To the editor:
The liver expression of the key iron regulator hepcidin is controlled by the bone morphogenetic protein (BMP)/SMAD pathway. BMP signaling requires the ligand (BMP6 or BMP2),1,2 type I (activin receptor-like kinase 2 [ALK2] and ALK3) and type II receptors (ACVR2 and BMPR2), and coreceptor hemojuvelin (HJV) to phosphorylate SMAD proteins.3 The transmembrane serine protease matriptase-2 (MT2) encoded by TMPRSS64 inhibits hepcidin by cleaving HJV from the hepatocyte surface.5 Loss-of-function TMPRSS6 mutations cause microcytic iron deficiency anemia (IRIDA), characterized by excessive hepcidin levels and refractoriness to iron treatment.6 Patients with IRIDA are homozygous or compound heterozygous for TMPRSS6 mutations; only a minority, usually adults, are reported to have a single mutation.6
Mutations in activin receptor 1A (ACVR1A), which encodes the BMP type I receptor ALK2, cause fibrodysplasia ossificans progressiva (FOP), a rare dominant disorder leading to progressive ectopic bone formation in soft tissues with consequent severe impairment of body movements because of extraskeletal bone bridges that entrap the entire skeleton.7 ACVR1 mutations responsible for FOP cause dysregulation of SMAD-dependent downstream signaling and confer to the mutated receptor the ability to respond to a noncanonical ligand, activin A,8-10 triggering ectopic bone formation in tissue-resident, multipotent mesenchymal cells.11,12
We previously reported the case of a girl (patient B II-1 in Table 2 of the De Falco et al13 report) who had all the features of IRIDA, including microcytic iron-deficient anemia and high hepcidin levels in the presence of compound heterozygosity for MT2 I212T and R271Q mutations.13 This was an atypical TMPRSS6 genotype, because functional studies showed that although I212T was a causal mutation with impaired hepcidin inhibition, R271Q was silent and behaved as the wild type control (Figure 4 in De Falco et al13 report), a finding later confirmed by other studies.14 Mild microcytic anemia unresponsive to iron treatment persisted at follow-up (Figure 1A), as a result of serum hepcidin levels inappropriately high for iron deficiency. At 5 years of age, because of the occurrence of submandibular swelling, a lesion in the right suprascapular region, and bilateral malformation of the big toes, FOP was suspected (see supplemental Data, available on the Blood Web site). The patient was referred to a molecular genetics laboratory in Genoa to search for ACVR1 gene mutations. Sequencing identified a c.774G>C heterozygous mutation leading to R258S substitution in the kinase domain of the ALK2 protein, a mutation previously described in an unrelated Italian patient.15 On the basis of family studies, R258S was determined to be a de novo mutation.
ALK2 mutants increase hepcidin expression in vitro. (A) Hematologic parameters of the proband at 5 and 10 years of age. §Hematologic data at age 5 years are from De Falco et al.13 Hb, hemoglobin; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; PTL, platelet; RBC, red blood cell; Transf Sat, transferrin saturation; WBC, white blood cell. (B) Plasma membrane localization of wild-type ALK2 (ALK2wt), ALK2R206H, and ALK2R258S was studied in HeLa cells labeled with a membrane-impermeable biotin. Cell-surface proteins were pulled down by using streptavidin beads and loaded onto a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. ALK2 was detected by using the anti-FLAG antibody. Plasma membrane protein pull down and loading were normalized using anti–pan cadherin antibody. TCL, total cell lysates. (C-D) Hep3B cells were transfected with the hepcidin promoter luciferase (Hamp-Luc) vector, thymidine kinase promoter–Renilla luciferase reporter plasmid for normalization, ALK2wt, or empty vector and incubated with BMP6 (1 ng/mL) for 18 hours (C) or cotransfected with HJV (D). Forty-two hours after transfection, cells were lysed, and luciferase activity was measured. (E) Hep3B cells were transfected with HJV as described in panel D in the presence of ALK2wt or ALK2R206H or ALK2R258S mutant. All the experiments were performed 3 times in triplicate. A representative experiment is shown for each panel. **P < .01; ***P < .001; ****P < .0001. RQ, relative quantification.
