Key Points
Complex karyotype and fludarabine refractoriness are key risk factors for progression of CLL on venetoclax.
Bruton tyrosine kinase inhibitors are active in patients with CLL after prior therapy with venetoclax.
Abstract
The BCL2 inhibitor venetoclax achieves responses in ∼79% of patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (RR-CLL/SLL), irrespective of risk factors associated with poor response to chemoimmunotherapy. A limitation of this targeted therapy is progressive disease (PD) in some patients. To define the risk factors for progression, the clinicopathological features of PD, and the outcomes for patients after venetoclax failure, we analyzed 67 heavily pretreated patients on 3 early phase clinical trials. Investigations at progression included positron emission tomography scan and biopsy. Twenty-five (37%) patients manifested PD on therapy: 17 with Richter transformation (RT) and 8 with progressive CLL/SLL. RT occurred significantly earlier (median 7.9 months) than progressive CLL (median 23.4 months) (P = .003). Among patients who received the recommended phase 2 dose of venetoclax or higher (≥400 mg/d), fludarabine refractoriness and complex karyotype were associated with progression (hazard ratio 7.01 [95% confidence interval 1.7-28.5]; P = .002 and 6.6 [1.5-29.8]; P = .005, respectively), whereas del(17p) and/or TP53 mutation were not (P = .75). Median postprogression survival was 13 (<1-49.9) months. Bruton tyrosine kinase inhibitors were active in progressive CLL, but outcomes were mixed. Patients with disease that is fludarabine refractory or who have complex cytogenetics should have occult RT excluded before initiating venetoclax therapy.
Introduction
Venetoclax (ABT-199/GDC-0199; AbbVie, Chicago, IL) is an orally bioavailable small molecule inhibitor of BCL2 that has shown promise in the management of relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (RR-CLL/SLL).1,2 Constitutive high-level expression of BCL2 is a hallmark of CLL and SLL, assisting the malignant cells to resist apoptosis and resulting in accumulation of CD5+CD19+ B lymphocytes.3,4 Venetoclax selectively targets BCL25 and has greater efficacy and tolerability than its predecessor, navitoclax, which was associated with dose-limiting thrombocytopenia induced by concomitant BCLxL inhibition.6,7 In a phase 1 trial, venetoclax achieved an objective overall response of 79% among patients with poor prognosis RR-CLL/SLL, including fludarabine-refractory (F-refractory) deletions affecting the short arm of chromosome 17 (del(17p)) and unmutated immunoglobulin variable heavy chain gene (IGHV) disease.1 Across a range of doses, 20% of patients achieved complete remissions (CRs) with ongoing therapy, including 5% that tested negative for minimal residual disease (MRD) in the bone marrow by flow cytometry. The US Food and Drug Administration approved venetoclax in April 2016 based on an objective overall response of 79% by the Independent Review Committee for patients with del(17p) CLL in the phase 2 trial.8
However, despite these deep responses, progression during ongoing therapy occurs in some patients. In the phase 1 trial, 27% of patients progressed within 15 months.1 As previously observed with ibrutinib9 and combination chemoimmunotherapy,10 some progressions present as Richter transformation (RT).1,8 Understanding the nature of disease progression on venetoclax has important implications for its clinical application. We provide the first report of the clinical features, risk factors, and outcomes of patients with heavily pretreated RR-CLL/SLL who manifest disease progression on venetoclax.
Methods
Study design
We retrospectively reviewed data from the first 67 consecutive patients with RR-CLL/SLL treated with venetoclax at 2 institutions (Royal Melbourne Hospital [RMH] and Peter MacCallum Cancer Centre [PMCC]) between June 2011 and March 2016. Patients were enrolled on 1 of 3 ongoing trials: M12-175 phase 1 monotherapy (NCT01328626; 42 patients), M13-365 phase 1b venetoclax plus rituximab (NCT01682616; 16 patients), or M13-982 phase 2 venetoclax monotherapy (NCT01889186; 9 patients) (supplemental Table 1, available on the Blood Web site). Patients received 150 to 1200 mg/d of venetoclax ± 6 doses of monthly rituximab. Eligibility criteria for each of these trials have been published1,2,8 ; patients were required to have progressive RR-CLL/SLL with an indication for therapy.11 There was no specific protocol-mandated screening for RT with positron emission tomography (PET) scanning at trial entry. Patients with RT detected at screening or with a past history of RT were not eligible.
Progression on venetoclax was defined as per International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines.11 Treating clinicians pursued a pathological diagnosis in all cases. Patients who had discordant responses, B symptoms, elevated serum lactate dehydrogenase, or PET findings suggestive of transformed disease typically had an excisional or core lymph node biopsy. Only histologically proven transformation is included as RT in this analysis.
Study patients
Baseline characteristics were recorded at enrollment, including age, the number of prior lines of therapy, whether refractory to fludarabine-containing regimens (F-refractory, defined as failure to respond to, or progression within 6 months of, fludarabine-based therapy12 ), the presence of bulky lymphadenopathy (defined as lymph nodes >5 cm either by clinical examination or by computed tomography [CT] scanning13 ), and genetic features: del(17p), del(11q), complex karyotype (defined as ≥3 clonal chromosomal aberrations on conventional metaphase karyotype14,15 ), TP53 mutation, and unmutated IGHV.
For the purposes of analysis, patients were subdivided by the cohort dose of venetoclax they received for the majority of their time on study: either ≥400 mg per day (400 mg being the recommended phase 2 dose1 ) or <400 mg per day (patients in the dose escalation phase of phase 1 studies), and as to whether they received monotherapy or venetoclax in combination with rituximab (patients in the M13-365 trial). Patients were also categorized by their best response to study drug as judged by the clinical investigators according to iwCLL 2008 criteria,11 including CT scanning.
The clinical characteristics of patients at time of disease progression on venetoclax were collected, including the presence of B symptoms, lymphadenopathy, PET findings, bone marrow biopsy results, and immunohistochemistry (IHC) results. Postprogression data, including details of subsequent treatment, response, and postprogression survival (PPS), were recorded.
All patients included in the analysis provided written informed consent, and the studies were performed in line with the Declaration of Helsinki and with the approval and monitoring of the Human Research Ethics Committees/Institutional Review Boards of the participating institutions: RMH (2011.044; 2012.092; and 2013.016) and PMCC (11/18; 12/45; and 13/163).
Pathological diagnosis
All pathology was reviewed by independent pathologists and reported according to standard World Health Organization 2008 Classification. Immunohistochemical staining was performed in each laboratory according to institutional guidelines and reported by an independent pathologist. Samples were considered positive if >20% of the tumor cells were positive for the specific antigen. Cytogenetics and fluorescence in situ hybridization (FISH) were performed by the Victorian Cancer Cytogenetic Service using established methods.16 TP53 mutation and IGHV testing were performed as previously described.17
Statistical analysis
Time to progression (TTP) with either CLL or RT was measured from the date of the first dose of drug to the date of progression on venetoclax and estimated by the method of Kaplan and Meier. Patients who discontinued therapy for reasons unrelated to CLL/SLL progression were censored at time of treatment cessation, or study exit, whichever was later. The cutoff date for postprogression follow-up was October 1, 2016. PPS was measured from date of documented progression to date of death from any cause. Survivors were censored at the date of their last follow-up.
Associations between clinical, pathological, and response variables and TTP were tested using the log-rank test, with an α level set at 0.05. Where a variable had >2 groups, post hoc pairwise comparisons were performed if the overall log-rank test indicated significant differences. Significant variables on univariate survival analysis were interrogated for interactions using Classification and Regression Tree (CART) analysis. All data were analyzed using Stata 14.1 for Mac (StataCorp, College Station, TX) and GraphPad Prism version 6.0h for Mac (GraphPad Software, La Jolla, CA).
Results
Patient characteristics
The median age of the patients studied was 68 (20-87) years, and the majority was heavily pretreated with chemoimmunotherapy (Table 1). At study entry, 94% of patients had 1 or more risk factors associated with unfavorable outcomes with chemoimmunotherapy (adverse FISH, unmutated IGHV, F-refractory, bulky adenopathy). Ninety-seven percent had received alkylator-based regimens, 96% anti-CD20 antibodies (rituximab, ofatumumab, or obinutuzumab), and 91% fludarabine-based regimens (81% fludarabine, cyclophosphamide, and rituximab [FCR]). Forty-nine percent of patients had F-refractory disease. All patients were Bruton tyrosine kinase inhibitor (BTKI) naive as reflective of practice in Australia at the time of enrollment in the trials. FISH analysis was available for all, and 40% had del(17p) CLL/SLL. In 58 patients, informative data were also available for TP53 mutation analysis; 34/58 (59%) had at least 1 abnormality of TP53, either deletion or mutation. Cytogenetics were known for CLL in 38 patients, and 42% of these had a complex karyotype (see supplemental Table 2). Sixteen patients (24%) received concurrent rituximab for up to 6 doses and 49 (73%) received the recommended phase 2 dose of venetoclax or higher (ie, ≥400 mg daily). PET scans were not performed routinely prior to study entry. Only 3 out of the 67 patients had a PET scan performed within 3 months of study entry, and in none was there evidence of RT. The best response to venetoclax therapy was CR or CR with incomplete count recovery in 17 (25%) patients, partial response (PR) in 41 (61%), and stable disease (SD) in 9 (13%) cases, as assessed by iwCLL criteria.
Baseline clinical and genetic features of CLL/SLL in 67 venetoclax-treated patients, subgrouped by outcome
| . | All patients . | Nonprogressors . | RT (Hodgkin) . | RT (DLBCL) . | CLL progression . |
|---|---|---|---|---|---|
| Number (N) | 67 | 42 | 3 | 14 | 8 |
| Age, y, median (range) | 68 (20-87) | 68 (20-87) | 62 (54-73) | 62 (47-74) | 70 (65-78) |
| Prior therapies, median (range) | 3 (1-12) | 3 (1-12) | 2 (1-3) | 4 (2-8) | 4 (1-7) |
| F-refractory, n (n/N%) | 33 (49) | 16 (38) | 2 (67) | 8 (57) | 7 (88) |
| Bulky disease, n (n/N%) | 32 (48) | 16 (38) | 1 (33) | 10 (71) | 5 (63) |
| Venetoclax <400 mg/d, n (n/N%) | 18 (27) | 6 (14) | 1 (33) | 8 (57) | 3 (38) |
| Concurrent rituximab, n (n/N%) | 16 (24) | 12 (29) | 2 (67) | 1 (7) | 1 (13) |
| Del11q, n (n/N%) | 17 (25) | 12 (29) | 0 (0) | 3 (21) | 2 (25) |
| Del17p, n (n/N%) | 27 (40) | 17 (40) | 0 (0) | 8 (57) | 2 (25) |
| Del17p and/or TP53 mutation, n/N (%) | 34/58 (59) | 20/35 (57) | 0/1 (0) | 10/14 (71) | 4/8 (50) |
| Complex karyotype, n/N (%) | 16/38 (42) | 6/23 (26) | 1/1 (100) | 5/8 (63) | 4/6 (67) |
| IGHV unmutated, n/N (%) | 28/35 (80) | 14/18 (78) | 2/2 (100) | 8/10 (100) | 4/5 (100) |
| . | All patients . | Nonprogressors . | RT (Hodgkin) . | RT (DLBCL) . | CLL progression . |
|---|---|---|---|---|---|
| Number (N) | 67 | 42 | 3 | 14 | 8 |
| Age, y, median (range) | 68 (20-87) | 68 (20-87) | 62 (54-73) | 62 (47-74) | 70 (65-78) |
| Prior therapies, median (range) | 3 (1-12) | 3 (1-12) | 2 (1-3) | 4 (2-8) | 4 (1-7) |
| F-refractory, n (n/N%) | 33 (49) | 16 (38) | 2 (67) | 8 (57) | 7 (88) |
| Bulky disease, n (n/N%) | 32 (48) | 16 (38) | 1 (33) | 10 (71) | 5 (63) |
| Venetoclax <400 mg/d, n (n/N%) | 18 (27) | 6 (14) | 1 (33) | 8 (57) | 3 (38) |
| Concurrent rituximab, n (n/N%) | 16 (24) | 12 (29) | 2 (67) | 1 (7) | 1 (13) |
| Del11q, n (n/N%) | 17 (25) | 12 (29) | 0 (0) | 3 (21) | 2 (25) |
| Del17p, n (n/N%) | 27 (40) | 17 (40) | 0 (0) | 8 (57) | 2 (25) |
| Del17p and/or TP53 mutation, n/N (%) | 34/58 (59) | 20/35 (57) | 0/1 (0) | 10/14 (71) | 4/8 (50) |
| Complex karyotype, n/N (%) | 16/38 (42) | 6/23 (26) | 1/1 (100) | 5/8 (63) | 4/6 (67) |
| IGHV unmutated, n/N (%) | 28/35 (80) | 14/18 (78) | 2/2 (100) | 8/10 (100) | 4/5 (100) |
The nonprogressor subgroup (n = 42) includes 6 patients who came off study for reasons other than disease progression (see main text for details). N, number per group (denominator); n, number of patients with a specific variable (numerator). Where not all patients were tested for a specific variable (last 3 rows), the denominator has been adjusted for that analysis, so that N = number of patients tested for that variable.
