Key Points
BDR is a chemotherapy-free, non-stem-cell–toxic regimen associated with high response rates and long-term remissions.
The long-term safety profile of BDR is favorable, with high probability of response to reintroduction of rituximab-based regimens at relapse.
Abstract
In this phase 2 multicenter trial, we evaluated the efficacy of the combination of bortezomib, dexamethasone, and rituximab (BDR) in 59 previously untreated symptomatic patients with Waldenström macroglobulinemia (WM), most of which were of advanced age and with adverse prognostic factors. BDR consisted of a single 21-day cycle of bortezomib alone (1.3 mg/m2 IV on days 1, 4, 8, and 11), followed by weekly IV bortezomib (1.6 mg/m2 on days 1, 8, 15, and 22) for 4 additional 35-day cycles, with IV dexamethasone (40 mg) and IV rituximab (375 mg/m2) on cycles 2 and 5, for a total treatment duration of 23 weeks. On intent to treat, 85% responded (3% complete response, 7% very good partial response, 58% partial response). After a minimum follow-up of 6 years, median progression-free survival was 43 months and median duration of response for patients with at least partial response was 64.5 months. Overall survival at 7 years was 66%. No patient had developed secondary myelodysplasia, whereas transformation to high-grade lymphoma occurred in 3 patients who had received chemoimmunotherapy after BDR. Thus, BDR is a very active, fixed-duration, chemotherapy-free regimen, inducing durable responses and with a favorable long-term toxicity profile (www.ClinicalTrials.gov #NCT00832234).
Introduction
Waldenström macroglobulinemia (WM) is a lymphoplasmacytic lymphoma, characterized by the production of monoclonal immunoglobulin M (IgM).1,2 Median survival is ∼8 to 10 years, and the disease varies from one with indolent course to a disease that needs rapid control.3-5 WM cells express CD20 surface antigen; therefore, rituximab has become a key treatment component; however, it is associated with a modest response rate of ∼30% and long time to response.6-8 Thus, it is recommended that primary treatment should consist of rituximab-based combinations, to increase response rates and shorten time to response.5 Nevertheless, rituximab-based combinations are associated with time to first response of 2 to 3 months. Bortezomib affects NF-κB signaling in WM cells9 and enhances the cytotoxic activity of rituximab in antibody-dependent cellular cytotoxicity assays.10 Furthermore, bortezomib monotherapy has shown significant, rapid activity.11-13 Therefore, the combination of rituximab and bortezomib may lead to synergistic, rapid activity. In a phase 2, multicenter trial we evaluated the activity of bortezomib, dexamethasone, and rituximab (BDR) in 59 newly diagnosed WM patients.14 WM has a prolonged course, therefore extensive follow-up is needed to evaluate the outcomes after a specific therapy. Here, we present the results from the long-term follow-up of this study after a minimum follow-up of 6 years.
Study design
This multicenter, phase 2 study enrolled 59 patients from 10 European sites, within the context of the European Myeloma Network (EMN). The study was approved by all institutional review boards and was registered at ClinicalTrials.gov (#NCT00832234). Patients gave informed consent in accordance with the Declaration of Helsinki. To reduce the need for plasmapheresis and prevent the rituximab “IgM flare,” single-agent IV bortezomib 1.3 mg/m2 was administered on days 1, 4, 8, and 11 of the first 21-day cycle. Then, on cycles 2 to 5, IV bortezomib 1.6 mg/m2 was administered on days 1, 8, 15, and 22 in four 35-day consecutive cycles. On cycles 2 and 5, IV dexamethasone 40 mg and IV rituximab 375 mg/m2 were given on days 1, 8, 15, and 22. Treatment was scheduled to be completed in 23 weeks without maintenance. Monoclonal IgM levels were evaluated before each cycle. Computed tomography scans were performed within 3 months of enrollment, after BDR completion or if progressive disease was suspected. Patients were followed every 3 months for 2 years, and every 6 months thereafter. Cause of death was defined as WM-related in case of disease progression or treatment complications, or WM-unrelated in all other cases. Unrelated deaths were considered competing events to WM-related deaths, progression, or second-line therapy initiation.15,16
Results and discussion
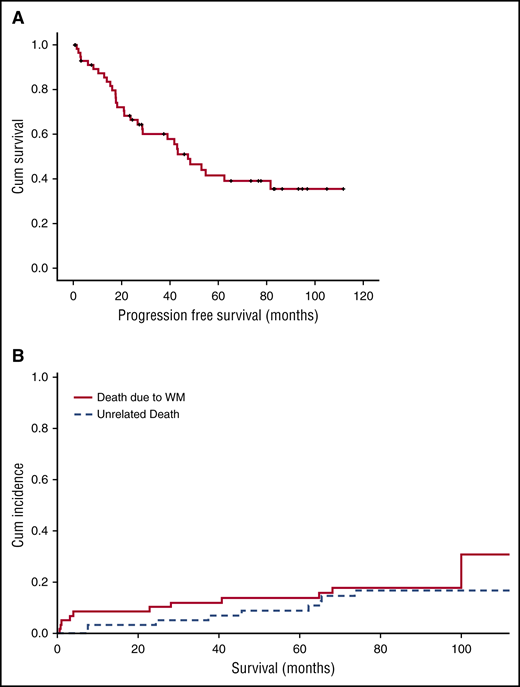
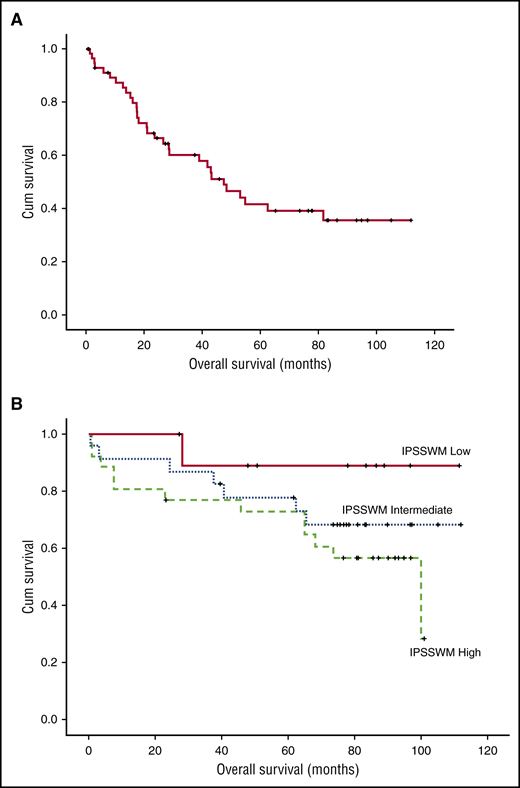
Patients were enrolled between March 2007 and June 2010. Their characteristics, response rates and toxicity were reported previously.14 Briefly, most were of advanced age (61% were >65 years) with adverse prognostic factors (hemoglobin <11.5 g/dL in 82% and β2-microglobulin ≥3 mg/dL in 64%), so that 45.5% and 40% were rated as high and intermediate risk per the International Prognostic Scoring System for WM (IPSSWM).3 On intent to treat, 85% of the patients responded (3% complete response [CR], 7% very good partial response [VGPR], 58% partial response [PR], and 17% minor response [MR]), for a major response rate (≥PR) of 68%. Median time to first response (≥MR) was 3 months and median time to best response was 5 months; however, 4 (8%) of the responders achieved their best response 6 months after treatment completion. Minimum follow-up of living patients is 72 months and median follow-up for all patients is 86 months. Peripheral neuropathy was recorded in 46% (grade 2 in 17% and grade >3 in 7%) and neuropathic pain in 20% (grade 3 in 1 patient). At the time of last follow-up, peripheral neuropathy resolved completely or to grade 1 in all patients. One patient developed herpes zoster while on treatment after discontinuing valacyclovir prophylaxis; there were no late herpes zoster episodes. Forty patients (68%) progressed or died as a result of WM, whereas 9 patients (15%) died of unrelated causes. Median progression-free survival (PFS) was 43 months (95% confidence interval [CI], 23-63) (Figure 1A) and 10 patients (17%) are still in remission after a median of 90 months (range, 73.5-112). Per IPSSWM stage, 7-year PFS was 62.5%, 42%, and 15% for low-, intermediate-, and high-risk disease, respectively. Median duration of response for patients who achieved at least PR was 64.5 months. Response rates were not affected by IPSSWM, and depth of response did not correlate with PFS, but the number of patients who achieved CR/VGPR was relatively small. Among the 40 patients who progressed,21 received second-line treatment with rituximab-based regimens (N = 17, R-CHOP: 7, DRC: 5, FCR: 3, R-bendamustine: 2), one with rituximab monotherapy, one fludarabine, and two alemtuzumab. Median time to next treatment was 73 months. Overall, 17 (80.9%) patients responded to second-line treatment (15 [71.4%] PR, 1 [4.75%] MR, and 1 [4.75%] CR). Thus, BDR given for 23 weeks induced responses even in high-risk patients with a long PFS of 43 months. Moreover, patients who relapse after BDR have high probability of response to other rituximab-based regimens. Treon et al also evaluated BDR in a phase 2 trial; after a median follow-up of 8.5 years, median time to progression was 5.5 years, the estimated 5-year PFS rate was 57% and the 5-year OS was 95%. The higher PFS and OS rates are probably a result of rituximab maintenance and the less-high-risk population enrolled in this study. However, these results confirm BDR efficacy.17 Up to June 2016, 20 patients (34%) have died; the 7-year WM-related death rate was 18.5% and unrelated death rate was 15.5% (Figure 1B). Overall survival (OS) rate at 7 years was 66% (Figure 2A) and was 87.5% for low-, 68.2% for intermediate-, and 48% for high-risk patients per ISSWM (Figure 2B). Myelodysplasia did not develop in any patient, whereas transformation to DLBCL occurred in 3 patients (5%): 2 had received chemoimmunotherapy after BDR (R-CHOP and alemtuzumab) and 1 received rituximab monotherapy. Until the date of data cut-off, 1 patient died during surgery for lung cancer and 1 as a result of colorectal cancer. This is one of the largest phase 2 studies conducted in a frontline setting with long-term follow-up, using a contemporary treatment. BDR is a chemotherapy-free regimen associated with rapid, deep, and durable responses. Although the treatment did not include maintenance, it provided long treatment-free interval, which translates to better quality of life. Importantly, toxicity was mild both in the short and long term. Thus, a strategy incorporating proteasome inhibitors in the first line is safe and effective. DRC is a regimen widely used in the frontline setting. BDR achieved modestly better PFS compared with DRC (43 vs 35 months); however, time to next treatment was significantly better (73 vs 51 months). Bendamustine with rituximab have also shown a PFS of 69 months, but the numbers were small.18 Ibrutinib was recently approved for treatment-naïve and relapsed/refractory WM patients; however, available data are based only on previously treated patients and long-term follow up is still missing. Furthermore, ibrutinib requires continuous treatment.19 Given the activity of BDR, the European WM Consortium is conducting a phase 3 study comparing DRC with or without bortezomib. Future studies may also evaluate combinations of BTK inhibitors with proteasome inhibitors and investigate whether fixed-duration treatment may be equally effective. Such combinations may improve disease control and increase the CR rate in potentially “curable” patients.
Time-to-event curves after therapy with BDR. (A) PFS and (B) cumulative (Cum) incidence of related and unrelated deaths (1 for related and 2 for unrelated deaths).
Time-to-event curves after therapy with BDR. (A) PFS and (B) cumulative (Cum) incidence of related and unrelated deaths (1 for related and 2 for unrelated deaths).
OS rate curves. (A) OS and (B) OS after therapy with BDR per ISSWM stage.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Bortezomib was provided by Janssen-Cilag, and rituximab was provided by Roche.
This study was conducted with the support of the European Myeloma Network.
Authorship
Contribution: M.G. analyzed and collected data, performed statistical analysis, and wrote the manuscript; R.G.-S. analyzed and collected data and critically reviewed the manuscript; E.K. analyzed and collected data, performed statistical analysis, and critically reviewed the manuscript; P.M. analyzed and collected data, and critically reviewed the manuscript; M.-C.K., E.M., Z.K., X.L., G.P., A.T., D.G., and G.M. analyzed and collected data and critically reviewed the manuscript; P.S. designed the study, analyzed and collected data, and critically reviewed the manuscript; and M.A.D. designed the study, analyzed and collected data, and wrote the manuscript.
Conflict-of-interest disclosure: E.K. received honoraria from Janssen, Celgene, Genesis Pharma, and Takeda. R.G.-S. received research support from Novartis SA and honoraria from Millennium-Takeda, Janssen-Cilag, and Pharmacyclics. G.P. received honoraria from Millennium-Takeda and Pfizer, and research support from Pfizer. G.M. received honoraria from Janssen and Prothena. P.S. received honoraria from Janssen and Celgene, and research support from Janssen, Celgene, Millennium-Takeda, and Amgen. M.A.D. received honoraria from Janssen, Celgene, Genesis-Pharma, Takeda, Millennium, Roche, Novartis, and Amgen, and research support from Janssen, Celgene, Millennium-Takeda, and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Maria Gavriatopoulou, Department of Clinical Therapeutics, National and Kapodistrian University of Athens School of Medicine, 80 Vas Sofias Ave, 115 28 Athens, Greece; e-mail: mariagabria@gmail.com.