ALK2 mutants increase hepcidin expression in vitro. (A) Hematologic parameters of the proband at 5 and 10 years of age. §Hematologic data at age 5 years are from De Falco et al.13 Hb, hemoglobin; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; PTL, platelet; RBC, red blood cell; Transf Sat, transferrin saturation; WBC, white blood cell. (B) Plasma membrane localization of wild-type ALK2 (ALK2wt), ALK2R206H, and ALK2R258S was studied in HeLa cells labeled with a membrane-impermeable biotin. Cell-surface proteins were pulled down by using streptavidin beads and loaded onto a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. ALK2 was detected by using the anti-FLAG antibody. Plasma membrane protein pull down and loading were normalized using anti–pan cadherin antibody. TCL, total cell lysates. (C-D) Hep3B cells were transfected with the hepcidin promoter luciferase (Hamp-Luc) vector, thymidine kinase promoter–Renilla luciferase reporter plasmid for normalization, ALK2wt, or empty vector and incubated with BMP6 (1 ng/mL) for 18 hours (C) or cotransfected with HJV (D). Forty-two hours after transfection, cells were lysed, and luciferase activity was measured. (E) Hep3B cells were transfected with HJV as described in panel D in the presence of ALK2wt or ALK2R206H or ALK2R258S mutant. All the experiments were performed 3 times in triplicate. A representative experiment is shown for each panel. **P < .01; ***P < .001; ****P < .0001. RQ, relative quantification.
The BMP receptor ALK2 is involved in the regulation of hepcidin in response to iron increase.16 We investigated the effect of two FOP mutants, ALK2R258S and the more common ALK2R206H,7,15,17 identified in patients receiving hepcidin in Hep3B cells transfected with the Hamp-Luc vector (expressing the luciferase complementary DNA under the control of the hepcidin promoter).18 As shown in the supplemental Data, first we confirmed that both ALK2 mutants are exported to the cell surface with the same efficiency of ALK2wt (Figure 1B). ALK2wt activates hepcidin only in the presence of the ligand BMP6 (Figure 1C), whereas both ALK2 mutants strongly upregulate hepcidin even in the absence of BMP6 (Figure 1E), suggesting that they are constitutively active in basal conditions.
The BMP-coreceptor HJV is essential for hepcidin activation,19 and accordingly, its overexpression in hepatoma cells upregulates hepcidin (Figure 1D). ALK2wt is not required for HJV response, as shown by comparable levels of hepcidin expression in cells transfected or not with ALK2wt (Figure 1D). However, in cells transfected with ALK2 mutants (Figure 1E), HJV further increases hepcidin expression, suggesting that the coreceptor levels drive the entity of hepcidin transcriptional response induced by ALK2 mutants.
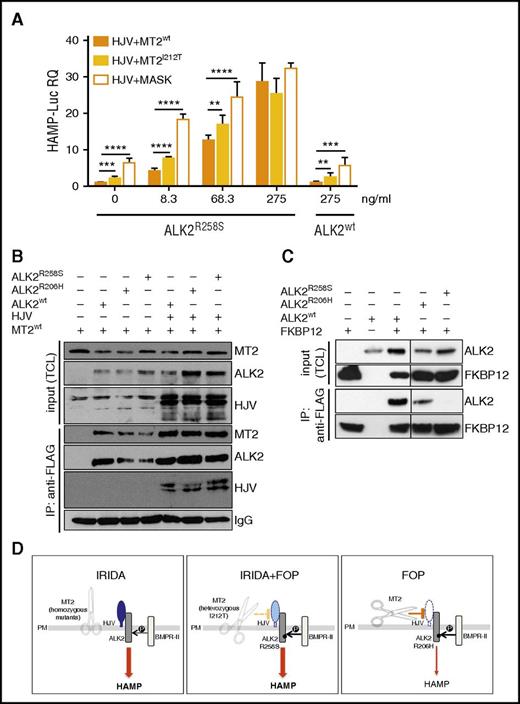
To demonstrate the role of ALK2R258S in IRIDA development in the index patient case, we reconstructed in vitro the proband genotype, expressing mutants TMPRSS6 and ACVR1 in the presence of HJV and assessing hepcidin activation. To this aim, Hep3B cells were transfected with the Hamp-Luc vector, HJV and MT2wt or MT2I212T and with increasing concentration of ALK2wt or ALK2R258S. ALK2wt does not change hepcidin expression, which remains similar to that of untreated cells even at the highest ALK2wt concentration. Conversely, ALK2R258S strongly upregulates hepcidin expression in a dose-dependent manner, even at low concentrations (Figure 2A). In cells expressing ALK2R258S and the partially inactive MT2I212T protease, hepcidin is significantly higher than in the presence of MT2wt, although lower than in the presence of a fully inactive (MASK) protease variant. These results suggest that ALK2R258S maintains high hepcidin expression in the presence of MT2I212T, supporting our interpretation of a novel IRIDA molecular pathogenesis.
Molecular pathogenesis of the novel form of IRIDA. (A) Hep3B cells were transfected with Hamp-Luc and thymidine kinase promoter–Renilla luciferase reporter plasmid, with fixed concentrations of HJV and MT2wt or MT2I212V and with increasing concentrations (from 8.3-275 ng/mL) of ALK2R258S. As a negative control of MT2 function, we used the inactive protease MT2MASK, the human homolog of the mouse variant that lacks the protease domain4 and does not inhibit hepcidin.5 Cells transfected with the highest (275 ng/mL) concentration of ALK2wt were used as control. Forty-two hours after transfection, cells were lysed, and luciferase activity was measured. The experiments were performed three times in triplicate. A representative experiment is shown. **P < .01; ***P < .001; ****P < .0001. (B) HeLa cells were transfected with MT2HA in the presence or absence of HJV. When indicated, cells were also transfected with ALK2wt-FLAG, ALK2R206H-FLAG, ALK2R258S-FLAG, or empty vector. Forty-two hours after transfection, cells were lysed, and ALK2 was pulled down by using the anti-FLAG M2 affinity gel. Total cell lysates (TCLs) and pull-down proteins were loaded onto a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and processed for Western blot analysis. HJV, MT2, and ALK2 were detected by using the anti-HJV,20 anti-HA, and anti-FLAG antibodies, respectively. Immunoglobulin G (IgG) was used to normalize protein pull down. Arrows indicate proteins recognized by anti-FLAG antibody. IP, immunoprecipitation. (C) HeLa cells were cotransfected with FKBP12MYC-FLAG and ALK2wt-MYC, ALK2R206H-MYC, ALK2R258S-MYC, or empty vector. Forty-two hours after transfection, cells were lysed, and FKBP12 was pulled down by using the anti-FLAG M2 affinity gel. TCLs and pull-down proteins were loaded onto a 12% SDS-PAGE and processed for Western blot analysis. FKBP12 and ALK2 were detected by using the anti-FLAG and anti-MYC antibodies, respectively. Arrows indicate proteins recognized by anti-FLAG antibody. (D) Schematic representation of the TMPRSS6 (MT2) effect on HJV and BMP receptors in the liver. In IRIDA, the inactivation of TMPRSS6 (the scissor) renders the BMP pathway hyperactive because HJV (in blue) is not cleaved from the membrane and acts as a BMP coreceptor; thus, both the signaling pathway and hepcidin expression (thick arrow) are high. In FOP/IRIDA, TMPRSS6 protease is only partially active (dotted yellow line), and some HJV (light blue) remains on cell surface, and together with the constitutively active ALK2, this upregulates hepcidin (thick arrow). In patients with FOP, ALK2R206H is constitutionally active, but the cleavage of HJV (white) by the normal protease (solid orange line) allows hepcidin regulation (thin arrow). HAMP, hepcidin; P, phosphorylation; PM, plasma membrane.
Molecular pathogenesis of the novel form of IRIDA. (A) Hep3B cells were transfected with Hamp-Luc and thymidine kinase promoter–Renilla luciferase reporter plasmid, with fixed concentrations of HJV and MT2wt or MT2I212V and with increasing concentrations (from 8.3-275 ng/mL) of ALK2R258S. As a negative control of MT2 function, we used the inactive protease MT2MASK, the human homolog of the mouse variant that lacks the protease domain4 and does not inhibit hepcidin.5 Cells transfected with the highest (275 ng/mL) concentration of ALK2wt were used as control. Forty-two hours after transfection, cells were lysed, and luciferase activity was measured. The experiments were performed three times in triplicate. A representative experiment is shown. **P < .01; ***P < .001; ****P < .0001. (B) HeLa cells were transfected with MT2HA in the presence or absence of HJV. When indicated, cells were also transfected with ALK2wt-FLAG, ALK2R206H-FLAG, ALK2R258S-FLAG, or empty vector. Forty-two hours after transfection, cells were lysed, and ALK2 was pulled down by using the anti-FLAG M2 affinity gel. Total cell lysates (TCLs) and pull-down proteins were loaded onto a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and processed for Western blot analysis. HJV, MT2, and ALK2 were detected by using the anti-HJV,20 anti-HA, and anti-FLAG antibodies, respectively. Immunoglobulin G (IgG) was used to normalize protein pull down. Arrows indicate proteins recognized by anti-FLAG antibody. IP, immunoprecipitation. (C) HeLa cells were cotransfected with FKBP12MYC-FLAG and ALK2wt-MYC, ALK2R206H-MYC, ALK2R258S-MYC, or empty vector. Forty-two hours after transfection, cells were lysed, and FKBP12 was pulled down by using the anti-FLAG M2 affinity gel. TCLs and pull-down proteins were loaded onto a 12% SDS-PAGE and processed for Western blot analysis. FKBP12 and ALK2 were detected by using the anti-FLAG and anti-MYC antibodies, respectively. Arrows indicate proteins recognized by anti-FLAG antibody. (D) Schematic representation of the TMPRSS6 (MT2) effect on HJV and BMP receptors in the liver. In IRIDA, the inactivation of TMPRSS6 (the scissor) renders the BMP pathway hyperactive because HJV (in blue) is not cleaved from the membrane and acts as a BMP coreceptor; thus, both the signaling pathway and hepcidin expression (thick arrow) are high. In FOP/IRIDA, TMPRSS6 protease is only partially active (dotted yellow line), and some HJV (light blue) remains on cell surface, and together with the constitutively active ALK2, this upregulates hepcidin (thick arrow). In patients with FOP, ALK2R206H is constitutionally active, but the cleavage of HJV (white) by the normal protease (solid orange line) allows hepcidin regulation (thin arrow). HAMP, hepcidin; P, phosphorylation; PM, plasma membrane.
MT2 interacts with its substrate, HJV.5 We next investigated whether ALK2 participates in the same functional complex using HeLa cells transiently transfected with HJV, MT2HA, and FLAG-tagged ALK2wt or mutant ALK2. HJV and MT2 are both coimmunoprecipitated with ALK2wt and mutant ALK2 (Figure 2B). Interestingly, although MT2 does not cleave the receptor, it interacts with ALK2 even in the absence of HJV (Figure 2B).
The immunophilin FKBP12 was reported to bind BMP type I receptors to block leaky signaling, and decreased binding to FKBP12 was reported for FOP mutant ALK2R206H in C2C12 cells.21 On the basis of the crystallographic structure residue, R206 is in the ALK2 glycine-serine–rich domain that interacts with FKBP12.21 R258 lies in the kinase domain, and mutation at this level might indirectly destabilize the FKBP12 binding. Both ALK2 mutants constitutively active in FOP show a decreased binding to FKBP12 in HeLa (Figure 2C) and hepatoma cells (data not shown), almost undetectable for R258S. In agreement with the ability of the 2 ALK2 mutants to activate hepcidin, drugs that bind and sequester FKBP12 activate hepcidin in hepatic cells, providing evidence for a new level of control of hepcidin expression (S.C., A.P., Mariateresa Pettinato, Irene Artuso, Antonella Nai, C.C., and L.S., manuscript submitted 20 April 2017).
The deregulated signaling of ALK2R258S provides a novel clue to explain the high hepcidin levels and iron deficiency in the proband. However, as shown by a series (13 patients) of patients with FOP, all of whom were carriers of ALK2R206H, this mutation does not cause microcytic anemia or iron alterations (data not shown). We concluded that IRIDA in the index patient case resulted from digenic inheritance of TMPRSS6 and ACVR1 mutations, as illustrated in Figure 2D.
According to our results, HJV increases hepcidin expression in cells transfected with mutant ALK2. Thus, although the BMP pathway is inhibited when normal MT2 cleaves HJV, in the presence of both ALK2wt and mutant ALK2, the heterozygosity for the inactive MT2I212T may leave enough membrane HJV to allow persistent hepcidin activation in the proband.
We expect that this new form of IRIDA is quite rare, because mutations in both TMPRSS6 and ACVR1 are rare. However, besides having diagnostic implications for IRIDA, our study may provide novel insights into hepcidin activation in vivo, reveal an unsuspected link between activation of bone and liver BMP type I receptors, and further emphasize the importance of MT2 in controlling hepatic BMP/SMAD signaling.
This digenic model suggests that isolated heterozygous mutations of TMPRSS6 are unlikely to cause IRIDA. Finally, our data provide a new perspective on a previously unsuspected role for FKBP12 as a modulator of ALK2 in the liver and potentially as a novel therapeutic target for hepcidin manipulation.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors gratefully acknowledge FOP Italia and the patients and their families for supporting this study.
This work was supported in part by Telethon Grant GGP15064 (L.S.).
Contribution: S.C. performed research; C.D. contributed family description and data discussion; R.B., M.B., and R.R. contributed patient data and paper writing; A.P. and L.S. outlined the experimental design, performed research, and drafted the paper; and C.C. contributed to discussion of results and writing the paper.
Conflict-of-interest disclosure: C.C. is a member of the advisory board of Vifor Pharma. The remaining authors declare no competing financial interests.
S.C. is currently a PhD student at Heidelberg University Hospital, Heidelberg, Germany.
Correspondence: Clara Camaschella, Vita Salute University & San Raffaele Scientific Institute, Via Olgettina, 58- 20132 Milan, Italy; e-mail: camaschella.clara@hsr.it.
References
Author notes
L.S. and C.C. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal