Key Points
Cdk5 function is required for optimal lymphocyte activation and migration following allo-HCT.
Targeting Cdk5 may be a particularly attractive strategy to reduce GVHD and maintain antitumor activity.
Abstract
Molecular intermediates in T-cell activation pathways are crucial targets for the therapy and prevention of graft-versus-host disease (GVHD) following allogeneic hematopoietic cell transplantation (allo-HCT). We recently identified an essential role for cyclin-dependent kinase 5 (Cdk5) in T-cell activation and effector function, but the contribution of Cdk5 activity to the development of GVHD has not been explored. Using an established, preclinical, murine, GVHD model, we reveal that Cdk5 activity is increased in key target organs early after allo-HCT. We then generated chimeric mice (Cdk5+/+C or Cdk5−/−C) using hematopoietic progenitors from either embryonic day 16.5 Cdk5+/+ or Cdk5−/− embryos to enable analyses of the role of Cdk5 in GVHD, as germ line Cdk5 gene deletion is embryonically lethal. The immunophenotype of adult Cdk5−/−C mice is identical to control Cdk5+/+C mice. However, transplantation of donor Cdk5−/−C bone marrow and T cells dramatically reduced the severity of systemic and target organ GVHD. This phenotype is attributed to decreased T-cell migration to secondary lymphoid organs (SLOs), reduced in vivo proliferation within these organs, and fewer cytokine-producing donor T cells during GVHD development. Moreover, these defects in Cdk5−/− T-cell function are associated with altered CCR7 signaling following ligation by CCL19, a receptor:ligand interaction critical for T-cell migration into SLOs. Although Cdk5 activity in donor T cells contributed to graft-versus-tumor effects, pharmacologic inhibition of Cdk5 preserved leukemia-free survival. Collectively, our data implicate Cdk5 in allogeneic T-cell responses after HCT and as an important new target for therapeutic intervention.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is the only curative therapy for many malignant and nonmalignant disorders. In addition to delivering effective anticancer treatment, the therapeutic potential of allo-HCT for hematologic malignancies relies on graft-versus-tumor (GVT) effects.1 Successful HCT outcomes are limited by several life-threatening complications; graft-versus-host-disease (GVHD) and malignant relapse are the 2 primary contributors to mortality. Unfortunately, GVT effects are closely associated with GVHD. Thus, the development of novel strategies that modulate immune dysregulation to reduce GVHD, preserve GVT activity, and facilitate immune reconstitution remain critical to enhancing survival after allo-HCT.
The pathophysiology of acute GVHD is complex.2-4 Experimental and clinical data support the hypothesis that immune dysregulation during GVHD occurs in distinct phases3 involving diffuse damage and inflammation from conditioning regimens, activation of donor T cells by host-derived antigen-presenting cells,5-7 and target organ injury by soluble and cellular effectors. Since its inception, components of this paradigm have been challenged and refined.4,8 Hence, this hypothesis continues to provide a conceptual framework within which novel therapeutic approaches can be explored.
Cyclin-dependent kinase 5 (Cdk5) is a unique, ubiquitously expressed, proline-directed, serine/threonine kinase whose diverse substrates include transcription factors, cytoskeletal proteins, and signaling molecules.9 Although Cdk5 shares homology with other cyclin-dependent kinases, it does not depend on association with cyclins for activity. Rather, Cdk5 interacts with its obligate partner proteins, p35 and p39,10-13 whose constitutive expression in postmitotic neurons is essential for the many known functions of Cdk5 in the regulation of cytoarchitecture, synaptic function, and dopamine signaling in the central nervous system. Physiologic Cdk5 activity is essential in neuronal development,14-17 memory, and neurogenesis,16 whereas aberrant hyperactivity of Cdk5 has been linked with neurodegenerative disorders11,18-21
Data from our laboratory and others have implicated Cdk5 in immune dysregulation13,22,23 and inflammatory pain signaling activated by tumor necrosis factor α (TNFα) through mechanisms that include transcriptional upregulation of p35.24,25 We recently demonstrated a role for Cdk5 in posttranslational modification of proteins triggered by T-cell receptor (TCR) and chemokine receptor signaling and required for optimal immune synapse formation, cellular activation, and migratory capacity. Protein phosphorylation by Cdk5 and other kinases can effect conformational changes through modification of binding motifs essential for recruiting proteins into signaling networks or by placing enzymes within proximity to substrates.26 Both tyrosine27 and serine/threonine28 kinases are key modulators during lymphocyte activation, and several novel small molecules designed to inhibit these kinases are currently under clinical investigation.29,30 Cdk inhibitors have shown activity in experimental models of inflammation31 and in improving outcomes in preclinical models of allo-HCT.32 However, the specific role of Cdk5 as a mediator of these events remains an important area of investigation.
Here, we report a key role for Cdk5 activity in the development of allogeneic T-cell responses after HCT. We developed a novel, chimeric, mouse model in which hematopoietic stem cells from Cdk5-deficient (Cdk5−/−) embryos are used to reconstitute lethally irradiated, adult mice (Cdk5−/−C). Transplantation of donor Cdk5−/−C bone marrow (BM) and T cells was associated with a significant reduction in the severity of systemic and target organ GVHD. We demonstrate decreased Cdk5−/−C T-cell migration to secondary lymphoid organs (SLOs), reduced Cdk5−/−C T-cell proliferation within these organs, and fewer cytokine-producing donor Cdk5−/−C T cells early after HCT. CCR7 signaling following ligation by CCL19 is altered in Cdk5−/−C T cells, which may contribute to defective migration into SLOs. Finally, although Cdk5 activity in donor T cells contributes to GVT effects, pharmacologic inhibition of Cdk5 optimized leukemia-free survival in a manner that includes suppression of Cdk5 activity in leukemia cells in vitro.
Material and methods
Mice, HCT, lymphocyte assays, and statistical analysis
All animal studies were approved by the institutional animal care and use committees at Case Western Reserve University (protocol 2010-0076) and Johns Hopkins University (protocol MO13M346). Female C57BL/6J (B6; H-2b) and B6D2F1 (F1; H-2bxd) mice aged 8 to 12 weeks were purchased from The Jackson Laboratory (Bar Harbor, ME). Detailed methods for the generation of Cdk5-null hematopoietic chimeras,13 immunoprecipitation and Cdk5 kinase activity assay,25 T-cell isolation and BM transplant (BMT) procedures,33 mixed lymphocyte reactions, T-cell proliferation and cytolytic activity,33,34 assessment of clinical35 and target organ GVHD,33 in vivo graft-versus-leukemia (GVL) models,33,34 bioluminescent imaging techniques,34,36 and statistical analysis have been previously described and are outlined in supplemental Methods (available on the Blood Web site).
In vivo T-cell migration, proliferation, and cytokine secretion
Assessment of in vivo T-cell migration, proliferation, and cytokine secretion is described in the legends of Figure 3 (migration and proliferation) and Figure 4 (cytokine secretion), respectively, and in supplemental Methods. To identify apoptotic cells, single-cell suspensions were stained with Annexin V–allophycocyanin (APC), 7 amino-actinomycin D (7AAD), and antibodies against CD4 or CD8, prior to analysis by Accuri C6 (BD Biosciences, San Jose, CA).
Western blot analysis
All tissues and single-cell suspensions were treated with Triton X-100 lysis buffer containing complete protease inhibitors (Roche, Indianapolis, IN) to generate protein lysate. Protein levels were measured using the Protein BCA Assay (Life Technologies, Grand Island, NY), and 2 to 5 µg of protein from cells were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions. Proteins were transferred to nitrocellulose membranes and probed with antibodies against extracellular signal-regulated kinase 1/2 (Erk1/2) (rabbit polyclonal; Cell Signaling Technology, Danvers, MA), phosphorylated Erk1/2 (pErk1/2) (Thr202/Tyr204, clone D13.14.4E; Cell Signaling Technology), phosphorylated MEK1/2 (pMEK1/2) (clone 41G9; Cell Signaling Technology), Cdk5 (clone sc-173; Santa Cruz Biotechnology, Dallas TX ), p35 (2680S; Cell Signaling Technology), β-actin (5125S; Cell Signaling Technology), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (clone EPR16891; Abcam, Cambridge, MA). Antibody detection was performed using West Dura (Life Technologies) and analysis using ChemiDoc and Quantity One software (Bio-Rad, Hercules, CA).
Results
Cdk5−/− T cells develop normally in vivo, exhibit mildly reduced alloreactivity and normal CTL function in vitro
Germ line deletion of the Cdk5 gene in mice is associated with embryonic lethality. Therefore, we generated Cdk5-null immune chimeric mice (Cdk5−/−C) as described in supplemental Methods.13 Three months after embryo transfer, spleen, lymph nodes (LNs), and thymus were harvested from fully engrafted, Cdk5+/+C and Cdk5−/−C animals and evaluated anatomically (spleen and LNs; Figure 1A) or by flow cytometry (spleen and thymus; Figure 1B). No differences between groups were noted in the architecture, cellularity, CD4+ and CD8+ phenotypes, or in the numbers of splenic natural regulatory T cells (nTregs) (Figure 1C). Similarly, no differences in Vβ usage were identified between groups (Figure 1D). In parallel experiments, spleens were collected from Cdk5+/+C and Cdk5−/−C mice and depleted of any remaining CD45.1 cells. T cells were then purified and stimulated in vitro. Functional characterization of Cdk5−/−C T cells in response to alloantigen stimulation showed only modest reductions in T-cell proliferation to higher concentrations of splenic dendritic cells (DCs) collected from B6D2F1 mice (Figure 1E), and no differences in cytolytic T lymphocyte (CTL) activity (Figure 1F) or cytokine production (data not shown) were noted between groups.
Immune reconstitution is intact in Cdk5−/−Chematopoietic chimeras. The spleen, LNs, and thymus were harvested from naive, fully engrafted, Cdk5+/+C (WT), and Cdk5−/−C (KO) mice and either sectioned and stained for anatomical evaluation (spleen and LN [A]) or enzymatically digested, dispersed into single-cell suspensions, stained for CD4 and CD8, and examined by flow cytometry (spleen and thymus [B]). No differences between groups were noted in architecture, cellularity, or CD4+ and CD8+ phenotypes. The numbers of nTregs in spleen were determined by staining single-cell suspension with CD4 and CD25 on surface followed by intracellular staining of FoxP3 (C). Vβ usage in T-cell populations was determined by staining single-cell suspensions of splenocytes with Vβ antibodies in combination with anti-CD4 and anti-CD8 (D). No differences in splenic Treg numbers or Vβ usage were noted. N = 3 to 6 mice per group. CD4+ and CD8+ T cells from or Cdk5−/−C mice were cultured with B6D2F1 splenic DCs for 96 hours and examined for proliferative capacity (E) and cytotoxicity (F) when 3H-thymidine–labeled, allogeneic (P815) or syngeneic (EL4) tumor cells were added. Data are representative of 1 of at least 3 replicate experiments. *P < .05. CPM, counts per minute.
Immune reconstitution is intact in Cdk5−/−Chematopoietic chimeras. The spleen, LNs, and thymus were harvested from naive, fully engrafted, Cdk5+/+C (WT), and Cdk5−/−C (KO) mice and either sectioned and stained for anatomical evaluation (spleen and LN [A]) or enzymatically digested, dispersed into single-cell suspensions, stained for CD4 and CD8, and examined by flow cytometry (spleen and thymus [B]). No differences between groups were noted in architecture, cellularity, or CD4+ and CD8+ phenotypes. The numbers of nTregs in spleen were determined by staining single-cell suspension with CD4 and CD25 on surface followed by intracellular staining of FoxP3 (C). Vβ usage in T-cell populations was determined by staining single-cell suspensions of splenocytes with Vβ antibodies in combination with anti-CD4 and anti-CD8 (D). No differences in splenic Treg numbers or Vβ usage were noted. N = 3 to 6 mice per group. CD4+ and CD8+ T cells from or Cdk5−/−C mice were cultured with B6D2F1 splenic DCs for 96 hours and examined for proliferative capacity (E) and cytotoxicity (F) when 3H-thymidine–labeled, allogeneic (P815) or syngeneic (EL4) tumor cells were added. Data are representative of 1 of at least 3 replicate experiments. *P < .05. CPM, counts per minute.
Allo-HCT using Cdk5−/−C donors results in significant reduction of GVHD
To define the impact of Cdk5 gene deletion on T-cell alloreactivity after HCT, we used an established, preclinical model wherein C57BL/6 (B6; H-2b) and B6D2F1 (H-2bxd) mice serve as HCT donors and recipients, respectively. In this model, both CD4+ and CD8+ T cells contribute to the development of reproducible systemic and target organ GVHD.33,34,37,38 We first assessed whether enhanced Cdk5 activity could be detected during early stages of GVHD as described in supplemental Methods.13 Cdk5 activity was notably increased by day 10 after allo-HCT in the spleen and small intestine, established sites of activation, and initial recruitment of donor T cells (Figure 2A).
Cd5k activity is critical for GVHD induction. Lethally irradiated B6D2F1 mice received HCT from either syngeneic B6D2F1 or allogeneic (B6) mice as described in supplemental Methods. Cdk5 kinase activity was significantly increased in the spleen and small intestine by day 10 after HCT (A). Allo-HCT using Cdk5−/−C (Allo Cdk5 ko) donors results in significant reduction in GVHD severity as measured by survival (B), clinical score (C), splenic T-cell expansion (D), serum IFNγ levels (E), and target organ histopathology (F) compared with recipients of Cdk5+/+C (Allo wt) donors. Data are from 2 to 3 comparable experiments: n = 3 to 4 mice per group (A), n = 12 to 24 mice per group (B-C), and 4 to 8 mice per group (D-F). *P < .01 for all comparisons.
Cd5k activity is critical for GVHD induction. Lethally irradiated B6D2F1 mice received HCT from either syngeneic B6D2F1 or allogeneic (B6) mice as described in supplemental Methods. Cdk5 kinase activity was significantly increased in the spleen and small intestine by day 10 after HCT (A). Allo-HCT using Cdk5−/−C (Allo Cdk5 ko) donors results in significant reduction in GVHD severity as measured by survival (B), clinical score (C), splenic T-cell expansion (D), serum IFNγ levels (E), and target organ histopathology (F) compared with recipients of Cdk5+/+C (Allo wt) donors. Data are from 2 to 3 comparable experiments: n = 3 to 4 mice per group (A), n = 12 to 24 mice per group (B-C), and 4 to 8 mice per group (D-F). *P < .01 for all comparisons.
BM and T cells isolated from Cdk5+/+C or Cdk5−/−C animals were transplanted into lethally irradiated B6D2F1 mice. HCT recipients of B6 Cdk5−/−C donors exhibited a dramatic reduction in mortality and systemic GVHD compared with mice receiving allo-Cdk5+/+C HCT (Figure 2B-C; P < .01). These findings were accompanied by a reduction in donor T-cell expansion on days 3, 7, and 14 in allo-Cdk5−/−C recipients (Figure 2D) correlating with decreases in day 7 serum interferon γ (IFNγ) levels (Figure 2E) and significant reductions in inflammation in key GVHD target organs (Figure 2F).
Although Cdk5−/−C mice show normal hematopoietic cell development,13 it remained possible that disruption of Cdk5 gene expression in myeloid-derived BM cells might contribute to the reduction in GVHD severity. We next performed mixing studies wherein T cells from Cdk5+/+C or Cdk5−/−C donors were added to or “mixed” with either Cdk5+/+C or Cdk5−/−C T-cell–depleted BM (in 1 of 4 possible combinations) prior to injection into irradiated F1 recipients. Our results indicate that Cdk5 activity in donor T cells and not BM cells was predominantly responsible for observed reduction in GVHD (Table 1).
Cdk5 activity in donor T cells is primarily responsible for reductions in GVHD
| Group . | GVHD score . | Survival, % . | ||
|---|---|---|---|---|
| Week 4 . | Week 5 . | Week 6 . | ||
| F1 → F1 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 100 |
| Allo WT T + Allo WT BM → F1 | 5.0 ± 0.4 | 5.2 ± 0.5 | 5.8 ± 0.1 | 13 |
| Allo WT T + Allo KO BM → F1 | 4.8 ± 0.6 | 4.6 ± 0.6 | 4.5 ± 0.5 | 33 |
| Allo KO T + Allo KO BM → F1 | 2.4 ± 0.3* | 2.5 ± 0.4* | 3.0 ± 0.5* | 73* |
| Allo KO T + Allo WT BM → F1 | 3.2 ± 0.8* | 2.6 ± 0.5* | 2.7 ± 0.4* | 60* |
| Group . | GVHD score . | Survival, % . | ||
|---|---|---|---|---|
| Week 4 . | Week 5 . | Week 6 . | ||
| F1 → F1 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 100 |
| Allo WT T + Allo WT BM → F1 | 5.0 ± 0.4 | 5.2 ± 0.5 | 5.8 ± 0.1 | 13 |
| Allo WT T + Allo KO BM → F1 | 4.8 ± 0.6 | 4.6 ± 0.6 | 4.5 ± 0.5 | 33 |
| Allo KO T + Allo KO BM → F1 | 2.4 ± 0.3* | 2.5 ± 0.4* | 3.0 ± 0.5* | 73* |
| Allo KO T + Allo WT BM → F1 | 3.2 ± 0.8* | 2.6 ± 0.5* | 2.7 ± 0.4* | 60* |
Lethally irradiated B6D2F1 received HCT from either syngeneic B6D2F1 or allogeneic Cdk5+/+C (Allo WT) or Cdk5−/−C (Allo KO) donors as described in Figure 2. Two additional allogeneic groups were evaluated in this “mixing” experiment as described in “Methods”: allogeneic KO BM cells mixed with allogeneic WT T cells and allogeneic WT BM cells mixed with allogeneic KO T cells. Survival was monitored daily and GVHD clinical scores were assessed weekly as described; n = 8 to 16 per group.
P < .01 compared with recipients of Cdk5+/+C HCT.
Disruption of Cdk5 gene expression impairs T-cell migration into SLO and T-cell activation in vivo
We next assessed the ability of Cdk5−/−C T cells to migrate to LNs and spleen after allo-HCT. Splenic T cells from Cdk5+/+C (wild-type [WT]) and Cdk5−/−C (knockout [KO]) mice were isolated and labeled with either carboxyfluorescein diacetate succinimidyl ester (CFSE) (WT) or SNARF-1 (KO) and 5 × 106 T cells from each group were coinjected into lethally irradiated F1 animals. A significant reduction in the percentage of Cdk5−/−C CD4+ and CD8+ T cells was observed in the spleen and LNs at 24 and 48 hours after injection (Figure 3A-B). In parallel, T cells from either Cdk5+/+C or Cdk5−/−C were labeled only with CFSE and separately injected (5 × 106) into lethally irradiated F1 mice. Spleens and LNs from recipients of Cdk5−/−C T cells analyzed at 72 hours showed significant reductions both in the percentage of cells dividing and the number of divisions observed (Figure 3C). When CFSE+ Cdk5+/+C or Cdk5−/−C T cells isolated 72 hours after injection were stained with Annexin V and 7AAD, we found no evidence for increased apoptosis in Cdk5−/−C T cells in spleen or LNs that would account for the marked reduction in proliferation seen in these organs (supplemental Figure 1).
Cdk5 regulates migrational and proliferative capacity of donor T cells after allo-HCT. Isolated Cdk5+/+C (WT) T cells were stained with 2.5 μL of CFSE and Cdk5−/−C (KO) T cells were stained with 5 μL of SNARF-1. Equal numbers of WT and KO T cells (3-5 × 106) were coinjected into lethally irradiated B6D2F1 mice. Spleens and LNs were isolated from recipient mice at 24 and 48 hours after injection and cells were stained with CD4 or CD8 fluorescent antibodies and examined by flow cytometry. The percentage of CD4+ and CD8+ from WT (CFSE+) and KO (SNARF-1+) and percentage reduction of cells from KO mice were determined (A-B). In vivo T-cell proliferation was assessed in parallel experiments. T cells from Cdk5+/+C or Cdk5−/−C were labeled with CFSE and 5 × 106 T cells were separately injected IV into lethally irradiated B6D2F1 mice. Seventy-two hours later, mice were sacrificed and the spleens were removed. Proliferating cells from WT or KO donors were identified based upon decreased staining for CFSE (C-D). Percentage reduction at 72 hours likely reflects effects of Cdk5 on both migration to SLOs and subsequent proliferation once present. N = 4 mice per group and represent 1 of at least 3 replicate experiments.
Cdk5 regulates migrational and proliferative capacity of donor T cells after allo-HCT. Isolated Cdk5+/+C (WT) T cells were stained with 2.5 μL of CFSE and Cdk5−/−C (KO) T cells were stained with 5 μL of SNARF-1. Equal numbers of WT and KO T cells (3-5 × 106) were coinjected into lethally irradiated B6D2F1 mice. Spleens and LNs were isolated from recipient mice at 24 and 48 hours after injection and cells were stained with CD4 or CD8 fluorescent antibodies and examined by flow cytometry. The percentage of CD4+ and CD8+ from WT (CFSE+) and KO (SNARF-1+) and percentage reduction of cells from KO mice were determined (A-B). In vivo T-cell proliferation was assessed in parallel experiments. T cells from Cdk5+/+C or Cdk5−/−C were labeled with CFSE and 5 × 106 T cells were separately injected IV into lethally irradiated B6D2F1 mice. Seventy-two hours later, mice were sacrificed and the spleens were removed. Proliferating cells from WT or KO donors were identified based upon decreased staining for CFSE (C-D). Percentage reduction at 72 hours likely reflects effects of Cdk5 on both migration to SLOs and subsequent proliferation once present. N = 4 mice per group and represent 1 of at least 3 replicate experiments.
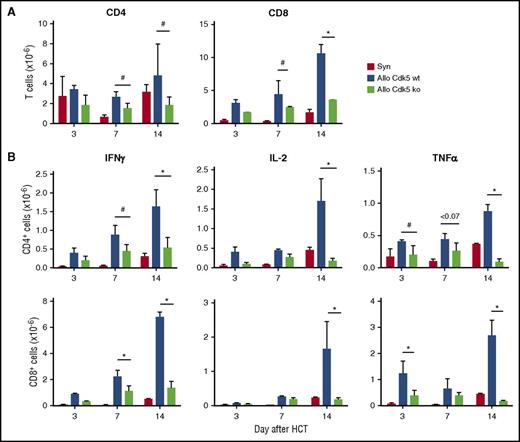
Finally, we determined the effect of Cdk5 activity on donor T-cell expansion and cytokine production 3, 7, and 14 days after transplant. We found fewer splenic CD4+ and CD8+ T cells in recipients of allo-Cdk5−/−C HCT (Figure 4A), and a reduction in the number of cells producing IFNγ, interleukin 2 (IL2), and TNFα compared with cells isolated from Cdk5+/+C HCT recipients (Figure 4B). Further evaluation of splenic lymphocytes collected from recipients of allo-Cdk5+/+C HCT on day 14 showed a preferential expansion of CD8+ cells with an activated phenotype (supplemental Figure 2). By day 42, the splenic T-cell compartment of syngeneic animals was predominately composed of CD4+ cells with a naive phenotype (CD44loCD62Lhi). Splenic T cells in surviving recipients of allo-HCT from Cdk5+/+C donors remained predominately CD44hiCD62lo CD8+, whereas recipients of Cdk5−/−C T cells showed a phenotype that was similar to syngeneic mice (supplemental Figure 2).
Loss of Cdk5 expression results in fewer donor-derived, cytokine-producing T cells early after HCT. Lethally irradiated B6D2F1 received HCT from either syngeneic (B6D2F1) or allogeneic Cdk5+/+C (Cdk5 wt) or Cdk5−/−C (Cdk5 ko) donors as described in Figure 2. HCT recipients were sacrificed and single-cell suspensions were prepared from spleen of mice 3, 7, 14 days posttransplant. Total numbers of donor-derived, CD4+ and CD8+ T lymphocytes were determined in Kd− or Kd+ cell populations in spleens of mice receiving allo- or syngeneic HCT, respectively (A). Cells were also incubated on anti-CD3–coated plates for 6 hours in RPMI1640 + 10% fetal bovine serum (FBS) containing 1 µg/mL brefeldin A, subsequently stained with anti-CD4 and anti-CD8, and incubated with fluorescent anti-cytokine antibodies. Total numbers of donor-derived, CD4+ and CD8+ T cells were counted and examined for the production of IFNγ, IL-2, and TNFα (B). Data are representative of 1 of 3 replicate experiments. N = 3 to 4 mice per group. *P < .01, #P < .05 or as otherwise noted.
Loss of Cdk5 expression results in fewer donor-derived, cytokine-producing T cells early after HCT. Lethally irradiated B6D2F1 received HCT from either syngeneic (B6D2F1) or allogeneic Cdk5+/+C (Cdk5 wt) or Cdk5−/−C (Cdk5 ko) donors as described in Figure 2. HCT recipients were sacrificed and single-cell suspensions were prepared from spleen of mice 3, 7, 14 days posttransplant. Total numbers of donor-derived, CD4+ and CD8+ T lymphocytes were determined in Kd− or Kd+ cell populations in spleens of mice receiving allo- or syngeneic HCT, respectively (A). Cells were also incubated on anti-CD3–coated plates for 6 hours in RPMI1640 + 10% fetal bovine serum (FBS) containing 1 µg/mL brefeldin A, subsequently stained with anti-CD4 and anti-CD8, and incubated with fluorescent anti-cytokine antibodies. Total numbers of donor-derived, CD4+ and CD8+ T cells were counted and examined for the production of IFNγ, IL-2, and TNFα (B). Data are representative of 1 of 3 replicate experiments. N = 3 to 4 mice per group. *P < .01, #P < .05 or as otherwise noted.
Cdk5 activity regulates CCR7 signaling
Our data indicate that Cdk5 activity is required for optimal lymphocyte migration and activation in vivo (Figures 3 and 4). We have previously shown that Cdk5 gene deletion impairs T-cell migration to CCL19 in vitro.13 CCL19 specifically binds to CCR7 and is important in directing the migration of naive T cells to SLOs.39 Intracellularly, the ERK pathway contributes to CCL19- and CCL21-generated signals governing cell survival and migration,40-42 and it is known that Cdk5 can suppress ERKs through direct action on a novel site in the MAPK/ERK kinase (MEK).43 Although ligation of CCR7 by either CCL19 or CCL21 activates the ERK1/2 pathway, CCL19 has been shown to be fourfold more potent in promoting ERK phosphorylation.44 We found no difference in the surface expression of CCR7 on peripheral blood T cells from Cdk5+/+C and Cdk5−/−C mice when examined by flow cytometry (Figure 5A). To determine how Cdk5 contributes to CCL19-induced changes in ERK1/2 phosphorylation, purified T cells from Cdk5+/+C (WT) or Cdk5−/−C (KO) mice were incubated with CCL19, and cell lysates were examined by western blot to measure expression of pERK1/2 and total ERK1/2 (Figure 5B1). The relative increase in the expression of pERK/ERK in both Cdk5+/+C and Cdk5−/−C T cells (Figure 5B2) and the percentage reduction of pERK expression in Cdk5−/−C T cells were determined at each time point (Figure 5B3). Both Cdk5+/+C and Cdk5−/−C T cells expressed similar levels of total ERK-1/2 protein. However, analysis of the kinetics of ERK1/2 phosphorylation shows both delayed and reduced total phosphorylation of ERK1/2 in Cdk5−/−C T cells when compared with Cdk5+/+C T cells. ERK activation is regulated by MEK, and the MEK-ERK axis is also known to be modulated by Cdk5.45,46 We observed a similar delay in the kinetics of MEK phosphorylation in Cdk5−/−C T cells following exposure to 100 ng/mL CCL19 (Figure 5C).
Patterns of phosphorylation of known intermediates of CCR7 intracellular signaling are altered in CDk5-deficient T cells. Cell surface expression of CCR7 on peripheral blood CD4+ and CD8+ T cells was examined by flow cytometry after labeling cells with CCL19-Fc followed by anti-human immunoglobulin G (IgG)-phycoerythrin (PE) and either CD4-APC or CD8-APC (A). Next, purified T cells from Cdk5+/+C (Cdk5 WT) or Cdk5−/−C (Cdk5 KO) mice were incubated with 100 ng/mL CCL19 for 0 to 4 minutes and then lysed with Triton X-100 buffer containing phosphatase/protease inhibitors. Total cell lysate (4 μg) was separated on a 4% to 12% Bis-Tris gel and transferred to nitrocellulose. Cell lysates were examined by western blot using antibodies against pERK1/2 to measure levels of phosphorylated protein and ERK1/2 to measure levels of pERK1/2 and total ERK1/2 loaded (B1). The relative increase in expression of pERK/ERK in lysates from either WT or KO T cells was determined at each time point using time 0 for respective samples (eg, 100%) as baseline (B2). The percentage reduction of pERK expression in KO T cells was also determined at each time point (B3). In separate experiments, purified T cells from WT or KO mice were again incubated with 100 ng/mL CCL19 and cell lysates were examined for expression of pMEK1/2 and GAPDH (C). Data shown are from 1 of 3 replicate experiments.
Patterns of phosphorylation of known intermediates of CCR7 intracellular signaling are altered in CDk5-deficient T cells. Cell surface expression of CCR7 on peripheral blood CD4+ and CD8+ T cells was examined by flow cytometry after labeling cells with CCL19-Fc followed by anti-human immunoglobulin G (IgG)-phycoerythrin (PE) and either CD4-APC or CD8-APC (A). Next, purified T cells from Cdk5+/+C (Cdk5 WT) or Cdk5−/−C (Cdk5 KO) mice were incubated with 100 ng/mL CCL19 for 0 to 4 minutes and then lysed with Triton X-100 buffer containing phosphatase/protease inhibitors. Total cell lysate (4 μg) was separated on a 4% to 12% Bis-Tris gel and transferred to nitrocellulose. Cell lysates were examined by western blot using antibodies against pERK1/2 to measure levels of phosphorylated protein and ERK1/2 to measure levels of pERK1/2 and total ERK1/2 loaded (B1). The relative increase in expression of pERK/ERK in lysates from either WT or KO T cells was determined at each time point using time 0 for respective samples (eg, 100%) as baseline (B2). The percentage reduction of pERK expression in KO T cells was also determined at each time point (B3). In separate experiments, purified T cells from WT or KO mice were again incubated with 100 ng/mL CCL19 and cell lysates were examined for expression of pMEK1/2 and GAPDH (C). Data shown are from 1 of 3 replicate experiments.
The contribution of Cdk5 to GVT activity
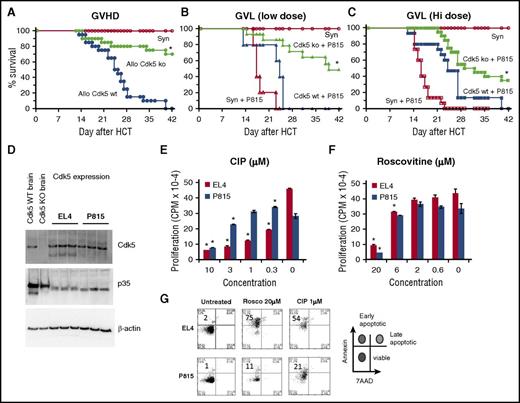
Given the observed impact disruption of Cdk5 activity had on donor T-cell responses after allo-HCT, it was critical to define how loss or inhibition of Cdk5 activity would affect GVT effects. Irradiated B6D2F1 mice received HCT from either allogeneic B6 (Cdk5−/−C or Cdk5+/+C) or syngeneic (F1) donors, and a reduction in systemic GVHD was again noted (Figure 6A). Next, an initial dose of 250 P815 tumor cells (H-2d) was coinfused with the BM inoculum on day 0. Survival was monitored daily and the cause of each death was determined to be either GVHD or tumor. All syngeneic HCT recipients uniformly died of widely disseminated, P815 tumor cell infiltration by day 25 whereas recipients of Cdk5+/+C allo-HCT effectively rejected their tumor but died of GVHD. By contrast, allo-HCT recipients of Cdk5−/−C donors exhibited a consistent reduction in GVHD severity, preservation of GVT activity, and improved survival (Figure 6B). To better ascertain the potency of these GVT effects, we doubled the tumor cell inoculum. Allo-HCT from Cdk5−/−C donors still resulted in significant antitumor activity, but the survival advantage was reduced (Figure 6C).
Effects of Cdk5 gene deletion on GVL activity. Lethally irradiated B6D2F1 mice received HCT from either syngeneic (B6D2F1) or allogeneic Cdk5+/+C (Allo Cdk5 WT) or Cdk5−/−C (Allo Cdk5 KO) donors as described in Figure 4. Consistent with previous experiments, animals receiving HCT from Cdk5−/−C donors have significantly reduced mortality from GVHD (A). In subsequent experiments, groups of HCT recipients received 250 or 500 P815 tumor cells at time of transplant. Recipients of BM and T cells from Cdk5 KO donors effectively eliminate low-dose tumor challenge and show improved leukemia-free survival (B). At higher tumor dose, significant GVL activity remains, but some Cdk5 KO HCT recipients succumb to tumor (C). The expression of Cdk5 and p35 was determined on P815 and EL4 tumor cell lysate using western blot analysis (D). Tumor cells (104 cells per well) were incubated with 3H-Thy for 24 hours in the presence or absence of roscovitine or CIP and cell proliferation was determined (E-F). In parallel experiments, tumor cells incubated with roscovitine or CIP for 24 hours were stained with Annexin V and 7AAD and the percentage of early apoptotic cells was determined (G). Data are from at least 2 combined experiments: n = 8 to 12 per group (A-C), or 1 of at least 2 comparable experiments (E-F). *P < .01.
Effects of Cdk5 gene deletion on GVL activity. Lethally irradiated B6D2F1 mice received HCT from either syngeneic (B6D2F1) or allogeneic Cdk5+/+C (Allo Cdk5 WT) or Cdk5−/−C (Allo Cdk5 KO) donors as described in Figure 4. Consistent with previous experiments, animals receiving HCT from Cdk5−/−C donors have significantly reduced mortality from GVHD (A). In subsequent experiments, groups of HCT recipients received 250 or 500 P815 tumor cells at time of transplant. Recipients of BM and T cells from Cdk5 KO donors effectively eliminate low-dose tumor challenge and show improved leukemia-free survival (B). At higher tumor dose, significant GVL activity remains, but some Cdk5 KO HCT recipients succumb to tumor (C). The expression of Cdk5 and p35 was determined on P815 and EL4 tumor cell lysate using western blot analysis (D). Tumor cells (104 cells per well) were incubated with 3H-Thy for 24 hours in the presence or absence of roscovitine or CIP and cell proliferation was determined (E-F). In parallel experiments, tumor cells incubated with roscovitine or CIP for 24 hours were stained with Annexin V and 7AAD and the percentage of early apoptotic cells was determined (G). Data are from at least 2 combined experiments: n = 8 to 12 per group (A-C), or 1 of at least 2 comparable experiments (E-F). *P < .01.
Recent studies have uncovered a specific role for Cdk5 in cancer biology and tumor invasiveness47-49 and Cdk5 is highly expressed in a variety of tumors.47,50-52 These observations suggest that pharmacologic inhibition of Cdk5 activity might optimize outcomes after HCT not only through suppression of T-cell alloreactivity and GVHD, but also through direct antitumor effects. The latter may in fact serve to compensate for any reduction in GVT activity as a consequence of Cdk5 gene deletion. Western blot analysis demonstrated the expression of Cdk5 and p35 on P815 and EL4 tumor cell lysates (Figure 6D). To assess the impact of Cdk5 inhibition on tumor cell replication and viability in vitro, tumor cells (104 cells per well) were incubated with 3H-Thy for 24 hours in the presence or absence of a nonselective Cdk inhibitor (roscovitine) or the Cdk5 inhibitory peptide (CIP), which is specific for Cdk5.53 As shown in Figure 6E-F, a dose-dependent reduction in proliferation of both cell lines was observed in each scenario. In parallel experiments, we found that exposure to these Cdk5 inhibitors also increases the percentage of early apoptotic cells as measured by Annexin V and 7AAD (Figure 6G).
In a final set of studies, irradiated F1 mice received HCT from either syngeneic (F1) or allogeneic (naive B6) donors. The P815 cells used in these experiments expressed a luciferase reporter36 and were added to the donor cells on day 0. Subsets of HCT mice received roscovitine 10 mg intraperitoneally daily for 21 days and all animals were followed for survival, clinical score, and tumor burden by bioluminescence imaging as described in supplemental Methods. As anticipated, susceptible, syngeneic animals rapidly succumbed to tumor whereas allo-HCT recipients benefited from significant GVT activity (Figure 7A). Consistent with previous reports,32 allogeneic mice treated with roscovitine maintained antitumor effects comparable to allogeneic controls and had a significant reduction in GVHD clinical score (Figure 7B). Importantly, the dose and schedule of roscovitine administered nonselectively inhibited Cdk5 activity in spleens of allo-HCT recipients 7 days after HCT (Figure 7C) and had no significant effect on survival of syngeneic mice receiving P815; all animals died of tumor dissemination before day 20 (median survival time, 16 days for syngeneic + P815 + roscovitine and syngeneic + P815).
Pharmacologic inhibition of Cdk5 after HCT reduces clinical GVHD severity and while maintaining potent GVL effects. Lethally irradiated B6D2F1 mice received HCT from either syngeneic B6D2F1 or allogeneic (B6) mice as described in Figure 2. Groups of mice received 500 P815 cells previously transduced using a lentiviral vector carrying a luciferase reporter that allows visualization of proliferating cells using bioluminescence imaging (BLI). Syngeneic and allo-HCT recipients were monitored for tumor progress (A) and clinical GVHD severity (B). Roscovitine, at the dose and schedule administered after HCT effectively inhibited Cdk5 activity measured in spleens of mice collected on day 7 posttransplant (C). Data shown are representative of 2 replicate experiments: n = 5 to 12 mice per group; P < .01. Note: A single mouse in the allo-HCT group receiving roscovitine unexpectedly died following anesthesia for BLI and was censored “C”. Radiance is expressed as p/sec/cm2/sr. Color scale: Min = 2.00e4, Max = 2.00e5.
Pharmacologic inhibition of Cdk5 after HCT reduces clinical GVHD severity and while maintaining potent GVL effects. Lethally irradiated B6D2F1 mice received HCT from either syngeneic B6D2F1 or allogeneic (B6) mice as described in Figure 2. Groups of mice received 500 P815 cells previously transduced using a lentiviral vector carrying a luciferase reporter that allows visualization of proliferating cells using bioluminescence imaging (BLI). Syngeneic and allo-HCT recipients were monitored for tumor progress (A) and clinical GVHD severity (B). Roscovitine, at the dose and schedule administered after HCT effectively inhibited Cdk5 activity measured in spleens of mice collected on day 7 posttransplant (C). Data shown are representative of 2 replicate experiments: n = 5 to 12 mice per group; P < .01. Note: A single mouse in the allo-HCT group receiving roscovitine unexpectedly died following anesthesia for BLI and was censored “C”. Radiance is expressed as p/sec/cm2/sr. Color scale: Min = 2.00e4, Max = 2.00e5.
Discussion
GVHD and malignant disease relapse remain the primary causes of death following allo-HCT, and novel strategies that modulate alloreactivity without compromising GVT activity are being pursued. We examined whether targeted disruption of Cdk5, a serine-threonine kinase known to be involved in neurodegenerative and inflammatory disorders, might mitigate the toxicity of GVHD while preserving antitumor effects. We first generated chimeric mice that lack Cdk5 expression in all immune cells because germ line deletion of Cdk5 is embryonically lethal.13 Immunophenotyping of theses hematopoietic chimeras (Cdk5−/−C) showed no difference in structure, cellularity, or lineage distribution in hematopoietic organs (BM, thymus, spleen, or LN) compared with Cdk5+/+C mice. Although immunologically intact, our prior studies demonstrated that Cdk5−/−C mice are less susceptible to experimental autoimmune encephalomyelitis.13 Proteomic analysis of activated T cells revealed that a major target of Cdk5 phosphorylation is coronin-1a, an adapter protein that links cytoskeleton dynamics to the TCR and TCR signaling. Specifically, phosphorylation of a critical threonine 418 was absent in T cells of Cdk5−/−C mice and associated with a reduction in F-actin–mediated polarization of the T-cell membrane following TCR ligation. As with lymphocytes deficient in coronin-1a,54 pharmacologic suppression and genetic deletion of Cdk5 resulted in reduced migration in response to CCL19, a major ligand for CCR7.
Data presented herein demonstrate that although lymphocytes from Cdk5−/−C mice exhibit slight reductions in proliferation to alloantigen and no decrements in cytolytic function in vitro, they are significantly impaired in their capacity to initiate and sustain the clinical hallmarks of GVHD in vivo. These findings were associated with a reduction in T-cell migration into SLOs, diminished proliferation, and lower numbers of cytokine-producing T cells in these tissues. The difference in the number of Cdk5−/−C T cells present in SLOs did not reflect changes in T-cell survival during the early phase of activation; both Cdk5+/+C and Cdk5−/−C T cells show similar percentages of cells that are Annexin V+. Analysis of splenic T cells from mice surviving 6 to 7 weeks after HCT suggests a failure of Cdk5−/−C T cells to maintain an effector phenotype, and donor chimerism is robust in both Cdk5+/+C and Cdk5−/−C BMT recipients (97% ± 3% vs 99% ± 0.7%, respectively) at this time. Although Cdk5+/+C and Cdk5−/−C T cells were predominately CD8+ and CD44hi 2 weeks after HCT, there was a shift toward CD44lo cells by 6 weeks posttransplant with Cdk5−/−C T cells. The reduction in the expression of CD44 suggests that these cells are naive, as a transition from effector to memory cells will maintain CD44 expression.55 In aggregate, our results demonstrate that Cdk5 activity contributes to optimal lymphocyte activation and migration and suggest a potential role for Cdk5 in the generation and maintenance of memory T cells. Studies are planned to more definitely test this hypothesis.
The SLOs are a major site of T-cell activation during GVHD. Factors that can influence interaction between host antigen-presenting cells and donor T cells in SLOs reduce GVHD severity. Several chemokines contribute to the recruitment of lymphocytes to SLOs in HCT and non-HCT settings (reviewed in Cooke et al39 ). CCR7 is a G protein–coupled receptor expressed on naive T cells (both conventional and regulatory), B cells, and DCs that binds to 2 structurally unique ligands, CCL19 and CCL21.56-58 CCR7 is the central regulator of homing and trafficking of lymphocytes into SLOs including LNs and the splenic white pulp. Ligation of CCR7 on T cells by CCL21 allows for lymphocyte firm arrest on LN high endothelial venules.56 After egress from the circulation, CCR7 also direct T cells to appropriate subregions within LNs and spleen in response to CCL19.39 Our observations are very similar to those reported using mice deficient in CCR7 as allo-HCT donors.59 CCR7−/− T cells also exhibit an impaired ability to traffic to recipient LNs and a reduced ability to undergo in vivo expansion in the spleen.59 Moreover, we found that phosphorylation patterns of key downstream intermediates of CCR7 signaling (following CCL19 binding) are significantly altered (ERK1/ERK2) or delayed (MEK) in T cells isolated from Cdk5−/−C mice (Figure 7). We did not complete similar studies in peripheral tissues. However, semiquantitative subset analyses show that mononuclear cell infiltration into the gut and liver was reduced in mice receiving BMT from Cdk5−/−C donors compared with littermate controls (intestine, 5.0 ± 0.4 vs 8.6 ± 0.5; liver, 2.3 ± 0.3 vs 4.4 ± 0.2; P < .05). It remains unresolved whether a reduction of lymphocytes into GVHD target organs is secondary to diminished donor T-cell expansion in SLOs or a direct effect of Cdk5 on effector cell migration to inflamed tissue.
An emerging body of data implicate Cdk5 activity in processes and conditions as diverse as neurodegenerative disease,11,17 inflammation,13,22,23,31 cancer biology,47,50-52 and tumor invasiveness.48,49 Cdk5 was the top hit of 37 genes that when silenced synergistically potentiate effects of proteosome inhibition in myeloma cells,47 and a cell-based, high-content, screening assay determined that Cdk5 was the biologic target of several cyclin-depending kinase inhibitors that could modulate cancer cell invasion capacity.48 Cdk5 is highly expressed in a variety of tumors47,50-52 and several clinical trials studying the use of roscovitine and other nonselective Cdk5 inhibitors in patients with cancer have either been completed or are ongoing.60-66 Our studies demonstrated that the Cdk5-specific inhibitor CIP resulted in a dose-dependent reduction in tumor cell proliferation and an increase in the percentage of early apoptotic cells in culture (Figure 6). The optimal formulation of CIP for in vivo administration has yet to be determined. A limitation to our in vivo GVL studies is that roscovitine is a broadly acting Cdk inhibitor potently inhibiting Cdk1, 2, 5, and 7.67 Via its effects on Cdk2, roscovitine has been shown to reduce human alloreactive responses without impairing key responses to tumor (WT-1) or viral (cytomegalovirus, Epstein-Barr virus) antigens.68 Furthermore, defective activation of Cdk2 (along with Cdk4 and 6) has been associated with tolerance induced by IL-10 and transforming growth factor β69 and with a reduction in experimental GVHD.32 These data notwithstanding, the administration of roscovitine at the dose and schedule delivered did effectivity abrogate Cdk5 activity and, consistent with a previous report,32 was associated with a reduction in clinical GVHD and preservation of GVL effects (Figure 7). Finally, any strategy to modulate GVHD severity that focuses on T-cell activation or migration (to SLOs or peripheral organs) may influence (negatively) responses to opportunistic infection. Although Nellore and colleagues assessed the effects of roscovitine on human T-cell responses to cytomegalovirus and Epstein-Barr virus in vitro, future lines of investigation to study the impact Cdk5 gene deletion has on infectious immunity in vivo would have significant merit.68
The potential utility of these agents in the setting of inflammatory and immune disorders is high given the increasing appreciation for the roles that cyclin-dependent kinases play in T-cell biology.70 For example, Foxp3 stability is regulated by Cdk2 through phosphorylation of 4 cyclin-dependent kinase motifs (Ser/Thr-Pro) within the N-terminal repressor domain,71 and we have shown the capacity of IL-6 to suppress activation of Foxp3 expression by transforming growth factor β requires Cdk5-dependent phosphorylation of STAT3 on serine 727.72 Moreover, we have also shown that Cdk5 controls IL-2 gene expression via repression of the mSin3a–histone deacetylase complex during T-cell activation.73 Finally, Cdk5 activity may also significantly contribute to inflammation engendered by the innate immune response, which is also operative during GVHD.33 Cdk5 activity was notably increased in macrophages stimulated by lipopolysaccharide through the synthesis of its binding partner p35. Specifically, Cdk5 activity enhances the inflammatory function of macrophage by regulating MAPK-dependent production of suppressor of cytokine signaling-3 and IL-10.74 Although experiments mixing BM from Cdk5−/−C donors with Cdk5+/+C T cells resulted in a modest improvement in survival after allo-HCT (33% vs 13%, Table 1), systemic GVHD as measured by clinical score was not different between groups underscoring the predominant role of Cdk5 activity in the T-cell compartment as a determinant of GVHD severity.
In summary, the accumulating evidence regarding Cdk5-dependent T-cell function has important implications for a broad spectrum of immune and inflammatory disorders. The data presented here suggest that targeting Ckd5 may be a particularly attractive strategy to reduce GVHD and maintain (or enhance) antitumor activity. Hence, our results not only further illuminate the role and mechanism of Cdk5 in modulating GVHD but also provide a rationale for developing innovative strategies using (selective or nonselective) small-molecule inhibitors of Cdk5 to prevent or treat this lethal complication in the context of carefully controlled clinical trials. The relevance of the latter is quite high with an expanding industry effort to develop specific Cdk inhibitors, including small molecules already in early-phase cancer trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Matthew Keller, Roccio Guirdo-Wolff, and Jay Meyers for their technical assistance with experiments contributing to this manuscript, and Chen Liu for coded review of target organ histopathology.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grant R01HL111682-03 (J.J.L., A.Y.H., K.R.C.) and the Spark of Hope Foundation (K.R.C.). J.J.L. was supported by the Jane and Lee Seidman Chair in Pediatric Cancer Innovation; K.R.C. was supported by the Herman and Walter Samuelson Chair in Oncology; and D.A. was supported by the Steven G. AYA Cancer Research Fund.
Authorship
Contribution: D.A., J.J.L., A.Y.H., T.K.P., and K.R.C. conceived and designed the study; D.A., T.K.P., S.E., S.G., M.T., J.J.L., and K.R.C. generated, collected, and/or assembled data; D.A., T.K.P., J.J.L., A.Y.H., and K.R.C. analyzed and interpreted data; D.A., J.J.L., and K.R.C. wrote the manuscript; K.R.C., J.J.L., and A.Y.H. provided financial support; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenneth R. Cooke, Bunting-Blaustein Cancer Research Building (CRB1), 1650 Orleans St, Room 207, Baltimore, MD 21287; e-mail: kcooke5@jhmi.edu.
References
Author notes
J.J.L. and K.R.C. are joint senior authors.

![Figure 1. Immune reconstitution is intact in Cdk5−/−C hematopoietic chimeras. The spleen, LNs, and thymus were harvested from naive, fully engrafted, Cdk5+/+C (WT), and Cdk5−/−C (KO) mice and either sectioned and stained for anatomical evaluation (spleen and LN [A]) or enzymatically digested, dispersed into single-cell suspensions, stained for CD4 and CD8, and examined by flow cytometry (spleen and thymus [B]). No differences between groups were noted in architecture, cellularity, or CD4+ and CD8+ phenotypes. The numbers of nTregs in spleen were determined by staining single-cell suspension with CD4 and CD25 on surface followed by intracellular staining of FoxP3 (C). Vβ usage in T-cell populations was determined by staining single-cell suspensions of splenocytes with Vβ antibodies in combination with anti-CD4 and anti-CD8 (D). No differences in splenic Treg numbers or Vβ usage were noted. N = 3 to 6 mice per group. CD4+ and CD8+ T cells from or Cdk5−/−C mice were cultured with B6D2F1 splenic DCs for 96 hours and examined for proliferative capacity (E) and cytotoxicity (F) when 3H-thymidine–labeled, allogeneic (P815) or syngeneic (EL4) tumor cells were added. Data are representative of 1 of at least 3 replicate experiments. *P < .05. CPM, counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/2/10.1182_blood-2016-05-702738/4/m_blood702738f1.jpeg?Expires=1767707439&Signature=LNNNo-dOjZWLFxN5htSvi4O62JvOVkOdYcqsOoLteYrFMDwUX8UVzRT2hCnKLsBwkYL0ERbB73~60A-y1AmOn9sIeSfke-xbNmuj~ZGXfcT2gRe3~s7TcYJM0-tdVJZ52L7KMlI~m8lXYrt3F3Axm81g8qlLZaL4EF4Lf4C2-fqKq9zl~gHs9Mp2DuEezW4rPfWnQEK1zjDSrGPxFy4mE8iwtO~9af8Mw2gNI4sjkAEzU4h5VA2Z-w-AR1BtRSbzi4W-woOfVeRFKSBCWbwgXyOTpIQ7lWZioBF9HLVfmEyEDEjKJ4rKD9TH93RO6Z7hMzqJ0ZN4beePj80F4wCGwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)