Abstract
Autoimmune hemolytic anemia (AIHA) is an uncommon entity that presents diagnostic, prognostic, and therapeutic dilemmas despite being a well-recognized entity for over 150 years. This is because of significant differences in the rates of hemolysis and associated diseases and because there is considerable clinical heterogeneity. In addition, there is a lack of clinical trials required to refine and update standardized and evidence-based therapeutic approaches. To aid the clinician in AIHA management, we present four vignettes that represent and highlight distinct clinical presentations with separate diagnostic and therapeutic pathways that we use in our clinical practice setting. We also review the parameters present in diagnostic testing that allow for prognostic insight and present algorithms for both diagnosis and treatment of the AIHA patient in diverse situations. This is done in the hope that this review may offer guidance in regard to personalized therapy recommendations. A section is included for the diagnosis of suspected AIHA with negative test results, a relatively infrequent but challenging situation, in order to assist in the overall evaluation spectrum for these patients.
Introduction and history
The diagnosis, prognosis, and management of autoimmune hemolytic anemia (AIHA) continue to be challenging in current practice. This is related to an incomplete understanding of the pathophysiology of the disease process, complexity of initiating factors, and a lack of evidence-based standardized therapies. There is no completely validated and standard therapeutic approach to AIHA, because randomized clinical trials are difficult to implement.
Key historical events of AIHA include original descriptions of an AIHA-like disease in the 19th century and subsequently more definitive descriptions in the seminal publications of J. Donath and K. L and steiner in the early 20th century.1-4 The direct antiglobulin test (DAT) was described by Robin Coombs and A. Mourant in 1945 and is a laboratory-based assay still of great utility in the diagnosis of AIHA.5 A positive DAT result, along with no other obvious cause of hemolysis, is the defining clinical signature of AIHA. Antibodies directed against self-erythrocytes capable of induction of hemolysis at excessive or uncompensated rates result in an entity known as AIHA. These antibodies are usually immunoglobulin G (IgG) in nature, capable of fixing complement, and are detected by the DAT. The DAT is based on specific antibodies to IgG and/or C3d (fragment of the third component of complement) capable of binding to these components on the erythrocyte surface. If the latter molecules are present in sufficient quantity on the erythrocyte membrane, the result is a visible agglutination by cross-linking erythrocytes. DAT techniques that enhance the sensitivity of this test beyond visual agglutination have been developed but are not routinely used. If more commonly employed, these enhanced tests would increase the detection of autoantibodies but are likely to lead to questions about their exact relationship to clinically important disease. These tests are important in the setting of DAT-negative AIHA, an uncommon form of AIHA (see case 4). In contrast to the DAT, the indirect antiglobulin (indirect Coombs) test is used to detect erythrocyte antibodies in patient serum. This is done by incubating patient serum with a panel of erythrocytes of known antigens and observing whether agglutination results. The “super-Coombs” test is an enhanced direct Coombs test that utilizes different methods to generate erythrocyte agglutination (also reviewed in Table 4 below) and performed when the standard DAT result is negative.
The incidence of AIHA is considered uncommon, with prior estimates of 1 to 3 in 100 000 population annually.6 AIHA affecting children and adults and warm-reacting antibodies are the primary pathogenic etiology in the majority of cases (∼75% and ∼90%, respectively).7,8 AIHA can be subdivided into warm- or cold-mediated disease based on the thermal optimum used to detect anti–erythrocyte antibodies. Primary AIHA comprises ∼50% of cases, while secondary AIHA is usually associated with B-cell malignancies, autoimmune diseases, or drugs. Primary (idiopathic) AIHA occurs when no disease is clearly associated with the hemolysis, whereas secondary AIHA occurs when hemolytic anemia is directly associated with another disease or drug believed to induce or promote the hemolysis. The progression of events that need to be dealt with in the management of AIHA includes using the appropriate methodologies for diagnosing AIHA, defining whether AHIA is primary or secondary in type, and identifying the most effective treatment for a given patient.9
Serology that matters in warm AIHA (WAIHA) and cold agglutinin disease (CAD) evaluation
The essence of AIHA is that it is caused by the increased destruction of erythrocytes by anti–erythrocyte autoantibodies. This can occur with or without complement fixation and activation. Here is a primer for the clinician to aid in the fundamental understanding of immune mediators in AIHA, diagnosis, and prognostic risk.
Advances in understanding the pathophysiology of AIHA and how to use anti–CD20 antibodies with or without immunosuppressive agents has augmented treatment approaches.