After a median follow-up of 23 months (range 2-46), 25 (37%) patients had developed disease progression on therapy: 14 with RT to diffuse large B-cell lymphoma (DLBCL) (56%), 3 with RT to Hodgkin lymphoma (HL) (12%), and 8 with progressive CLL/SLL (32%). Forty-two patients had not progressed at the time of the data cutoff, including 6 patients who discontinued venetoclax without progression. The reasons for discontinuation without progression were death due to strangulated hernia (n = 1), withdrawal of consent (n = 1), cessation due to attainment of CR (n = 1), intercurrent malignancy (n = 2), and poor performance status (n = 1).
Clinicopathological features of RT on venetoclax
RT is a recognized manifestation of CLL evolution, particularly for heavily pretreated disease.18 The clinical and radiological features observed in patients progressing with biopsy-proven RT are summarized in Table 2. Of the 17 patients, 4 had neither symptoms nor signs and were first identified by routine imaging. The diagnosis was heralded by B symptoms in 6 and by emergent cytopenias in 4 (2 had both B symptoms and cytopenias). Other pertinent clinical features specifically prompting investigation for RT included isolated progressive (or discordant response with residual) adenopathy (n = 6), pleural effusion (n = 1), and abnormal liver function tests (n = 1). In total, 14 had progressive lymphadenopathy detected radiologically, including 6 with clinically observable progressive lymphadenopathy.
Clinical, radiological and pathological features of RT in patients receiving venetoclax at progression
| Case no. . | F-refractory . | Prognostic factors at entry . | . | . | . | Nodal PD . | . | PET features . | BM at time of RT . | IHC of RT . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complex . | Del17p . | TP53 mutated . | Best response to Ven . | TTP (mo) . | B symptoms . | Clin . | CT . | Other features . | No. avid nodes . | SUVmax . | % Large cells . | % CLL . | p53 . | BCL2 . | MYC . | Ki67 . | EBV . | ||
| RT–DLBCL | |||||||||||||||||||
| 1 | − | ND | + | + | PR | 4.1 | − | + | + | Rapid multifocal PD | ND | ND | ND | ND | + | + | ND | 95% | − |
| 2 | + | + | + | + | PR | 4.4 | + | − | − | Cytopenias; BM involved | 6 | 22 | + | Heavy | + | + | + | 80% | ND |
| 3 | − | ND | − | − | PR | 5.3 | − | + | + | Localized nodal PD | ND | ND | − | <5% | + | + | + | 95% | + |
| 4 | + | + | − | + | SD | 5.6 | − | − | + | Cytopenias; mixed nodal response | 3 | 11 | − | 20% | + | + | ND | 60% | ND |
| 5 | + | + | + | + | PR | 7.1 | − | + | + | Multiple-nodal PD | ND | ND | − | 1% | ND | + | + | 60-70% | ND |
| 6 | − | − | − | − | SD | 7.9 | + | − | − | Abnormal LFTs | 6 | 14 | − | 10-15% | + | + | − | ND | ND |
| 7 | + | ND | − | − | PR | 8.5 | − | − | + | Pleural; PET-avid BM | EN | ND | − | 20% | + | + | ND | 30% | ND |
| 8 | + | − | + | + | PR | 10.6 | − | − | + | Mixed nodal response | 11 | 25.2 | − | 5-10% | + | + | ND | ND | ND |
| 9 | + | + | + | − | PR | 15.7 | − | − | + | Single-node PD | 1 | * | − | 5-10% | ND | ND | 25% | − | |
| 10 | + | − | + | − | PR | 15.8 | − | + | ND | Tonsillar mass | 6 | 44 | − | 5% | ND | + | + | 90% | − |
| 11 | − | ND | + | − | PR | 16.9 | − | − | + | 6 | 10 | − | <5% | + | + | + | 33% | ND | |
| 12 | − | ND | − | + | PR | 17.4 | − | − | + | Cytopenias | 12 | 23 | + | 0 | − | + | ND | ND | ND |
| 13 | + | ND | + | − | PR | 22.3 | + | − | + | Cytopenias | ND | ND | + | MRD+ | − | + | ND | ND | ND |
| 14 | − | + | − | − | CR | 23 | + | − | + | Multiple-nodal PD | ND | ND | − | 30% | + | + | − | 25% | − |
| RT-HL | |||||||||||||||||||
| 15 | + | + | − | ND | SD | 1 | + | − | − | 9 | 15 | ND | ND | ND | − | ND | ND | + | |
| 16 | − | ND | − | − | SD | 5.7 | + | + | + | 3 | 16 | − | <5% | ND | − | ND | ND | + | |
| 17 | + | ND | − | ND | PR | 5.8 | − | + | + | Single-node PD | 1 | 9.2 | ND | ND | ND | − | ND | ND | + |
| Case no. . | F-refractory . | Prognostic factors at entry . | . | . | . | Nodal PD . | . | PET features . | BM at time of RT . | IHC of RT . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complex . | Del17p . | TP53 mutated . | Best response to Ven . | TTP (mo) . | B symptoms . | Clin . | CT . | Other features . | No. avid nodes . | SUVmax . | % Large cells . | % CLL . | p53 . | BCL2 . | MYC . | Ki67 . | EBV . | ||
| RT–DLBCL | |||||||||||||||||||
| 1 | − | ND | + | + | PR | 4.1 | − | + | + | Rapid multifocal PD | ND | ND | ND | ND | + | + | ND | 95% | − |
| 2 | + | + | + | + | PR | 4.4 | + | − | − | Cytopenias; BM involved | 6 | 22 | + | Heavy | + | + | + | 80% | ND |
| 3 | − | ND | − | − | PR | 5.3 | − | + | + | Localized nodal PD | ND | ND | − | <5% | + | + | + | 95% | + |
| 4 | + | + | − | + | SD | 5.6 | − | − | + | Cytopenias; mixed nodal response | 3 | 11 | − | 20% | + | + | ND | 60% | ND |
| 5 | + | + | + | + | PR | 7.1 | − | + | + | Multiple-nodal PD | ND | ND | − | 1% | ND | + | + | 60-70% | ND |
| 6 | − | − | − | − | SD | 7.9 | + | − | − | Abnormal LFTs | 6 | 14 | − | 10-15% | + | + | − | ND | ND |
| 7 | + | ND | − | − | PR | 8.5 | − | − | + | Pleural; PET-avid BM | EN | ND | − | 20% | + | + | ND | 30% | ND |
| 8 | + | − | + | + | PR | 10.6 | − | − | + | Mixed nodal response | 11 | 25.2 | − | 5-10% | + | + | ND | ND | ND |
| 9 | + | + | + | − | PR | 15.7 | − | − | + | Single-node PD | 1 | * | − | 5-10% | ND | ND | 25% | − | |
| 10 | + | − | + | − | PR | 15.8 | − | + | ND | Tonsillar mass | 6 | 44 | − | 5% | ND | + | + | 90% | − |
| 11 | − | ND | + | − | PR | 16.9 | − | − | + | 6 | 10 | − | <5% | + | + | + | 33% | ND | |
| 12 | − | ND | − | + | PR | 17.4 | − | − | + | Cytopenias | 12 | 23 | + | 0 | − | + | ND | ND | ND |
| 13 | + | ND | + | − | PR | 22.3 | + | − | + | Cytopenias | ND | ND | + | MRD+ | − | + | ND | ND | ND |
| 14 | − | + | − | − | CR | 23 | + | − | + | Multiple-nodal PD | ND | ND | − | 30% | + | + | − | 25% | − |
| RT-HL | |||||||||||||||||||
| 15 | + | + | − | ND | SD | 1 | + | − | − | 9 | 15 | ND | ND | ND | − | ND | ND | + | |
| 16 | − | ND | − | − | SD | 5.7 | + | + | + | 3 | 16 | − | <5% | ND | − | ND | ND | + | |
| 17 | + | ND | − | ND | PR | 5.8 | − | + | + | Single-node PD | 1 | 9.2 | ND | ND | ND | − | ND | ND | + |
The table summarizes the key clinical features, the PET findings, and the degree of bone marrow (BM) infiltration by CLL at the time of diagnosis of RT. Immunohistochemical analyses of the RT biopsy for each patient are also summarized.
EBV, Epstein-Barr virus; EN, extranodal disease only; MRD+, no morphological CLL infiltrate detected, positive for CLL only by 4-color flow cytometry; LFT, liver function test; NA, not applicable; ND, not done; SD, stable disease; Ven, venetoclax; +, present (see “Methods” for details); −, absent.
PET performed after core biopsy of only avid node, so SUVmax reading uncertain.
In 11 patients, the presentation of RT was considered acute by the treating physician with patients requiring expedient therapy for the transformed disease due to rapidly progressive cytopenias, B symptoms, biochemical derangement, or increasing lymphadenopathy. In the other 6 patients, the diagnosis of RT was made in otherwise well patients with a more indolent presentation that did not clinically suggest the presence of an aggressive transformation. Overall, lactate dehydrogenase was above the upper limit of normal in 13 (76%) cases. Only 1 patient had a lymphocytosis (>5.0 × 109/L) at time of RT diagnosis, and in only 3 was there evidence of transformed cells in the bone marrow biopsy. Among patients who developed RT, the best clinical response to venetoclax had been SD in 4 (24%), PR in 12 (71%), and CR in only 1 (6%) (Table 2).
In contrast, the clinical presentation of progressive CLL among the 7 of 8 patients who experienced this was more indolent. The 1 exception presented with fevers and cytopenias and was clinically suspected of having RT, but this was not proven despite investigation. Patients with progressive CLL typically presented with increasing lymphadenopathy (range 1.7-6.7 cm; n = 7) detected by CT scan.
Tissue confirmation of RT was accompanied by PET imaging in 12/17 (71%) cases. Fludeoxyglucose-avid sites were unifocal at RT diagnosis in 2 cases and multifocal in 10, including 1 patient who only had extranodal involvement (Table 2). The median number of highly PET-avid nodes per patient was 6 (range 1-12), and the median maximum standardized uptake value (SUVmax) was 15.5 (range 9.2-44).
In all patients with DLBCL RT, BCL2 expression by the transformed large cells was evident by IHC (Table 2). In contrast, BCL2 was not detectable in the Reed-Sternberg cells by IHC in any of the 3 cases of HL RT. Ki-67 expression by large cells ranged between 25% and 95% in the 10 patient biopsies evaluated. In 5 out of 7 patient biopsies tested, IHC for c-MYC was positive. IHC for P53 was positive in 9 of 11 patients tested (all known TP53 mutation positive) and Epstein-Barr virus chromogenic in situ hybridization was positive in 4 of 9 patients tested, including all 3 patients with HL RT.
Cytogenetic clonal evolution at progression was demonstrated in 2 cases: new del(17p) in 1 (progressive CLL) and new del(17p) plus complex karyotype in 1 patient previously known to be negative for del(17p) by FISH but without a prestudy karyotype performed (progression with RT). In another case of RT, complex karyotype was also demonstrated in a patient who had not previously had cytogenetic analysis performed. Therefore, 12 of the 25 patients (48%) with progression on venetoclax had complex karyotype CLL at trial entry or at progression, including 8 of the 17 with RT (47%). The only recurrent breakpoints seen at trial entry in the CLL cells among patients who developed RT were 3q29 and 9q13, both of which were noted in 2 cases. Tetraploidy was present in 1 case with RT DLBCL at the time of transformation.
Bone marrow biopsies were performed in 14/17 of the patients at the time of RT. Twelve (86%) had concomitant CLL infiltration, ranging from 1% to 80% by light microscopy, including IHC, and 1 other had only MRD-detectable CLL by flow cytometry. However, in the majority (12/14), there was evidence of ongoing response in the bone marrow (supplemental Figure 1), suggesting disparate behavior between the emergent RT and the underlying still-responsive CLL. Consistent with this, non-fludeoxyglucose-avid nodal disease showed ongoing response to venetoclax as the biopsy-proven RT node progressed when this was analyzable in a subset of patients with complete biopsy, PET, and serial CT data (supplemental Figure 2).
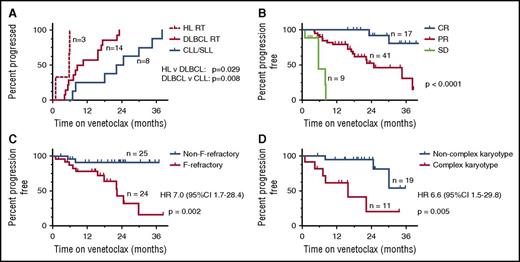
TTP
The tempo of progression varied according to the pathology at progression (with CLL, HL RT, or DLBCL RT) (Figure 1A). TTP with RT was significantly shorter than for progression with CLL/SLL (median 7.9 [1.0-23.0] months vs 23.4 [6.9-34.5] months, respectively; [P = .003]). Median time from trial entry to diagnosis of DLBCL RT was 9.5 (4.4-23.0) months and 5.7 (1.0-5.8) months to HL RT (P = .029; Figure 1A). Lymphadenopathy that was ultimately proved to harbor RT showed primary progression in some patients, while in others, initial reductions during venetoclax therapy were followed by progression (supplemental Figure 2). These short timeframes for presentation with RT suggest that some patients entered the trials with preexisting RT, especially as patients were not screened specifically for this prior to enrollment.
TTP. (A) TTP by histopathology in the 25 patients who progressed during venetoclax therapy. (B-D) The associations between TTP for patients with CLL/SLL receiving venetoclax therapy and (B) depth of clinical response: CR vs PR (P = .002) and PR vs SD (P < .001); (C) F-refractoriness (P = .005); and (D) karyotypic complexity (complex vs not complex; P = .005). In panel B, data from the whole cohort of n = 67 are presented. In panels C-D, only data from patients who received ≥400 mg/d are presented to exclude the response-modifying effect of use of low-dose venetoclax. Log-rank testing was used for comparisons. HR, hazard ratio.
TTP. (A) TTP by histopathology in the 25 patients who progressed during venetoclax therapy. (B-D) The associations between TTP for patients with CLL/SLL receiving venetoclax therapy and (B) depth of clinical response: CR vs PR (P = .002) and PR vs SD (P < .001); (C) F-refractoriness (P = .005); and (D) karyotypic complexity (complex vs not complex; P = .005). In panel B, data from the whole cohort of n = 67 are presented. In panels C-D, only data from patients who received ≥400 mg/d are presented to exclude the response-modifying effect of use of low-dose venetoclax. Log-rank testing was used for comparisons. HR, hazard ratio.
Consistent with the phase 1 data,1 earlier progression of any kind appeared related to receipt of less than the recommended phase 2 dose of venetoclax (HR 2.1 [95% confidence interval, CI, 0.9-4.6], P = .071), but a statistically significant association was not proven (supplemental Figure 3). As may be expected, risk for progression of any kind was inversely correlated with depth of objective response to venetoclax (P < .0001; Figure 1B).
Other potential risk factors for progression of any kind on venetoclax were explored in the 49 patients who received ≥400 mg/d. Univariate survival analyses of dichotomous variables, including age ≥65 years, ≥4 prior lines of therapy, F-refractory status, bulky lymphadenopathy, the absence of rituximab coadministration, del(11q), del(17p), del(17p), and/or TP53 mutation and complex karyotype (Table 3), were performed. F-refractory status (7.01 [95%CI 1.7-28.5]; P = .002) and complex karyotype (6.6 [1.5-29.8]; P = .005) were strongly associated with higher risk of progression (Figure 1C-D), whereas del(17p) and/or TP53 mutation were not (HR 1.23 [0.3-4.4]; P = .752).
Putative risk factors for failure and TTP
| Variable . | N . | Median TTP (mo) . | HR (95% CI) . | Log-rank P value . |
|---|---|---|---|---|
| Age ≥65 y | ||||
| Y | 31 | 30 | 0.88 (0.28-2.72) | .819 |
| N | 18 | NR | ||
| ≥4 prior lines of therapy | ||||
| Y | 27 | 30 | 2.03 (0.64-6.49) | .222 |
| N | 22 | NR | ||
| F-refractory | ||||
| Y | 24 | 22 | 7.02 (1.73-28.45) | .002 |
| N | 25 | NR | ||
| Bulky disease | ||||
| Y | 21 | 25 | 1.83 (0.61-5.49) | .276 |
| N | 28 | NR | ||
| Rituximab combination studies | ||||
| Y | 12 | NR | 0.50 (0.12-2.21) | .355 |
| N | 37 | 30 | ||
| Deletion 11q | ||||
| Y | 13 | NR | 0.34 (0.07-1.62) | .154 |
| N | 36 | 30 | ||
| Deletion 17p | ||||
| Y | 21 | 30 | 1.24 (0.40-3.87) | .709 |
| N | 28 | NR | ||
| Deletion 17p and/or TP53 mutation | ||||
| Y | 24 | 30 | 1.23 (0.34-4.41) | .752 |
| N | 16 | NR | ||
| Complex karyotype | ||||
| Y | 11 | 16 | 6.61 (1.47-29.75) | .005 |
| N | 19 | NR |
| Variable . | N . | Median TTP (mo) . | HR (95% CI) . | Log-rank P value . |
|---|---|---|---|---|
| Age ≥65 y | ||||
| Y | 31 | 30 | 0.88 (0.28-2.72) | .819 |
| N | 18 | NR | ||
| ≥4 prior lines of therapy | ||||
| Y | 27 | 30 | 2.03 (0.64-6.49) | .222 |
| N | 22 | NR | ||
| F-refractory | ||||
| Y | 24 | 22 | 7.02 (1.73-28.45) | .002 |
| N | 25 | NR | ||
| Bulky disease | ||||
| Y | 21 | 25 | 1.83 (0.61-5.49) | .276 |
| N | 28 | NR | ||
| Rituximab combination studies | ||||
| Y | 12 | NR | 0.50 (0.12-2.21) | .355 |
| N | 37 | 30 | ||
| Deletion 11q | ||||
| Y | 13 | NR | 0.34 (0.07-1.62) | .154 |
| N | 36 | 30 | ||
| Deletion 17p | ||||
| Y | 21 | 30 | 1.24 (0.40-3.87) | .709 |
| N | 28 | NR | ||
| Deletion 17p and/or TP53 mutation | ||||
| Y | 24 | 30 | 1.23 (0.34-4.41) | .752 |
| N | 16 | NR | ||
| Complex karyotype | ||||
| Y | 11 | 16 | 6.61 (1.47-29.75) | .005 |
| N | 19 | NR |
Univariate analyses of associations between putative risk factors for failure and TTP for all patients who received ≥400 mg/d of venetoclax. Variables were dichotomized: Y, present; N, absent; n, number with data for each variable group (where testing was not performed, data are not included).
NR, not reached.
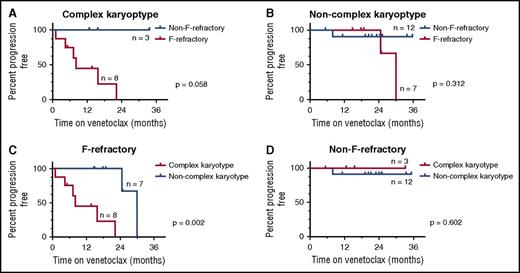
The modest number of progressions in our cohort precluded multivariate analysis using the Cox proportional hazards model. CART analysis was therefore used to investigate the relationship between F-refractoriness and complex karyotype as predictors of progression of any kind. This CART analysis involved a series of subgroup analyses. First, the cohort was divided into patients whose disease harbored or lacked a complex karyotype, and a log-rank method survival analysis was performed to assess the impact of F-refractoriness on TTP in each subgroup (Figure 2A-B). Reciprocally, the cohort was also divided into patients whose disease was F-refractory or non–F-refractory, and a survival analysis of complex karyotype on TTP was performed (Figure 2C-D). Among patients with complex karyotype, F-refractoriness was associated with additional risk of progression (P = .058; Figure 2A), but not in patients with CLL/SLL without a complex karyotype (P = .312; Figure 2B). Similarly, the presence of a complex karyotype augmented the risk of progression among patients with F-refractory disease (P = .002; Figure 2C) but had little impact on the favorable prognosis of patients with non–F-refractory disease (P = .602; Figure 2D). This CART analysis suggests a 3-tier risk model, with non–F-refractory CLL/SLL being low-risk, F-refractory, noncomplex karyotype disease being intermediate risk, and F-refractory disease with complex karyotype defining high risk (median TTP respectively: not reached, 30 and 8 months; P < .0001). Of the 8 patients who had F-refractory and complex karyotype disease at trial entry and received the recommended phase 2 dose, 6 (75%) progressed over a median follow-up period of 7.6 (1-22) months. Five of the 8 patients had del(17p); TP53 mutations were present, absent, and unknown in the 3 remaining subjects, respectively. The best objective responses to venetoclax documented in these patients were PR (5) and SD (3).
CART analysis. CART analysis of F-refractory status and complex karyotype in patients who received ≥400 mg/d. Patients with unknown karyotype were excluded from analysis. TTP of any kind (both CLL and RT) according to (A) F-refractory status in patients with complex karyotype; (B) F-refractory status in patients with noncomplex karyotype; (C) karyotype complexity in patients with F-refractory disease; and (D) karyotype complexity in patients with non-F-refractory disease.
CART analysis. CART analysis of F-refractory status and complex karyotype in patients who received ≥400 mg/d. Patients with unknown karyotype were excluded from analysis. TTP of any kind (both CLL and RT) according to (A) F-refractory status in patients with complex karyotype; (B) F-refractory status in patients with noncomplex karyotype; (C) karyotype complexity in patients with F-refractory disease; and (D) karyotype complexity in patients with non-F-refractory disease.
Outcomes after progression on venetoclax
Six of 8 patients with progressive CLL/SLL on venetoclax were treated with ibrutinib as their first postprogression therapy. Five achieved a PR, and 3 remain alive on therapy at last follow-up (6, 13, and 16 months post-CLL progression), with 2 dying of toxicity and 1 dying of progressive disease (PD; Figure 3A; Table 4). Treatments for RT were varied and included salvage with high-dose chemotherapy followed by autograft (n = 2), allograft (n = 2), or radiotherapy (n = 2); multiagent salvage chemotherapy alone (n = 10); or conservative management (n = 1) (Table 4; Figure 3A). The responses of patients with transformed disease to salvage therapies were CR (5), PR (3), and no response in 8 (50%). Seven patients with RT remain alive, including all 3 patients with HL RT (29, 30, and 50 months postprogression), 1 of whom had a subsequent progression with CLL and is receiving ongoing treatment with ibrutinib. Three patients with DLBCL RT who responded to salvage (2 CR, 1 PR) subsequently progressed with CLL/SLL and then received BTKI therapy. They remain alive on BTKIs at 37, 41, and 45 months posttransformation, after 19 to 21.4 months of BTKI therapy for secondary CLL progression (Figure 3A; Table 4).
Outcomes after progression. (A) Outcomes of patients post-CLL progression or RT on venetoclax. Patients are grouped according to histology at progression (progressive CLL, progression with chronic lymphocytic leukemia only). Each patient line begins at time of progression on venetoclax. A light green bar represents time from progression prior to next treatment. Treatment of progression is marked by a red, yellow, or blue bar, representing salvage chemotherapy for RT, or FCR or BTKI therapy for progressive CLL, respectively. (B) Survival of patients whose disease progressed on venetoclax. Data are grouped according to histology at progression and differences are assessed by log-rank testing. TOX, death from toxicity.
Outcomes after progression. (A) Outcomes of patients post-CLL progression or RT on venetoclax. Patients are grouped according to histology at progression (progressive CLL, progression with chronic lymphocytic leukemia only). Each patient line begins at time of progression on venetoclax. A light green bar represents time from progression prior to next treatment. Treatment of progression is marked by a red, yellow, or blue bar, representing salvage chemotherapy for RT, or FCR or BTKI therapy for progressive CLL, respectively. (B) Survival of patients whose disease progressed on venetoclax. Data are grouped according to histology at progression and differences are assessed by log-rank testing. TOX, death from toxicity.
Treatments and outcome for patients with PD
| Case no. . | Treatment . | Response . | Later CLL PD (treatment) . | Status . | PPS (mo) . |
|---|---|---|---|---|---|
| RT–DLBCL | |||||
| 1 | R-CHOP | PD | — | Dead | 2.3 |
| 2 | No treatment | — | — | Dead | 0.9 |
| 3 | Vin/Gem | PR | — | Alive | 32.3 |
| 4 | R-CHOP | SD | — | Dead | 24.6 |
| 5 | HyperCVAD | PD | — | Dead | 14.9 |
| 6 | R-CHOP | PD | — | Dead | 5.6 |
| 7 | OFAR | PD | — | Dead | 10.9 |
| 8 | CHOP + AlloSCT | PR | — | Dead | 13 |
| 9 | R-ICE + AuSCT | CR | + (Novel BTKI on trial) | Alive | 36.9 |
| 10 | R-ICE + AuSCT | CR | + (Ibr) | Alive | 45.0 |
| 11 | XRT + R-MVP | PR | + (Ibr) | Alive | 40.5 |
| 12 | R-CHOP | SD | — | Dead | 9.3 |
| 13 | R-CHOP | Death | — | Dead | 1 |
| 14 | R-ICE | SD | — | Dead | 10.7 |
| RT-HL | |||||
| 15 | ABVD | CR | — | Alive | 29.3 |
| 16 | R-CHOP + AlloSCT | CR | — | Alive | 49.9 |
| 17 | CHEP + XRT | CR | + (Ibr) | Alive | 30.2 |
| CLL progression | |||||
| 1 | No treatment | — | — | Dead | <1.0 |
| 2 | Ibr | SD | + | Dead | 11.4 |
| 3 | Ibr | PR | — | Alive | 6.2 |
| 4 | FCR | Unk | — | Dead | 5.6 |
| 5 | Ibr | PR | — | Dead (toxicity) | 8.6 |
| 6 | Ibr | PR | — | Alive | 15.7 |
| 7 | Ibr | PR | — | Alive | 13.2 |
| 8 | Ibr | PR | — | Dead (toxicity) | 8.3 |
| Case no. . | Treatment . | Response . | Later CLL PD (treatment) . | Status . | PPS (mo) . |
|---|---|---|---|---|---|
| RT–DLBCL | |||||
| 1 | R-CHOP | PD | — | Dead | 2.3 |
| 2 | No treatment | — | — | Dead | 0.9 |
| 3 | Vin/Gem | PR | — | Alive | 32.3 |
| 4 | R-CHOP | SD | — | Dead | 24.6 |
| 5 | HyperCVAD | PD | — | Dead | 14.9 |
| 6 | R-CHOP | PD | — | Dead | 5.6 |
| 7 | OFAR | PD | — | Dead | 10.9 |
| 8 | CHOP + AlloSCT | PR | — | Dead | 13 |
| 9 | R-ICE + AuSCT | CR | + (Novel BTKI on trial) | Alive | 36.9 |
| 10 | R-ICE + AuSCT | CR | + (Ibr) | Alive | 45.0 |
| 11 | XRT + R-MVP | PR | + (Ibr) | Alive | 40.5 |
| 12 | R-CHOP | SD | — | Dead | 9.3 |
| 13 | R-CHOP | Death | — | Dead | 1 |
| 14 | R-ICE | SD | — | Dead | 10.7 |
| RT-HL | |||||
| 15 | ABVD | CR | — | Alive | 29.3 |
| 16 | R-CHOP + AlloSCT | CR | — | Alive | 49.9 |
| 17 | CHEP + XRT | CR | + (Ibr) | Alive | 30.2 |
| CLL progression | |||||
| 1 | No treatment | — | — | Dead | <1.0 |
| 2 | Ibr | SD | + | Dead | 11.4 |
| 3 | Ibr | PR | — | Alive | 6.2 |
| 4 | FCR | Unk | — | Dead | 5.6 |
| 5 | Ibr | PR | — | Dead (toxicity) | 8.6 |
| 6 | Ibr | PR | — | Alive | 15.7 |
| 7 | Ibr | PR | — | Alive | 13.2 |
| 8 | Ibr | PR | — | Dead (toxicity) | 8.3 |
ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; AlloSCT, allogeneic stem cell transplant; AuSCT, autologous stem cell transplant; BGB, BGB-3111, an experimental BTK inhibitor, as part of a phase 1 clinical trial (NCT02343120); CHEP, cyclophosphamide, doxorubicin, epirubicin, prednisolone; Gem, gemcitabine; Ibr, ibrutinib; OFAR, oxaliplatin, fludarabine, cytarabine, and rituximab; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone; R-ICE, rituximab, ifosfamide, carboplatin, etoposide; R-MVP, rituximab, mitoxantrone, vincristine, prednisolone; Unk, unknown; Vin, vinorelbine; XRT, involved-field radiation therapy.
Postprogression, the median follow-up was 11.4 (0-49.9) months. The median PPS for all progressors was 13 (0-49.9) months and an estimated 37% at 2 years. Median PPS for HL RT, DLBCL RT, and progressive CLL/SLL was not reached, 11.9 and 8.6 months, respectively (P = .138) (Figure 3B). For the 10 patients treated with BTKI for progressive CLL developing either on venetoclax (n = 6) or after treatment of RT (n = 4), the median survival on BTKI therapy has not been reached after a median follow-up on BTKI of 11.3 (4-21.4+) months (Table 4).
Discussion
In this first report of the clinicopathological features and outcomes of patients whose CLL/SLL progresses on venetoclax, we identified F-refractoriness and complex karyotype as the dominant risk factors for progression despite ongoing treatment with the BCL2 inhibitor. These features are particularly relevant for assessment of heavily pretreated patients, as typified in these early phase clinical trials, and may be less relevant for (and certainly less prevalent among) patients receiving venetoclax earlier in their disease course. Our data align with those for relapsed CLL/SLL patients treated with ibrutinib.19 Thompson et al reported significant associations between complex karyotype and F-refractory disease and inferior survival among patients treated with ibrutinib in the relapsed and refractory setting.19 These findings suggest that complex karyotype may hold greater prognostic significance than TP53 aberrations (del(17p) and mutations) in heavily previously treated patients receiving novel agents, possibly reflecting greater genomic instability among this group. These findings must be interpreted in the light of modest patient numbers, which limited the power to detect additional predictive features by CART and univariate analysis, and precluded multivariate analysis. Future pooled analysis of longer-term data across all early phase venetoclax trials will significantly add to the power of analyses designed to predict which patients are at significant risk of progression on venetoclax monotherapy.
Our data provide the first detailed description of the clinical features of patients who progress on venetoclax. In addition to progressive lymphadenopathy and B symptoms, clinicians may also be alerted to potential RT by cytopenias and mixed nodal response. Although a significant rate of RT should be expected in our heavily pretreated and del(17p)-enriched cohort,18 clinicians should nevertheless be cognizant of this mechanism of progression and obtain PET imaging and biopsies whenever practicable. In a broader population of less heavily pretreated patients with RR-CLL/SLL, the development of RT during venetoclax therapy was less common, being observed in 12% of 387 patients treated with 400 mg/d.20
Postprogression outcomes are not routinely collected in early phase trials and, although our dataset only has modest numbers, for the foreseeable future this study will be the most comprehensive available for patients treated with venetoclax. Outcomes for patients who progress on venetoclax are not universally poor. Patients with HL RT may represent a prognostically favorable subgroup, as has been previously reported.18,21 Although historically DLBCL RT is often associated with dismal outcomes,22 some patients with RT emerging on venetoclax can achieve durable responses to salvage therapy. Patients in our cohort whose RT was controlled successfully by salvage therapy demonstrated a response in residual CLL with BTK inhibition, leading to prolonged survival. The PPS for patients treated with venetoclax reported here contrasts somewhat to the unfavorable prognosis reported for patients who progressed on ibrutinib, especially those who progressed with RT (median survival estimates of <4 months for transformed disease).9,23,24 This difference may be related in part to the broad availability of BTK inhibitors for salvage therapy of venetoclax failures among this cohort, and the absence of venetoclax for ibrutinib failures until recently. As none of the patients in this study had been previously exposed to BTK inhibitors, the applicability of our data is limited for patients with progressive CLL who had failed ibrutinib prior to venetoclax.
Our data make a compelling argument for the need to prospectively evaluate the risk factors for development of disease progression, in particular RT, among patients with RR-CLL/SLL being considered for treatment with venetoclax. Patients whose disease is F-refractory or who have complex karyotype are at significant risk of early progression with RT on venetoclax. We therefore recommend that these patients should have RT specifically excluded prior to treatment with venetoclax monotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families as well as the research nurses from RMH and PMCC. They also thank colleagues at all participating hospitals and AbbVie and Genentech for conduct of the trials and critical comment on the manuscript.
The clinical trials were funded by AbbVie and Genentech. This research was also supported by scholarships, fellowships, and grants from the Australian National Health and Medical Research Council (Research Fellowships, Program Grants 1016647, 1016701, Independent Research Institutes Infrastructure Support Scheme grant 9000220) (A.W.R., D.C.S.H.), the Leukemia and Lymphoma Society (SCOR grants 7001-13), the Victorian Cancer Agency, the Cancer Council Victoria, the Australian Cancer Research Foundation, and a Victorian State Government Operational Infrastructure Support grant. Past support by a fellowship from the Webster bequest and current support by the Snowdome Foundation is acknowledged (M.A.A.).
Authorship
Contribution: The research was designed by M.A.A., C.T., D.C.S.H., J.F.S., and A.W.R.; M.A.A., C.T., T.E.L., S.J., M.J., D.W., M.W., S.L., J.F.S., and A.W.R. generated the data; M.A.A., C.T., T.E.L., J.F.S., and A.W.R. analyzed the data, and A.G. provided statistical input. All authors contributed to manuscript preparation and approved the final version.
Conflict-of-interest disclosure: M.A.A., D.C.S.H., and A.W.R. are employees of Walter and Eliza Hall Institute of Medical Research, which receives milestone payments related to venetoclax. D.C.S.H. and A.W.R. received research funding from AbbVie, Genentech, and Servier. A.W.R. also received research funding from Janssen. C.T. received research funding and honoraria from AbbVie and Janssen, honoraria from Roche, Gilead, and Pharmacyclics, and travel support from Beigene. J.F.S. is a member of Advisory Boards for AbbVie, Roche, and Genentech. The remaining authors declare no competing financial interests.
Correspondence: Mary Ann Anderson, Department of Clinical Haematology and BMT, Royal Melbourne Hospital, Grattan St, Parkville, VIC, Australia 3050; e-mail: manderson@wehi.edu.au; and Andrew W. Roberts, Division of Cancer and Haematology, Walter and Eliza Hall Institute, 1G Royal Parade, Parkville, VIC, Australia 3052; e-mail: roberts@wehi.edu.au.
References
Author notes
M.A.A., C.T., and T.E.L. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal