD33 and may affect in vivo potency; however, like the CD33 BiTE, CLL1/CD3 TDB can kill low-copy-number AML tumor cell lines. A major concern is that the number and distribution of T-cell subsets in healthy individuals may differ substantially from that in patients, especially those with underlying disease and/or prior anticancer therapies. Patients with chemotherapy-induced cytopenia also have variable number and ratio of T cells (CD4/CD8) that are generally lower than healthy individuals. Despite this, the ability of T-cell recruiting bispecifics to elicit antitumor activity with minimal numbers of effector cells is supported by several observations. First, clinical data with blinatumomab for r/r-B-cell ALL has shown that even after intense chemotherapy in patients with severe leukemia, which may result in immune compromise, T-cell recruiting bispecifics can elicit antitumor activity. Second, despite low numbers, T-cell function in AML is preserved. For example, CD3+ T cells in untreated AML are comprised mostly of CD45RA+ naïve TCRαβ+ T cells, rather than CD45RO+ memory T cells and can be activated through the CD3/TCR complex in the presence of AML blasts.26 In addition, patients at diagnosis show similar expression profiles of inhibitory molecules (PD-1, 2B4, TIM-3, Lag-3, and CD160) compared with healthy donors, thus lack of T-cell responsiveness as a result of negative immune checkpoint molecules is less likely.27,28 Furthermore, the proliferative capacity of residual T cells in chemotherapy-induced cytopenic AML patients was preserved and higher than that observed in ALL.29 Finally, in relapsed patients after allogeneic stem cell transplantation, both PD-1 and 2B4 expression on T cells was higher and associated with a shift toward a differentiated T-effector memory phenotype.28 Overall, these observations suggest that intervention with a T cell redirecting therapeutic at early diagnosis or in combination with chemotherapy is a rational approach.
Targeted immunotherapy could change the landscape of AML treatments by harnessing the body’s immune system to destroy leukemic blasts. We provide evidence that a full-length humanized-IgG TDB-targeting human CLL-1 and human CD3ε is a feasible approach to eradicate CLL-1+ leukemic cells. The superior pharmacokinetic properties of an IgG antibody and low liability for off-target destruction of HSCs make this attractive as a potential targeted therapy for AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.G.P. designed experiments and co-wrote the manuscript; S.R.L. designed and performed experiments, performed analyses, and cowrote the manuscript; S. Sukumaran, B.R.V., and S.T.L. designed experiments, performed analyses, and contributed to writing the manuscript; G.L. managed the project and edited the manuscript; Y.-W.C., R.A.P., and K.L. designed experiments and review of data analyses; and M.H., K.T., S. Stainton, E.L., A.W., L.T., R.N., D.E., C.G., M.M., M.S.D., A.N., B.Z., and C.Z. designed experiments, performed experiments, performed analyses, and/or developed novel reagents.
Conflict-of-interest disclosure: All authors reside at Genentech, a member of the Roche Group.
Correspondence: Andrew G. Polson, Genentech, 1 DNA Way, MS 72A, South San Francisco, CA 94080; e-mail: polson@gene.com; and Steven R. Leong, Genentech, 1 DNA Way, MS 72A, South San Francisco, CA 94080; e-mail: leong.steven@gene.com.
References
Author notes
S.R.L. and S. Sukumaran contributed equally to this study.

![Figure 1. AML tumor cell growth suppression is dependent on affinity to CD3ε and effects of CLL1/CD3H TDB on AML BMMNCs. (A) Dose-dependent survival curves determined by Prism-6 using a nonlinear regression with sigmoidal dose response where X is the log [ ]. Percent survival is calculated by dividing the treatment live events by its untreated control replicate live events, and multiplying by 100. HL60 was performed in a separate experiment from the other cell lines (data not shown for CLL1/CD3H). (B) EOL-1 dose-dependent survival and percent of CD8+CD69+CD25+ effector cells. One of 3 experiments, each using a different blood donor. Similar results were observed for other AML cell lines. (C-D) CD8+ T cells were purified from peripheral blood of a healthy human donor (ALLCells, Alameda, CA) for donor 1 and a Genentech donor for donor 2 by Ficoll density gradient centrifugation and a human CD8+ T cell isolation kit from Miltenyi (130-096-495). Patient AML bone marrow was obtained from the Stanford Cancer Center and purified by Ficoll centrifugation. The AML blasts were preincubated for ∼6 hours with hIgG (EU = 0.07/mg) to reduce nonspecific binding to FcγRs. The E:T ratio was 3:1 (150 000 CD8 T cells to 50 000 blasts). Medium used for the assay was RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, Pen-Strep, 1X cytokine bullet (IL-3, IL-6, SCF, Flt3; StemCell Technologies, Vancouver, Canada), 0.1 μg/mL GM-CSF and 0.1 μg/mL G-CSF. The test articles were NT/CD3H and CLL1/CD3H diluted serially in threefold steps. The assay was set up in triplicate with either a 20-hour (donor 2; blue lines) or 40-hour (donor 1; red lines) exposure to test article. (C) Dot plot shows SSC/CD45 profile of AML patient donor 1 and donor 2. Histogram shows positive staining of CD11b–/CD34+ AML blasts for human CD33 (red) and CLL-1 (blue) compared with isotype control antibody. (D) Allogeneic CD8+ effector T cells in the presence of threefold serially diluted CLL1/CD3H shows concentration-dependent killing of AML blasts with an EC50 ∼0.45 ng/mL for donor 1 compared with the NT/CD3H TDB. Dotted line (NT/CD3H) and solid line (CLL1/CD3H). Donor 1 phenotype was consistent with AML LSCs—CD45+/CD34+/CD33+/CLL-1+/CD38– (99%) with <1% CD38+. Donor 2 phenotype was consistent with AML progenitors—CD45+/CD34+/CD33+/CLL-1+/CD38+ (∼97%).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/5/10.1182_blood-2016-08-735365/4/m_blood735365f1.jpeg?Expires=1763475429&Signature=RdLUg1i6Cjyjv1QjHo2ZOnuzWURVPcQg1jkp5rtgjZwnRkmYG9H7Ma3iMPS3GHMSScxAt43-yaXNlwfrfsFKNc46kaTiloqd2eZZ2OQAlxwPPcwtP6mXJgcmxVDU7x9kVvirTYvxKEbRI64~uZSxs77BmBPhCtkD82zI2efAg3tHUJYgW3eAAL6P5AyjhHnGSMjRqappfNAjSNOGMMOgdGjfzfuVN~vY~Be8ZyYWcrqoq8gtmB4F8Ji8FAKnNWo7M-Wg3bVRy12uQdpXzFdgyT-74K9~rpLl162wl8ZsYGixJ~OVniTabbtMiFb8AsOabMvqioohIBHMG3opJIpFBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal