The continuous turnover of erythrocyte iron requires intercommunication between multiple cell types for homeostasis, including cells participating in iron uptake (enterocytes), utilization (erythroid precursors), recycling (reticuloendothelial macrophages), and storage (hepatocytes). Coordination of iron flux between these cell types is determined by the regulated expression of the hepatocellular hormone hepcidin. In this issue of Blood, 2 research teams, Canali et al and Koch et al, independently demonstrate a key role in iron homeostasis by a cell type that might otherwise seem a bystander. Their studies provide convincing evidence that the source of bone morphogenetic proteins (BMPs) essential to basal and iron-regulated hepcidin expression is liver sinusoidal endothelial cells (LSECs).1,2
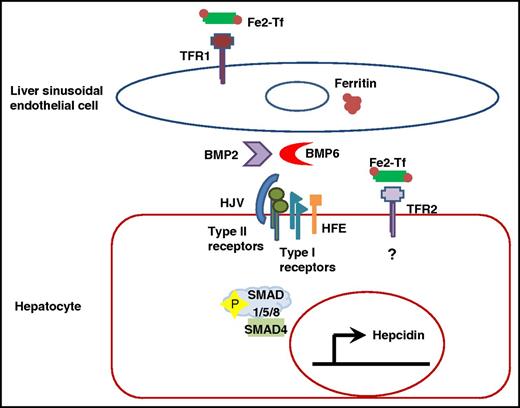
LSECs produce BMP2 and BMP6, possibly related to iron-loaded transferrin and ferritin. The BMPs regulate hepatocellular hepcidin expression in a paracrine fashion via BMP type II and type I receptors. Which specific receptor subtypes participate might be influenced by the BMP subtype; likewise, the ability of HJV to serve as a coreceptor might also depend on the BMP subtype. In both instances, type I receptor activation results in the recruitment and phosphorylation of SMAD1/5/8. Phosphorylated SMAD1/5/8 then forms a complex with the common SMAD, SMAD4, which translocates to the nucleus, where it binds the hepcidin promoter to induce expression. The roles of TFR2 and HFE are not completely defined, but may respond to iron-loaded transferrin to influence signaling through the SMAD (and possibly MAPK) pathways. Fe2-Tf, diferric transferrin.
LSECs produce BMP2 and BMP6, possibly related to iron-loaded transferrin and ferritin. The BMPs regulate hepatocellular hepcidin expression in a paracrine fashion via BMP type II and type I receptors. Which specific receptor subtypes participate might be influenced by the BMP subtype; likewise, the ability of HJV to serve as a coreceptor might also depend on the BMP subtype. In both instances, type I receptor activation results in the recruitment and phosphorylation of SMAD1/5/8. Phosphorylated SMAD1/5/8 then forms a complex with the common SMAD, SMAD4, which translocates to the nucleus, where it binds the hepcidin promoter to induce expression. The roles of TFR2 and HFE are not completely defined, but may respond to iron-loaded transferrin to influence signaling through the SMAD (and possibly MAPK) pathways. Fe2-Tf, diferric transferrin.
A role for members of the transforming growth factor β (TGF-β) superfamily in the regulation of hepcidin has been long recognized. Among these, murine knockout studies and, more recently, genetic analysis of patients with iron overload have clearly identified a role for BMP6.3-5 Because liver expression of hepcidin and of Bmp6 in murine systems each correlate with hepatocellular iron concentration,6 initial model systems proposed an autocrine role for BMP6 as the iron “stores” signal regulating hepcidin.5 Ex vivo hepatocellular culture systems moreover supported such a model. However, the observation that nonparenchymal cells in the liver are the predominant sites of basal and iron-regulated BMP6 expression raised the possibility that other, perhaps multiple, liver cell types were relevant sources.7,8 Canali et al demonstrate the limitations in interpreting observations from primary hepatocyte cell culture systems and definitively dissect among the candidate cell types utilizing Cre-driven cellular-specific murine knockout systems. They clearly identify LSECs as the essential source of Bmp6 for basal and iron-regulated liver hepcidin expression (see figure).
Although BMP6 clearly plays a central role in hepcidin regulation, studies utilizing crosses between Bmp6 knockout and other genetic murine models of hemochromatosis supported the possibility that additional member(s) of the TGF-β superfamily might also contribute.9 BMP2 has been suggested as a candidate, based on the observed upregulation of hepcidin by BMP2 in cell culture systems10 and evidence that BMP2 might serve as a genetic modulator of HFE-associated hemochromatosis.11 Utilizing another sinusoidal endothelial cell (SEC) promoter-driven Cre system, Koch et al report that ablating Bmp2 in these cells likewise results in suppressed liver hepcidin expression and hemochromatosis. It should be noted, however, that ablation of Bmp2 in SECs results in much less severe hepatic iron loading than that observed by Canali et al upon ablation of Bmp6. Likewise, extrahepatic iron loading was less severe. Whether ablation of SEC Bmp2 abrogates the regulation of liver hepcidin by iron was undetermined. Of note, liver Bmp6 was found to be upregulated in the mice lacking SEC Bmp2, raising the possibility that Bmp6 partially compensates for the loss of Bmp2. Taken together, these studies suggest BMP6 as the superior molecule to target pharmacologically, in agreement with prior in vivo studies of soluble inhibitors of these 2 BMPs.3
One of the major remaining challenges is determining how each of these BMPs participates with other molecules involved in the iron-mediated regulation of hepcidin. Conceptually it has been convenient to divide this regulation into an “iron stores” component, which appears to be directly regulated by BMPs and augmented by the BMP coreceptor hemojuvelin (HJV),3,5 and a “circulating iron” component involving transferrin receptor 2 (TFR2), possibly as a supercomplex with HFE, HJV, and BMP receptors.5,9,10 One proposed model suggests that BMP6 signals as the “iron stores” regulator, while BMP2 contributes to regulation by the “circulating iron” complex.12 BMPs bind to type II receptors (ACTR2a or BMPR2 in liver), which phosphorylate type I receptors (ALK2, ALK3), resulting in activation and nuclear translocation of the SMAD1/5/8 complex and induction of the hepcidin promoter.5 Both ALK2 and ALK3 can mediate BMP2 signaling in ex vivo systems.13 HFE has been reported to directly interact with and stabilize ALK3 but not ALK2.14 However, the modest liver iron load and pattern of hepatic iron distribution found in the SEC Bmp2 knockout mice are not consistent with loss of Alk3, suggesting that BMP2 is not independently regulating a pathway modulated by HFE. The mild phenotype of the BMP2 knockout also raises the possibility that rather than serving as an independent regulator of hepcidin expression, BMP2 instead modulates BMP6 signaling. Indeed, heterodimers of the TGF-β superfamily have been described with signaling capabilities that differ from homodimers.5
A second remaining challenge is identifying and characterizing the iron signal that induces liver BMP expression. It remains to be determined if BMP expression in LSECs directly reflects their iron status, iron flux across these cells, or a paracrine signal from yet another cell type. LSECs express transferrin receptor 1 and are well positioned to transduce a circulatory signal. In vivo studies in mice, however, suggest that ferri-transferrin can regulate hepcidin independently of changes in hepatic expression of Bmp6.6 The uptake or production of ferritin by LSECs is another potential means of signaling iron status.5 LSEC-specific ablation of molecules known to participate in cellular iron flux may be informative. The identification of the SEC as the relevant source of BMPs regulating hepcidin is a significant step forward in characterizing the regulation of systemic iron homeostasis. Although the precise nature of iron-mediated signaling to hepcidin in the liver remains elusive, investigators at least know in which cell type to look.
Conflict-of-interest disclosure: R.E.F. has served on the scientific advisory board for Protagonist, a biotechnology company developing products to manipulate the hepcidin-ferroportin axis. These products include hepcidin agonists, but not products to manipulate BMP action. N.L.P. declares no competing financial interests.