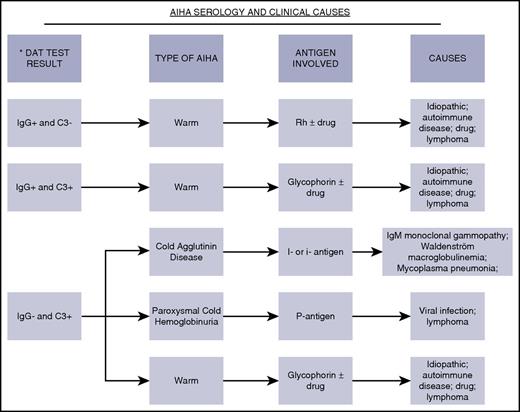
Figure 1 shows the pattern of antibodies and complement that are typically found on erythrocyte membranes in warm and CAD AIHA.10-13 It features the most well-characterized erythrocyte antigens involved in AIHA and the more typical diseases or drugs associated with specific AIHA subtypes. These autoantibodies can be of IgG, IgM, or IgA isotypes, but most commonly, the antibody is an IgG antibody in WAIHA and an IgM antibody in CAD. The IgA isotype is much less commonly involved in AIHA but may cause severe hemolysis.14,15 The ability of the anti–erythrocyte antibody to bind to the erythrocyte antigen at specific temperatures is fundamental to the diagnosis in terms of whether it is designated as warm (reacts maximally at 37°C) or cold (reacts maximally at <4°C) AIHA. The pathologic and clinical features of AIHA relate to the autoantibody class, thermal amplitude, and their efficiency in activating complement. It is known that the broader the thermal amplitude, the worse the rate of hemolysis for IgM cold agglutinins. With initiation of therapy, it is best to monitor restoration of the hemoglobin and reticulocyte levels over the first several weeks of therapy. Monitoring the DAT is routine, but even if the result remains positive, this may not reflect a lack of disease control. The extent of hemolysis must be matched by a robust marrow-based erythrocyte production rate but may be insufficient.16,17 Insufficient reticulocytosis may occur in children and in adults with very severe hemolysis. Recognition of this phenomenon has generated data indicating that the use of erythropoietin may be useful in managing situations like this and refractory AIHA.18
Direct antiglobulin test serology and clinical aspects. Shown are the spectrum of DAT serologic findings, autoimmune hemolytic classifications, antigen specificity, and medical/drug associations.10,11 Drugs most commonly implicated11-13,20 in drug-induced autoimmune hemolytic anemia are β lactam antibiotics (penicillin, ceftriaxone, cefotetan, and piperacillin), nonsteroidal anti-inflammatory drugs (tolmetin, sulindac, and diclofenac), quinine, purine nucleoside analogs (fludarabine and cladribine), and platinums (cisplatin and oxaliplatin).12,13
Direct antiglobulin test serology and clinical aspects. Shown are the spectrum of DAT serologic findings, autoimmune hemolytic classifications, antigen specificity, and medical/drug associations.10,11 Drugs most commonly implicated11-13,20 in drug-induced autoimmune hemolytic anemia are β lactam antibiotics (penicillin, ceftriaxone, cefotetan, and piperacillin), nonsteroidal anti-inflammatory drugs (tolmetin, sulindac, and diclofenac), quinine, purine nucleoside analogs (fludarabine and cladribine), and platinums (cisplatin and oxaliplatin).12,13
Case 1: idiopathic WAIHA
A 68-year-old previously healthy woman presented with new-onset fatigue. Physical examination was remarkable only for mild pallor. Laboratory test results (with reference ranges provided in brackets) were significant for hemoglobin (9.3 g/dL [12.0-15.5]), mean cell volume (MCV; 89.7 fL [81.6-98.3]), absolute reticulocyte count (119 × 109/L [38.1-112.6]), haptoglobin (<14 mg/dL [30-200]), lactate dehydrogenase (LDH; 267 U/L [122-222]), total bilirubin (0.8 mg/dL [0.1-1]), and blood smear showing polychromasia. The DAT showed 2+ anti-IgG and weakly positive anti-C3.
Should we routinely evaluate for an underlying lymphoid malignancy?
Contemporary series composed of unselected patients with WAIHA are scarce. A recent single-institutional study (N = 60) showed that an underlying condition could be found in 48% of patients at or preceding the diagnosis and in another 8% subsequently. The most common conditions were lymphoma or undefined lymphoproliferative disorder (54%) and autoimmune diseases (27%).19 Another report (N = 107) studied warm and cold AIHA cases initially considered idiopathic or associated with autoimmune disorders and found that 18% of patients developed lymphoma at a median of 27 months.20 In both studies, evaluation for lymphoma was not routinely performed at the time of AIHA diagnosis. Therefore, the prevalence of lymphoma is likely to be underestimated. It is reasonable to consider evaluating for lymphoid malignancies, including a computed tomographic scan of the chest, abdomen, and pelvis, as well as bone marrow biopsy in patients with newly diagnosed AIHA. Discovery of such malignancies upfront may open the option of non–glucocorticoid-based therapies, improve the chance of response, and minimize relapses.
How do we use glucocorticoids?
While glucocorticoids are considered the first-line treatment in WAIHA, this was empirically derived. Mechanisms of actions include suppression of autoantibody production, reduction in autoantibody affinity, and decreased destruction of erythrocytes by splenic macrophages, perhaps by diminished expression of Fcγ receptors.21,22 The first-ever randomized trial of newly diagnosed AIHA compared high-dose prednisone (1.5 mg/kg per day × 2 weeks, then tapered over 8-12 weeks), with or without rituximab, found that 50% of patients in the prednisone-only group achieved either a complete response (CR) or partial response (PR) at 3 months. However, nearly half of the responders relapsed within a year. This study was limited by small sample size (N = 64) and did not report the proportion of patients needing second-line treatment after relapse. The rituximab arm had higher rates of CR at 12 months (75% vs 36%) and relapse-free survival at 36 months (70% vs 45%).23 A prospective registry study of 308 patients showed a higher prednisone-based response rate of 80%. Half did not require subsequent treatment after a median follow-up of 33 months.24 The optimal starting dose and taper schedule of prednisone are unknown, although most reports and experts use starting doses of 1.0 to 1.5 mg/kg per day or a flat dose of 60 to 100 mg daily.25-27 The starting dose is maintained for at least 2 weeks and until achievement of hemoglobin >12 g/dL. Thereafter, we taper the prednisone by 20 mg every week until a dose of 20 mg daily is reached, followed by a slower taper over 4 to 8 weeks. We monitor hemoglobin levels on a weekly basis until the tapering process is complete. Thereafter, less frequent testing is needed. The success of high-dose dexamethasone (40 mg daily for 4 days) in the initial treatment of immune thrombocytopenia makes an attractive alternative option in the treatment of WAIHA, although further studies are needed.28 One study showed its efficacy in the setting of refractory AIHA.29 Because chronic hemolysis may potentially lead to folate deficiency due to increased utilization, it is customary to supplement with 1 mg folic acid daily when glucocorticoid is started.
What is the value of splenectomy?
Splenectomy is effective but has never been compared with other treatments in the second-line setting. The response rate of splenectomy in unselected patients is ∼60% to 90%, and approximately one-third will relapse, mostly within 1 to 2 months.24,30,31 Patients without an underlying autoimmune disease or hematologic malignancy are twice as likely to respond as those with such conditions (82% vs 19% complete response).32 Although rituximab has increasingly superseded splenectomy in recent retrospective studies and may be primary second line for some centers,33 we still consider splenectomy as the primary second-line option in idiopathic AIHA. In one study of 52 patients with AIHA, 64% were in unmaintained remission after a mean follow-up of 33 months. Relapse patterns were not reported separately for idiopathic and secondary AIHAs.30 For those with underlying medical conditions, alternative treatments such as rituximab and disease-specific therapies may be a better next option. It is absolutely essential to vaccinate against encapsulated bacterial organisms at least 14 days prior to (preferable) or at least 14 days after splenectomy to maximize immunity.34
How do we treat subsequent relapse or refractory disease?
With no standard definition for treatment responses reflecting refractory or relapsed AIHA, the definition proposed by Barcellini et al is useful.35 We consider a subsequent line of treatment in the following scenarios: (1) requirement of >20 mg of prednisone daily (or equivalent corticosteroid) to maintain hemolysis control; (2) clinically significant relapse (hemoglobin < 11g/dL or symptomatic anemia with ongoing evidence of hemolysis); or (3) intolerance to a currently effective treatment. If hemolysis continues that is well compensated after prednisone tapering, starting a second-line treatment may not be necessary. Similarly, DAT negativity is not essential with controlled hemolysis. The more commonly used treatments along with their corresponding dosing schedules are displayed in Table 1.36-42 Patterns of care studies are not available, but single-agent rituximab is perhaps the most commonly used treatment in this setting and is our first choice after splenectomy relapse. In a meta-analysis of 21 studies that investigated rituximab, the overall response (OR) and CR rates were 79% and 42%, respectively. The OR was similar regardless of whether it was idiopathic or secondary AIHA (67% vs 72%).43 In studies with >3 years of follow-up, the relapse rate was ∼50%. However, most patients responded to rituximab retreatment.19,44
Pharmacologic treatment options for relapsed or refractory warm autoimmune hemolytic anemia from a case series
| Treatment . | Initial dose(s) . | OR, % . | Median time to response (range) . | Median response duration (range) . | Relapse rate (at 1-2 y), % . | Comments . | Reference . |
|---|---|---|---|---|---|---|---|
| Azathioprine | 2-4 mg/kg orally once a day | 50-70 | NA | 11 mo (4-36) | 60 | N = 9-31 | 19, 24, 36, 37 |
| Cyclophosphamide (low dose) | 1-2 mg/kg orally once a day; or 50-150 mg orally once a day | 50-70 | NA | 11 mo (4-36) | 50 | N = 7-40 | 19, 24, 26, 37 |
| Cyclophosphamide (high dose) | 50 mg/kg IV days 1-4 with mesna and granulocyte colony stimulating factor rescue; or 1000 mg IV every 4 wk × 4 | 100 | 3 wk; 82% at 4 mo | 15 mo (4-29) | 0 | For the 50 mg/kg dose, 40% hospitalization due to complications (N = 9-17) | 38, 39 |
| Cyclosporine | 2.5 mg/kg orally twice a day | 60 | NA | 11 mo (4-36) | NA | Maintain serum level 200-400 ng/mL (N = 12) | 24 |
| Danazol | 200 mg orally 3-4 times daily | 60-80 | NA | 18 mo (7-77) | 30-75 | Maintenance dose 200-400 mg/day (N = 15-22) | 8, 40, 41 |
| Mycophenolate | 500-1000 mg orally twice a day | 25-70 | 5 mo (1-9) | 11 mo (4-36) | NA | N = 3-4 | 19, 24, 42 |
| Rituximab | 375 mg/m2 IV every week × 4; or 100 mg IV every week × 2-4 | 70-90 | 2 wk (1-12) | 20 mo (9-60) | 20-50 | N = 25-74 | 19, 24, 44 |
| Treatment . | Initial dose(s) . | OR, % . | Median time to response (range) . | Median response duration (range) . | Relapse rate (at 1-2 y), % . | Comments . | Reference . |
|---|---|---|---|---|---|---|---|
| Azathioprine | 2-4 mg/kg orally once a day | 50-70 | NA | 11 mo (4-36) | 60 | N = 9-31 | 19, 24, 36, 37 |
| Cyclophosphamide (low dose) | 1-2 mg/kg orally once a day; or 50-150 mg orally once a day | 50-70 | NA | 11 mo (4-36) | 50 | N = 7-40 | 19, 24, 26, 37 |
| Cyclophosphamide (high dose) | 50 mg/kg IV days 1-4 with mesna and granulocyte colony stimulating factor rescue; or 1000 mg IV every 4 wk × 4 | 100 | 3 wk; 82% at 4 mo | 15 mo (4-29) | 0 | For the 50 mg/kg dose, 40% hospitalization due to complications (N = 9-17) | 38, 39 |
| Cyclosporine | 2.5 mg/kg orally twice a day | 60 | NA | 11 mo (4-36) | NA | Maintain serum level 200-400 ng/mL (N = 12) | 24 |
| Danazol | 200 mg orally 3-4 times daily | 60-80 | NA | 18 mo (7-77) | 30-75 | Maintenance dose 200-400 mg/day (N = 15-22) | 8, 40, 41 |
| Mycophenolate | 500-1000 mg orally twice a day | 25-70 | 5 mo (1-9) | 11 mo (4-36) | NA | N = 3-4 | 19, 24, 42 |
| Rituximab | 375 mg/m2 IV every week × 4; or 100 mg IV every week × 2-4 | 70-90 | 2 wk (1-12) | 20 mo (9-60) | 20-50 | N = 25-74 | 19, 24, 44 |
NA, not available.
Information about response rates and their duration for less used drugs often varied and were frequently not reported. Many of these treatments were added to corticosteroids at relapse. It is impossible to determine the comparative efficacy of these treatments. We caution the treatment outcomes in Table 1 may look more optimistic due to reporting bias and small study sample sizes. The choice of treatment beyond rituximab will depend on the physician’s clinical judgment, the patient’s preference, and the drug’s side effect profile. In our experience using noncorticosteroid immunosuppressive agents, the response may take months to be evident. Therefore, it is reasonable continue treatment of at least 8 to 12 weeks, especially if the hemolysis rate is stable during treatment. A recent review article has more in-depth discussions of the individual treatment options for relapsed or refractory disease.26
Case 2: WAIHA associated with chronic lymphocytic leukemia (CLL)
A 62-year old male with a history of CLL presented with profound fatigue. He was severely anemic with hemoglobin of 5.8 g/dL. Additional laboratory test results (with reference ranges in parentheses) included MCV 93.5 fL, white cell count 4.0 × 109/L (3.5-10.5), platelet count 50 × 109/L (150-450), absolute lymphocyte count 1.6 × 109/L (0.9-2.9), absolute reticulocyte count 10 × 109/L, haptoglobin <14 mg/dL, LDH 420 U/L, total bilirubin 2.0 mg/dL, indirect bilirubin 1.5 mg/dL (<1.0), and blood smear showing polychromasia. DAT showed 2+ anti-IgG and no anti-C3. He was treated a year ago with a fludarabine/cyclophosphamide/rituximab combination regimen and achieved a PR.
When is erythrocyte transfusion indicated or contraindicated, and what needs to be considered when transfusing a patient?
Consideration of erythrocyte transfusion for hemodynamically stable patients with a hemoglobin of <7 g/dL is based on the AABB guidelines.45 This restrictive strategy applies to the relatively asymptomatic patient. For patients who are experiencing cardiopulmonary symptoms due to anemia, erythrocyte transfusion should not be withheld regardless of hemoglobin level. Because the major erythrocyte antigenic targets of the autoantibodies (Rh, Rh-related, band 3, or glycophorin) are nearly universally present in humans,27 special compatibility test procedures are necessary to rule out the presence of an alloantibody and proper crossmatching. These tests include removing the autoantibody from the patient’s serum, leaving behind any alloantibodies by utilizing either the patient’s (autologous) red blood cells or selected sets of donor red blood cells of known antigen type to adsorb the autoantibody. The adsorbed serum (adsorbate) is then used to identify any potential allogeneic antibodies by reacting it against panels of red blood cells of known antigen type (antibody screen) and to perform crossmatching to identify compatible erythrocytes. In severe anemia, there may be insufficient autologous erythrocytes to perform an autologous adsorption. To enhance the safety of transfused erythrocytes, it is possible to use erythrocytes phenotypically matched with the patient. This means determining the antigen profile of the patient’s erythrocytes using typing sera for antigens toward common alloantibodies. Antigen typing of the patient’s erythrocytes should be performed prior to transfusion or at least 3 months after the patient’s last transfusion to avoid potential inaccurate typing. Determining the “molecular phenotype” of a patient in order to provide antigen matched red blood cells is possible and can be used in transfused patients.
While phenotyping and providing phenotypically matched erythrocytes reduces the risk of hemolysis due to alloantibodies “hiding behind” an autoantibody, it does not eliminate it. More than 400 red cell antigens have been identified, and typing sera are available for a minority. Molecular phenotyping may also fail to correctly determine a patient’s antigen type because of gene silencing through mutations occurring outside of antigen coding regions. The presence of these other methods means that immediate discussion with the blood bank personnel is necessary to avoid delays or miscommunication and allow for timely testing. It is expected that the transfused erythrocytes, even if phenotypically matched, will have shorter half-lives. Nevertheless, there is no absolute contraindication to erythrocyte transfusion, as it remains a safe procedure.
Do we treat the CLL or AIHA or both?
We first determine whether there is an indication for the treatment of CLL based on the International Workshop on CLL guidelines for active disease.46 If chemotherapy is indicated, then any of the nonpurine nucleoside analog containing chemoimmunotherapy combinations or ibrutinib may be used, since they are not known to be associated with or cause hemolysis.47 The AIHA generally responds in parallel to CLL therapy. Otherwise, the treatment is similar to what is used for AIHA in the nonmalignant setting.
How commonly does AIHA occur during fludarabine treatment?
The incidence of AIHA among previously untreated patients receiving nonpurine nucleoside analog based treatment is ∼2%.48 This contrasts with the ∼6% incidence among those receiving fludarabine-based therapy.49-51 In the latter group, the majority of cases occur during the first 3 treatment cycles, although AIHA can occur at any time during treatment and reoccur after rechallenge. Hemolytic episodes can be severe enough to require transfusion, and fatalities have been reported.52 One randomized trial showed that the combination of fludarabine and cyclophosphamide might have a lower incidence of AIHA than fludarabine alone.50 However, this was not supported by other randomized trials.49,51 In another randomized trial, the incidence of AIHA was similar (∼1%) among those who received fludarabine and cyclophosphamide with or without rituximab.53 Thus, we generally discontinue fludarabine-based therapies in the setting of AIHA, especially when the hemolysis is severe. Purine nucleoside analogs such as pentostatin and cladribine are also associated with AIHA and should be avoided.54,55 If further chemotherapy is unnecessary, we use corticosteroids alone. If additional CLL treatment is needed, we prefer rituximab/cyclophosphamide/dexamethasone, bendamustine/rituximab, or novel signal inhibitors because of their safety in this setting.56-59 These 2 chemotherapy combination regimens have been shown to effectively treat steroid-refractory AIHA. In most (>80%) of the cases, there was sustained control of hemolysis and CLL.58,59 Among those who were receiving AIHA treatment, the initiation of ibrutinib frequently allowed discontinuation of AIHA treatment within 5 months.47
Are any of the recently approved CLL drugs associated with AIHA?
Five drugs have been recently approved for use in CLL: ibrutinib, idelalisib, obinutuzumab, ofatumumab, and venetoclax. There is no evidence from randomized trials to suggest that any of these agents increases the absolute risk of AIHA.48,60-62 There is a flare phenomenon described among patients with preexisting immune cytopenias treated with ibrutinib.57 Immune cytopenia may exacerbate shortly after starting ibrutinib (within a median of 3 weeks and ranging from 2 to 8 weeks). The flare episode can be managed by continuation of ibrutinib with or without the addition of corticosteroids.57
Case 3: CAD
A 54-year-old man with acute bronchitis was treated with azithromycin with resolution of respiratory symptoms. Because of continued fatigue, laboratory tests were performed, which showed hemoglobin 11.4 g/dL, MCV 100 fL, absolute reticulocyte count 266 × 109/L, haptoglobin <14 mg/dL, LDH 295 U/L, total bilirubin 1.3 mg/dL, and indirect bilirubin 1.0 mg/dL; the blood smear showed erythrocyte agglutination. DAT showed weak+ anti-IgG and 3+ anti-C3. Serum protein electrophoresis showed a small, unquantifiable amount of IgMκ monoclonal protein, while Mycoplasma pneumoniae serology was consistent with past exposure. Cold agglutinin titer was >512. Physical examination was unremarkable.
What should be the extent of workup for a hematologic malignancy at the time of diagnosis?
In half of the cases, an autoimmune condition or infection (M pneumoniae or Epstein-Barr virus) are identified as potential precipitating factors.63 In the remainder, CAD is commonly associated with an underlying clonal B-cell disorder similar to WAIHA. In a recent series of non–infection-related CAD patients (N = 89), 34% were found to have a B-cell lymphoma, and another 47% had monoclonal gammopathy of undetermined significance.64 The predominant monoclonal protein was IgMκ (95%), while the rest was either IgGλ or IgAλ.65 Therefore, a routine evaluation for non-Hodgkin lymphoma that includes bone marrow biopsy and body computed tomographic scan should be considered when there is no obvious infection.
Who should we treat?
Supportive measures aimed at avoiding cold exposures apply to all patients regardless of symptoms. Day-to-day practices include adequate clothing for cold weather, keeping indoor thermostat at higher set points, and avoidance of icy drinks and cold showers. If the patient is admitted due to a medical event or for surgery, the application of body-warming blankets and prewarming of IV fluids and blood products may minimize the exacerbation of the hemolysis. We consider systemic treatment when there are substantial or disabling signs or symptoms despite supportive measures. These include cold-induced manifestations such as acrocyanosis or Raynaud phenomenon and clinical sequelae of anemia and hemolysis. CAD associated with infections is usually self-limited and generally does not require treatment.66 It is necessary to treat the underlying lymphoproliferative disorder if this is present.
What is the efficacy of rituximab?
Despite the lack of randomized trials, single-agent rituximab is currently considered the first-line systemic therapy for CAD due to its superior efficacy and tolerability. Two relatively larger prospective studies that included newly diagnosed and previously treated patients (N = 20 and N = 27) have shown ORs ranging from 45% to 54% with median times to response between 1 and 2 months and median response durations of 8 to 11 months. However, CRs were uncommon (<5%). The responses were similar regardless of the presence or absence of an underlying lymphoid malignancy.67 Another prospective study (N = 19) used a combination of rituximab and prednisone and achieved a relatively shorter median time to response (2 weeks), more CRs (56%), and fewer relapses (33% at 12 months), suggesting a potential additive effect.35 Both standard-dose (375 mg/m2 IV weekly × 4) and low-dose (100 mg fixed dose IV weekly × 4) rituximab were effective.35,67,68
Do corticosteroids work?
Older studies showed poor responses to corticosteroids (<15% OR),65,69 but recent studies suggest that corticosteroids are still commonly used and have higher response rates. In one study, corticosteroids were used in 24 of 89 patients (27%), mostly (81%) as first-line treatment, and produced an OR of 36%. One-third of patients did not require additional therapy after long-term follow-up.64 Another study (N = 64) of patients receiving corticosteroids reported an OR of 69% (mostly PRs). Many patients had to be maintained on corticosteroids at higher doses compared with WAIHA patients.24 In one of these two recent large series, half of the patients had predominantly IgG cold agglutinin.64 Reports suggest long-term efficacy of corticosteroids in this latter subgroup.70,71
What is the value of splenectomy?
Splenectomy is generally not recommended as a treatment in CAD, as erythrocyte destruction is known to primarily occur in the liver.72 In the 3 largest series of primary CAD reported in recent years (total N = 259), only 11 patients (4.2%) were treated with splenectomy, although, surprisingly, 3 (27.3%) responded with a response duration between 5 and 15 months.24,64,65 The antibody specificities of the DAT in these patients were not reported. There are anecdotal reports that some patients with predominantly IgG cold agglutinin may achieve a durable response to splenectomy.70
How do we treat subsequent relapse or refractory disease?
Data on the efficacy of alternative systemic treatments are limited for CAD. A summary of case treatment series with reported efficacy in CAD are shown in Table 2.73 While most immunosuppressive or cytotoxic agents used in WAIHA have been tested in CAD, the efficacy is generally lower. Treatments reported to have nearly no response are azathioprine and cladribine.65,74 Treatments reported to be effective but published as single case reports are bortezomib, eculizumab, rituximab/bendamustine, rituximab/cyclophosphamide, and rituximab/fludarabine/cyclophosphamide.59,75-80
Pharmacologic treatment options for relapsed or refractory cold agglutinin disease from case series
| Treatment . | Initial dose . | OR, % . | Median time To response (range) . | Median response duration (range) . | Relapse rate (at 1-2 y), % . | Comments . | Reference . |
|---|---|---|---|---|---|---|---|
| Chlorambucil | 4-20 mg orally once a day | 16-46 | NA | 11 mo | NA | Included newly diagnosed and previously treated patients (N = 19-37) | 64, 65 |
| Cyclophosphamide | 50-150 mg orally once a day | ||||||
| Rituximab | 375 mg/m2 IV every week × 4 wk | 45-54 | 1.5 mo (1-2) | 10 mo (8-27) | 50-83 | Included newly diagnosed and previously treated patients (N = 20-32) | 64, 67, 68 |
| Rituximab + prednisone | Rituximab: 100 mg IV every week × 4 wk; Prednisone: 1 mg/kg per day orally × 30 d, then taper | 56 | 2 wk | NA | 33 | Included newly diagnosed and previously treated patients (N = 19) | 35 |
| Rituximab + fludarabine | Rituximab: 375 mg/m2 IV every 4 wk; Fludarabine: 40 mg/m2 orally on days 1-5 every 4 wk; both × 4 cycles | 76 | 4 mo | >66 mo (3-66) | 23 | Grade 3-4 hematologic toxicities in 41% (N = 29) | 73 |
| Treatment . | Initial dose . | OR, % . | Median time To response (range) . | Median response duration (range) . | Relapse rate (at 1-2 y), % . | Comments . | Reference . |
|---|---|---|---|---|---|---|---|
| Chlorambucil | 4-20 mg orally once a day | 16-46 | NA | 11 mo | NA | Included newly diagnosed and previously treated patients (N = 19-37) | 64, 65 |
| Cyclophosphamide | 50-150 mg orally once a day | ||||||
| Rituximab | 375 mg/m2 IV every week × 4 wk | 45-54 | 1.5 mo (1-2) | 10 mo (8-27) | 50-83 | Included newly diagnosed and previously treated patients (N = 20-32) | 64, 67, 68 |
| Rituximab + prednisone | Rituximab: 100 mg IV every week × 4 wk; Prednisone: 1 mg/kg per day orally × 30 d, then taper | 56 | 2 wk | NA | 33 | Included newly diagnosed and previously treated patients (N = 19) | 35 |
| Rituximab + fludarabine | Rituximab: 375 mg/m2 IV every 4 wk; Fludarabine: 40 mg/m2 orally on days 1-5 every 4 wk; both × 4 cycles | 76 | 4 mo | >66 mo (3-66) | 23 | Grade 3-4 hematologic toxicities in 41% (N = 29) | 73 |
NA, not available.
Case 4: DAT-negative AIHA
A 50-year-old male presented with a 2-week history of fatigue and jaundice. He denied alcohol use, risk factors for viral hepatitis, recent travel, and toxin exposure, and he was not taking any medication. Physical examination showed icteric sclerae and hepatosplenomegaly. Further evaluation showed hemoglobin 6.0 g/dL, absolute reticulocyte count 272 × 109/L, platelet count 245 × 109/L, LDH 1000 U/L, haptoglobin <14 mg/dL, total bilirubin 6.3 mg/dL, indirect bilirubin 4.7 mg/dL, 2 negative DAT results, no paroxysmal hemoglobinuria clone, and blood smear with marked spherocytosis. Bone marrow biopsy specimen demonstrated erythroid hyperplasia. Due to worsening symptomatic anemia, he was transfused with 4 U erythrocytes, which raised the hemoglobin to 10.0 g/dL, but within 24 hours, hemoglobin declined to 7.5 g/dL. Because of the high index of suspicion for WAIHA, enhanced DATs were performed and detected a low-affinity IgG antibody.
How common is DAT-negative WAIHA?
A total of 3% to 11% of patients with hemolytic anemia clinically consistent with WAIHA will have a negative DAT result.81,82 A negative test result, considered critical for the diagnosis of WAIHA, may lead physicians to reject the diagnosis, resulting in additional patient evaluation and delays in treatment. It is therefore important to recognize the existence of DAT-negative WAIHA. The most common “cause” of DAT-negative WAIHA is technical. Approximately 10% to 50% of patients with DAT-negative WAIHA will have a positive standard DAT result using anti-IgG and anti-C3d reagents retested at immunohematology reference laboratories.81,83,84 If suspicion of WAIHA remains high, DAT should be repeated, preferably by an immunohematology reference laboratory. The presenting clinical features and treatment responses of patients with DAT-negative WAIHA are similar to those of patients with DAT-positive WAIHA.85
How do we test for DAT-negative AIHA?
The identified mechanisms by which erythrocyte antibody escapes recognition by standard DAT are listed in Table 3.81,82 There are several “enhanced” DATs that can be performed to detect one or more of the described mechanisms. The more common enhanced DATs are shown in Table 4.81,82 No single enhanced DAT will detect all potential hemolytic mechanisms requiring a panel of tests.
Mechanisms involved in DAT-negative WAIHA
| 1. Erythrocyte-bound antibody below the limit of detection of standard DAT |
| Erythrocytes from healthy individuals have up to 35 molecules of IgG bound to their surface.Standard DAT can detect >300-500 bound IgG molecules.WAIHA can occur with as few as 70-434 bound IgG molecules. |
| 2. Low-affinity IgG antibodies |
| Loosely bound antibodies are dislodged during the washing of erythrocytes or when samples are left standing at room temperature. |
| 3. IgA antibodies |
| IgA antibodies may trigger phagocytosis and antibody-dependent cell cytotoxicity, resulting in hemolysis.Standard anti–human globulin reagents do not have anti-IgA activity, as most polyspecific reagents contain a mixture of monoclonal anti-IgG and anti-C3d. |
| 4. Warm-reacting IgM and monomeric IgM antibodies |
| IgM antibodies reacting at warm temperatures and monomeric IgM may not fix complement.Standard anti–human globulin reagents do not detect IgM. However, these antibodies will detect C3d if the IgM antibody fixes complement. |
| 1. Erythrocyte-bound antibody below the limit of detection of standard DAT |
| Erythrocytes from healthy individuals have up to 35 molecules of IgG bound to their surface.Standard DAT can detect >300-500 bound IgG molecules.WAIHA can occur with as few as 70-434 bound IgG molecules. |
| 2. Low-affinity IgG antibodies |
| Loosely bound antibodies are dislodged during the washing of erythrocytes or when samples are left standing at room temperature. |
| 3. IgA antibodies |
| IgA antibodies may trigger phagocytosis and antibody-dependent cell cytotoxicity, resulting in hemolysis.Standard anti–human globulin reagents do not have anti-IgA activity, as most polyspecific reagents contain a mixture of monoclonal anti-IgG and anti-C3d. |
| 4. Warm-reacting IgM and monomeric IgM antibodies |
| IgM antibodies reacting at warm temperatures and monomeric IgM may not fix complement.Standard anti–human globulin reagents do not detect IgM. However, these antibodies will detect C3d if the IgM antibody fixes complement. |
Enhanced DATs
| Name . | Description . | DAT-negative WAIHA detected . |
|---|---|---|
| Column agglutination | Erythrocytes are placed on a column of beads suspended in diluent containing anti–human globulin reagent and centrifuged. | Low-affinity autoantibodies |
| Agglutination of antibody-coated erythrocytes results in cells failing to migrate in the bottom of the column. | ||
| No cell washing is required, as serum/plasma is retained on top of the column. | ||
| 4°C Low-ionic-strength saline wash | Erythrocytes are washed with cold, low-ionic-strength saline to avoid removal of low-affinity antibodies. | Low-affinity autoantibodies |
| Polybrene | Polybrene induces aggregation of erythrocytes, which are dispersed by sodium citrate. | Erythrocyte-bound antibody below limit of detection of standard DAT |
| If antibody is present, erythrocytes will not disperse. | ||
| Flow cytometry | Erythrocytes incubated with anti–human globulin reagent are tagged with fluorescence and examined by flow cytometry. | Erythrocyte-bound antibody below limit of detection of standard DAT |
| Anti–human globulin reagents to IgG, IgA, and IgM are used. | IgA antibody | |
| Erythrocyte-bound IgM and monomeric IgM antibody | ||
| IgA | Anti-IgA is used instead of anti-IgG and anti-C3d. | IgA antibody |
| IgM | Anti-IgM is used instead of anti-IgG and anti-C3d. | IgM warm antibody and monomeric IgM |
| Name . | Description . | DAT-negative WAIHA detected . |
|---|---|---|
| Column agglutination | Erythrocytes are placed on a column of beads suspended in diluent containing anti–human globulin reagent and centrifuged. | Low-affinity autoantibodies |
| Agglutination of antibody-coated erythrocytes results in cells failing to migrate in the bottom of the column. | ||
| No cell washing is required, as serum/plasma is retained on top of the column. | ||
| 4°C Low-ionic-strength saline wash | Erythrocytes are washed with cold, low-ionic-strength saline to avoid removal of low-affinity antibodies. | Low-affinity autoantibodies |
| Polybrene | Polybrene induces aggregation of erythrocytes, which are dispersed by sodium citrate. | Erythrocyte-bound antibody below limit of detection of standard DAT |
| If antibody is present, erythrocytes will not disperse. | ||
| Flow cytometry | Erythrocytes incubated with anti–human globulin reagent are tagged with fluorescence and examined by flow cytometry. | Erythrocyte-bound antibody below limit of detection of standard DAT |
| Anti–human globulin reagents to IgG, IgA, and IgM are used. | IgA antibody | |
| Erythrocyte-bound IgM and monomeric IgM antibody | ||
| IgA | Anti-IgA is used instead of anti-IgG and anti-C3d. | IgA antibody |
| IgM | Anti-IgM is used instead of anti-IgG and anti-C3d. | IgM warm antibody and monomeric IgM |
Conclusion and challenges
The etiology of AIHA remains incompletely understood; however, the mechanisms of erythrocyte destruction and the clinical complications that accompany this disorder are well defined. The clinical heterogeneity of AIHA requires the clinician clearly define the nature of the disorder for each patient. In this article, we also emphasized approaches to AIHA where the DAT result is negative. The determination that the AIHA is warm or cold mediated does give significant insight into the potential clinical course and its management. We have provided the rationale and evidence basis for certain treatments and their relative hierarchy for use in the subtypes of AIHA. We have also highlighted the need to look for associated diseases, as their management may aid in AIHA therapy.
The usual options for treatment of warm-mediated AIHA with steroids with or without rituximab and or splenectomy can be considered to be standard practice and are very helpful in ∼80% of cases. However, the upfront management of CAD is typically less successful and remains a challenge. Tables 1 and 2 list the treatment options for relapsed or refractory warm- and cold-mediated AIHA, respectively. There is no clear consensus on the sequence or timing of these agents, so additional studies are needed to improve efficacy in the relapsed setting. Novel strategic therapies can be devised based on known pathophysiology that would improve on the decrease or removal of autoantibody production and/or reduce the phagocytosis of antibody/complement-coated erythrocytes. There is an ongoing phase 2 trial (NCT02612558) evaluating the use of a syk inhibitor, fostamatinib, in the therapy of refractory AIHA, a phase 3 trial of rituximab in upfront therapy of AIHA (NCT01181154), and a completed trial of low-dose rituximab plus prednisone (NCT01345708 and NCT00309881). Given the role of interaction of IgG with the Fc γ receptor [FcγR] or the neonatal Fc receptor [FcRn] in autoimmune disease, there is growing interest in blockade or modulation of these latter receptors with various formulations of intravenous immunoglobulin.86 These trials and investigations show that novel agents are being tested in AIHA to enhance our effective therapeutic repertoire and should expand our therapeutic repertoire in the coming years.
Authorship
Contribution: R.S.G., J.L.W., and N.E.K. designed research, performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Neil E. Kay, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kay.neil@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal