Key Points
Uremic solute IS increases platelet activity via activation of ROS/p38MAPK signaling.
Klotho counteracts IS-induced thrombosis by restraining platelet hyperactivity.
Abstract
Thrombosis is a common complication of chronic kidney disease (CKD), but the causes and mechanisms of CKD-associated thrombosis are not well clarified. Here, we show that platelet activity is remarkably enhanced in CKD mice, with increase of serum indoxyl sulfate (IS), a typical uremic toxin, which cannot be effectively cleared by routine dialysis. Ex vivo and in vitro experiments reveal that IS displays a distinct ability to enhance platelet activities, including elevated response to collagen and thrombin, increases in platelet-derived microparticles, and platelet-monocyte aggregates. The flow chamber assay and carotid artery thrombosis model demonstrate that IS-induced platelet hyperactivity contributes to thrombus formation. Further investigations disclose that reactive oxygen species (ROS)-mediated p38MAPK signaling plays a key role in IS-induced platelet hyperactivity. Moreover, we show that Klotho, which is expressed dominantly in the kidneys, has the capacity to counteract IS-induced platelet hyperactivity by inhibiting ROS/p38MAPK signaling, whereas Klotho reduction may aggravate the effect of IS on platelet activation in CKD and klotho+/− mice. Finally, we demonstrate that Klotho protein treatment can protect against IS-induced thrombosis and atherosclerosis in apoE−/− mice. Our findings uncover the mechanism of platelet hyperactivity induced by IS and provide new insights into the pathogenesis and treatment of CKD-associated thrombosis.
Introduction
Chronic kidney disease (CKD) is a serious health problem that affects millions of people worldwide.1 Thrombosis is a common complication of CKD and thrombosis-related damages are main causes of mortality in CKD patients.2-4 It is well known that platelet hyperactivity plays a fundamental role in thrombosis. Besides releasing granule contents and microparticles that are procoagulant,5,6 activated platelets are prone to bind leukocytes, preferentially monocytes, to form platelet-leukocyte/monocyte aggregates, which are also conducive to thrombus formation.7-9 However, little is known about changes in platelet activity in the CKD environment and their contribution to thrombosis.
In CKD patients, an important characteristic is that oxidative stress is progressively enhanced with the impairment of renal function. Interestingly, oxidative stress has been confirmed to be a key regulator for platelet activation.10 The enhanced oxidative stress is mainly ascribed to the accumulation of metabolites and the reduction of anti-oxidative factors produced by the kidney, such as the Klotho protein.11-13 It has been reported that oxidative stress plays an important role in the pathophysiological development of CKD and its complications, including thrombosis and atherosclerosis.13,14
Indoxyl sulfate (IS) is a typical uremic toxin derived from indole, a metabolite of tryptophan produced by the intestinal flora. More importantly, as a kind of protein (albumin)-bound uremic solute, IS cannot be effectively cleared by routine dialysis.15 So, the serum IS increases significantly during the progression of CKD in the patients with or without dialysis. It has been shown that IS has a strong pro-oxidative activity by inducing reactive oxygen species (ROS) production. Recent studies, including our own, revealed that IS-induced oxidative stress cannot only damage renal cells and aggravate the progression of CKD,16-18 but also has harmful effects on other tissues, such as inducing vascular endothelial damage and myocardial hypertrophy.19,20 In addition, serum IS associates with dialysis graft thrombosis after endovascular interventions.21 However, the effect of IS on platelet activity and its role in the pathogenesis of CKD-associated thrombosis are largely unknown.
In this study, we found that IS displayed a distinct ability to enhance platelet activity through activation of ROS/p38MAPK signaling, and IS-induced platelet hyperactivity was involved in CKD-associated thrombosis and atherosclerosis. We also showed that Klotho protein exhibited potent biological activity against IS-induced platelet hyperactivity. Our findings unmask the role of platelet hyperactivity induced by IS in CKD-associated thrombosis.
Materials and methods
Animals
Normal C57BL/6J mice were purchased from the Institute of Zoology (Chinese Academy of Sciences, Beijing, China). Klotho+/− mice were kindly gifted by Jun Gu (State Key Laboratory of Protein and Plant Gene Research, College of Life Science, Peking University, Beijing, China), and the mice were backcrossed to C57BL/6J background for more than 6 generations. B6.129P2-apoEtm1Unc/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). ApoE−/− mice were fed an atherogenic diet (D12108C; Research Diet Inc., New Brunswick, NJ).
The CKD mouse model was created as previously described.19 Briefly, male C57BL/6J mice at 8 to 10 weeks of age were first inflicted with 2/3 electrocoagulation of the right renal cortex and then received left total nephrectomy 2 weeks later. The CKD mice were housed with food and water available ad libitum without erythropoietin treatment. For IS (Sigma, St. Louis, MO) administration, normal C57BL/6J, klotho+/−, and wild-type (WT) mice were treated with a single intraperitoneal (IP) dose of 100 mg/kg daily for 8 weeks. For AST-120 (Kremezin; Kureha Chemical Industry Co. Ltd, Tokyo, Japan) administration, normal CKD mice were fed a diet containing 5% AST-120 after left nephrectomy for 8 weeks. For recombinant mouse Klotho (1819-KL-050; R&D Systems, Minneapolis, MN) administration, C57BL/6J and apoE−/− mice were treated with a dose of 10 μg/kg IP every other day for 8 weeks. For Klotho/IS administration, apoE−/− mice were firstly treated with Klotho IP, whereas IS was administered IP 6 hours after Klotho treatment on the same day. After anesthesia, mouse blood and arteries were collected to perform various experiments, and blood urea nitrogen and serum creatinine were measured. All of the experimental procedures were approved by the Animal Care Committee of the Third Military Medical University (Chongqing, China).
Platelet activity assay
Platelet activity was detected as previously reported.22 After anesthesia, mouse blood was collected from the inferior vena cava with acid citrate dextrose (ACD) (65 mM trisodium citrate, 70 mM citric acid, and 100 mM dextrose; pH 6.5) as the anticoagulant. To detect the expression of P-selectin and activated integrin GPIIb/IIIa on platelet surface, ACD-whole blood was diluted in modified Tyrode buffer (137 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.4 mM NaH2PO4, 5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5.6 mM glucose, and 0.35% bovine serum albumin; pH 7.4). The samples were pretreated with indicated concentrations of IS at room temperature for 15 minutes, and then stimulated with collagen (2 μg/mL) or thrombin (0.05 U/mL) in the presence of anti–P-selectin–phycoerythrin (BioLegend) or anti-activated integrin GPIIb/IIIa (JON/A-phycoerythrin; Emfret Analytics) and anti-CD61–allophycocyanin (BioLegend) for 15 minutes at room temperature. CaCl2 (1 mM final concentration) was added immediately before platelet activation studies. The reactions were stopped by the addition of 400 μL modified Tyrode buffer and analyzed using a fluorescence-activated cell sorter (FACS)verse (BD Biosciences, San Jose, CA) flow cytometer within 30 minutes. Platelets were selected on the basis of forward and side scatter characteristic, and CD61+ platelets were selected for analysis.
Platelet-monocyte aggregates assay
To detect the platelet-monocyte aggregates in vitro, anticoagulated blood was incubated in a constant temperature shaker at 1000 rpm, at 37°C in the presence of indicated concentrations of IS for 15 minutes. Then, collagen or thrombin was added to incubate for another 5 minutes. The reaction was stopped by adding 1 mL red cell lysis buffer and carefully washed with phosphate buffered saline. Then the cells were stained with CD45, CD115, Gr1, CD11b, and CD41 (all eBioscience) for 30 minutes on ice. The cells were washed and resuspended in FACS buffer and run on a FACSverse flow cytometer. Viable cells were selected based on the forward and side scatter characteristic, and CD45+ leukocytes were selected for further analysis. Platelet-monocyte aggregates were identified as CD115+Gr1hi(Ly6-Chi) and CD41+. Platelet-induced activation of monocytes was measured as CD11b mean fluorescence intensity after subtracting the expression of CD11b on monocytes stained negative for platelets (CD41−).
Platelet-derived microparticles (PMPs) detection
Platelet-free plasma was prepared using serial centrifugations as previously reported.23 Then, the plasma was diluted with Annexin V-binding buffer and incubated with antibodies to Annexin V (eBioscience) and CD41. Equal amounts of 1 μm beads (Invitrogen) were added to the sample as a size standard and analyzed by a FACSverse flow cytometer. PMPs were detected as particles <1 μm in size that stained positive for CD41 and Annexin V. Data were converted to the number of PMPs per 1 μL of whole blood.
Whole blood ex vivo flow chamber assay
Whole blood was incubated with the fluorescent dye rhodamine-6G (50 μL 0.05% per mL whole blood; Sigma) for 10 minutes at 37°C. The whole blood was then perfused over microcapillary glass tubes coated with type I collagen (150 μg/mL) or bovine serum albumin (background control) overnight at a controlled shear rate (1800 s−1) using a syringe pump for 3 minutes. Adherent platelets and aggregates in the chamber were washed and micrographs of adhered platelets were acquired using a fluorescent microscope. Flow chamber surface coverage by the thrombus was measured as the area of platelet adhesion on collagen using ImageJ software.
FeCl3-induced carotid artery thrombosis model
Mice were anesthetized and a cervical incision was made to expose the common carotid artery. A miniature Doppler flow probe (Transonic Systems Inc.) was placed on the carotid artery to monitor blood flow. Vessel wall injury was created by application of a piece of Whatman paper (2 mm × 2 mm) saturated with 10% FeCl3 to the surface of the carotid artery for 1 minute. The paper was then removed, and the carotid artery was rinsed with saline at 37°C and recorded for 30 minutes. The vessel occlusion time was set as cessation of blood flow for >30 seconds or no occlusion after 30 minutes.
Platelet aggregometry
Anticoagulated whole blood was diluted with physiologic saline (1:1) and pretreated with indicated concentrations of IS. Then, collagen (2 μg/mL) or thrombin (0.05 U/mL) was added, and aggregation was measured with constant stirring (1000 rpm) at 37°C using a luminoaggregometer Model 700 (ChronoLog, Havertown, PA).
Flow cytometry
For detection of platelets ROS production, whole blood was diluted with 37°C prewarmed modified Tyrode buffer and incubated with 10 μM dichlorodihy-drofluorescein diacetate (Sigma) for 30 minutes at 37°C. The platelets were then examined immediately. For detection of platelets p38MAPK activity, diluted whole blood was fixed using intracellular fixation buffer (eBioscience), and then permeabilized using permeabilization buffer (eBioscience) before incubation with anti-human/mouse phospho (P)-p38MAPK (Thr180/Tyr182; eBioscience) antibodies at room temperature for 30 minutes. Finally, samples were examined using a FACSverse flow cytometer.
Preparation of mouse platelets and western blot analysis
Blood from normal mice was collected in ACD buffer and centrifuged at 100 g for 10 minutes to obtain platelet-rich plasma. The platelet-rich plasma was stimulated with indicated concentrations of IS for 15 minutes, and then platelets were pelleted and lysed using an M-PER Mammalian Protein Extraction Reagent (Thermo). Western blot was performed and quantified as previously described.19 Protein expression was detected for P-p38MAPK (Thr180/Tyr182), p38MAPK (Cell Signaling Technology), and glyceraldehyde-3-phosphate dehydrogenase (Beyotime).
Measurements of serum IS and Klotho
The serum IS was measured by high-pressure liquid chromatography and the serum Klotho was measured using a mouse Klotho enzyme-linked immunosorbent assay kit as previously reported.19
Histology and morphometry analysis of atherosclerotic plaque
Evaluation of the atherosclerotic plaque area was performed as described.24 Firstly, the aortas were carefully freed of connective and adipose tissue, and perfused with 10% neutral formalin. After being embedded in paraffin, the aortic root was sectioned serially in 8-μm thick slices from the aortic valves. Ten sections were collected and subsequently placed on 10 slides that were 80 μm apart. The slides were then stained with hematoxylin and eosin. Finally, vessel sections were photographed under a microscope (Olympus Optical) and quantified using ImagePro software.
Immunofluorescence
For immunofluorescence analysis of platelet-monocyte aggregates in the blood, whole blood was incubated with anti-CD14–allophycocyanin (eBioscience) and anti-CD41–fluorescein isothiocyanate at room temperature for 15 minutes, and visualized by a laser confocal microscopy (Carl Zeiss).
Scanning electron microscopy (SEM) of mouse carotid artery
The arteries were fixed with 2.5% glutaraldehyde by cardiac perfusion. Then, the carotid artery was excised and fixed in 2.5% glutaraldehyde overnight. The fixed carotid artery was dehydrated in increasing concentrations of ethanol followed by hexamethyldisilazine, and immobilized onto a cover slip. The cover slip was sputtered with gold prior to observation at 15 kV under a Hitachi S-3400N SEM.
Hematologic parameter test
Mouse blood cell counts were measured as previously reported.25 Briefly, 20 μL of peripheral blood was collected from the tail veins of the mice, and diluted in 1% ethylene diamine tetraacetic acid solution and counted automatically by a Sysmex XT-2000iV hematology analyzer (Kobe, Japan).
Statistical analysis
Data were analyzed with Prism 6.0 (GraphPad Software). Experiments were repeated at least 3 times independently, and the results are expressed as mean ± standard deviation (SD). Comparisons between 2 groups were determined by 2-tailed Student t test, and 3 or more groups were compared by analysis of variance (ANOVA) followed by Tukey-Kramer post hoc analysis. P < .05 was considered statistically significant.
Results
Platelet hyperactivation is detected in CKD mice with a high level of serum IS
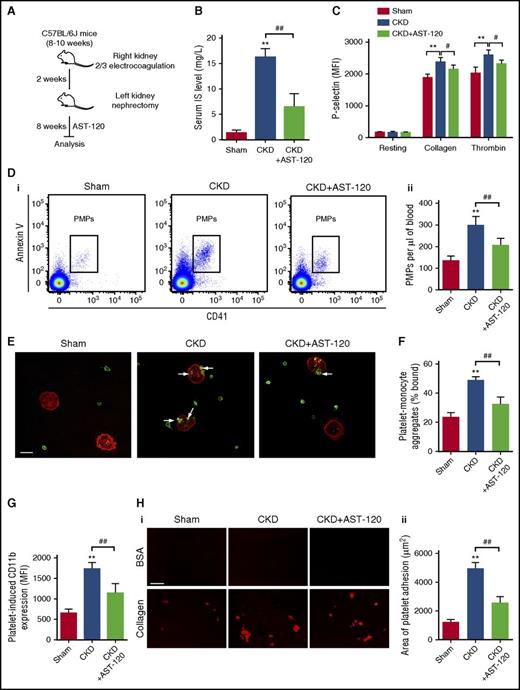
To investigate the changes of platelet activity in CKD, we created a 5/6 nephrectomy CKD mouse model (Figure 1A). As expected, blood urea nitrogen and serum creatinine dramatically increased in CKD mice, accompanied by a remarkable increase in serum IS (Figure 1B; supplemental Figure 1A-B, available on the Blood Web site). Eight weeks after left nephrectomy, there were no significant changes in blood cell counts, including white blood cells, red blood cells, and platelets (supplemental Figure 1C). We then investigated platelet activity by detecting P-selectin and activated GPIIb/IIIa expression on platelet membrane by flow cytometry. Although the resting platelets in CKD mice and sham-operated mice had comparable levels of P-selectin and activated GPIIb/IIIa, the increase in the expression of P-selectin and activated GPIIb/IIIa induced by collagen and thrombin was more pronounced in CKD mice (Figure 1C; supplemental Figure 1D). Meanwhile, the circulating level of PMPs significantly increased (Figure 1D), indicating a hyperactivation state of platelets in CKD mice. In addition, platelet-monocyte aggregates, another marker of platelet activation, also increased in the blood of CKD mice (Figure 1E-F), accompanied by enhanced expression of CD11b in the aggregated monocytes (Figure 1G). We then evaluated ex vivo thrombus formation using a flow chamber, and found that platelets in CKD mice had a higher tendency to adhere to a collagen-coated surface and form larger aggregates under shear-flow conditions (Figure 1H). However, AST-120, a charcoal adsorbent of IS, remarkably inhibited platelet hyperactivation presented in CKD mice (Figure 1). These data indicate that the platelet activity is enhanced significantly in CKD mice, possibly due to the high level of serum IS.
Platelet hyperactivation is detected in CKD mice possibly due to the high level of serum IS. (A) Scheme for creation of CKD mouse model and AST-120 treatment. (B) Serum IS levels were measured at 8 weeks after left nephrectomy (n = 8). (C) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen or 0.05 U/mL thrombin (n = 6). (D) Flow cytometric analysis of the concentration of PMPs in whole blood (i). Quantification of the number of PMPs per 1 μL of whole blood (n = 6) (ii). (B-D) Data are mean ± SD. **P < .01 vs sham group; #P < .05, ##P < .01 vs CKD group, ANOVA. (E) Representative immunostaining images of platelet-monocyte aggregates formation in vivo. The peripheral blood cells were labeled with anti-CD14 (red) and anti-CD41 (green), and visualized by confocal microscopy. Scale bar, 10 μm. White arrows indicate platelets associated with monocytes. (F) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in whole blood in vivo (n = 6). (G) Flow cytometric analysis of platelet-induced CD11b expression in platelet-associated monocytes (n = 6). (H) Representative images of thrombus formation on a collagen-coated flow chamber under shear-flow conditions (i). Scale bar, 50 μm. Quantification of the area of platelet adhesion for each group (n = 6) (ii). (F-H) Data are mean ± SD. **P < .01 vs sham group; ##P < .01 vs CKD group, ANOVA. BSA, bovine serum albumin; MFI, mean fluorescence intensity.
Platelet hyperactivation is detected in CKD mice possibly due to the high level of serum IS. (A) Scheme for creation of CKD mouse model and AST-120 treatment. (B) Serum IS levels were measured at 8 weeks after left nephrectomy (n = 8). (C) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen or 0.05 U/mL thrombin (n = 6). (D) Flow cytometric analysis of the concentration of PMPs in whole blood (i). Quantification of the number of PMPs per 1 μL of whole blood (n = 6) (ii). (B-D) Data are mean ± SD. **P < .01 vs sham group; #P < .05, ##P < .01 vs CKD group, ANOVA. (E) Representative immunostaining images of platelet-monocyte aggregates formation in vivo. The peripheral blood cells were labeled with anti-CD14 (red) and anti-CD41 (green), and visualized by confocal microscopy. Scale bar, 10 μm. White arrows indicate platelets associated with monocytes. (F) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in whole blood in vivo (n = 6). (G) Flow cytometric analysis of platelet-induced CD11b expression in platelet-associated monocytes (n = 6). (H) Representative images of thrombus formation on a collagen-coated flow chamber under shear-flow conditions (i). Scale bar, 50 μm. Quantification of the area of platelet adhesion for each group (n = 6) (ii). (F-H) Data are mean ± SD. **P < .01 vs sham group; ##P < .01 vs CKD group, ANOVA. BSA, bovine serum albumin; MFI, mean fluorescence intensity.
IS has a strong ability to increase platelet activity both in vitro and in vivo
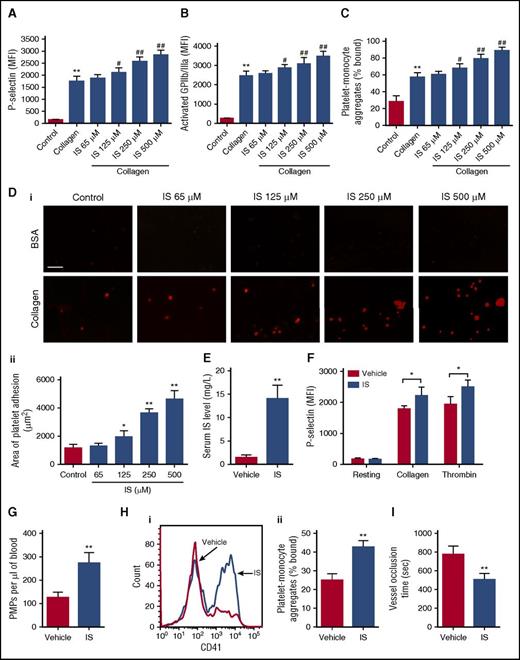
To explore the relationship between the increase in serum IS and platelet activity enhancement in CKD mice, we studied the effect of IS on platelet activation. Firstly, it was found that IS treatment could dose-dependently enhance P-selectin and activated GPIIb/IIIa expression in the platelets in presence of low concentrations of collagen and thrombin (Figure 2A-B; supplemental Figure 2A-B). Similarly, IS also induced the formations of platelet-monocyte aggregates, and platelet adhesion and aggregation in a dose-dependent manner (Figure 2C-D; supplemental Figure 2C-E). Then, we investigated the in vivo effect of IS on platelet activation. After 8 weeks of IS administration, a high level of serum IS was observed in the mice resembling that in CKD mice without affecting the survival of mice (Figure 2E), accompanied by enhanced P-selectin and activated GPIIb/IIIa expression in the platelets in presence of collagen and thrombin (Figure 2F; supplemental Figure 2F). Meanwhile, we found significant increases in PMPs and platelet-monocyte aggregates in the blood after IS treatment (Figure 2G-H; supplemental Figure 2G). Moreover, the in vivo thrombosis experiment demonstrated that the time for carotid artery occlusion was significantly shorter in IS-treated mice (Figure 2I). These findings disclose that IS has a distinct ability to increase platelet activity, which may contribute to thrombus formation.
IS has a strong ability to increase platelet activity both in vitro and in vivo. (A-C) The whole blood was pretreated with indicated concentrations of IS for 15 minutes and then stimulated with 2 μg/mL collagen. (A-B) Flow cytometric analysis of the expression of P-selectin and activated GPIIb/IIIa in platelets in response to 2 μg/mL collagen (n = 6). (C) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in vitro (n = 6). (D) Representative images of thrombus formation on a collagen-coated flow chamber under shear-flow conditions using whole blood pretreated with different concentrations of IS (i). Scale bar, 50 μm. Quantification of the area of platelet adhesion for each group (n = 6) (ii). (A-D) Data are mean ± SD. *P < .05, **P < .01 vs control group; #P < .05, ##P < .01 vs collagen group, ANOVA. (E-I) Mice were IP injected with IS (100 mg/kg per day) for 8 weeks. (E) Serum IS levels were measured by high-pressure liquid chromatography (n = 8). (F) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen or 0.05 U/mL thrombin (n = 6). (G) Flow cytometric analysis of the concentration of PMPs in whole blood (n = 6). (H) Flow cytometric analysis of platelet-monocyte aggregates formation in whole blood in vivo (i). The histogram shows the percentage of platelet-monocyte aggregates in whole blood (n = 6) (ii). (I) The time for carotid artery occlusion by thrombus in a FeCl3-induced thrombosis model (n = 6). (E-I) Data are mean ± SD. *P < .05, **P < .01 vs vehicle group, Student t test.
IS has a strong ability to increase platelet activity both in vitro and in vivo. (A-C) The whole blood was pretreated with indicated concentrations of IS for 15 minutes and then stimulated with 2 μg/mL collagen. (A-B) Flow cytometric analysis of the expression of P-selectin and activated GPIIb/IIIa in platelets in response to 2 μg/mL collagen (n = 6). (C) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in vitro (n = 6). (D) Representative images of thrombus formation on a collagen-coated flow chamber under shear-flow conditions using whole blood pretreated with different concentrations of IS (i). Scale bar, 50 μm. Quantification of the area of platelet adhesion for each group (n = 6) (ii). (A-D) Data are mean ± SD. *P < .05, **P < .01 vs control group; #P < .05, ##P < .01 vs collagen group, ANOVA. (E-I) Mice were IP injected with IS (100 mg/kg per day) for 8 weeks. (E) Serum IS levels were measured by high-pressure liquid chromatography (n = 8). (F) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen or 0.05 U/mL thrombin (n = 6). (G) Flow cytometric analysis of the concentration of PMPs in whole blood (n = 6). (H) Flow cytometric analysis of platelet-monocyte aggregates formation in whole blood in vivo (i). The histogram shows the percentage of platelet-monocyte aggregates in whole blood (n = 6) (ii). (I) The time for carotid artery occlusion by thrombus in a FeCl3-induced thrombosis model (n = 6). (E-I) Data are mean ± SD. *P < .05, **P < .01 vs vehicle group, Student t test.
ROS/p38MAPK signaling is involved in IS-induced platelet hyperactivation
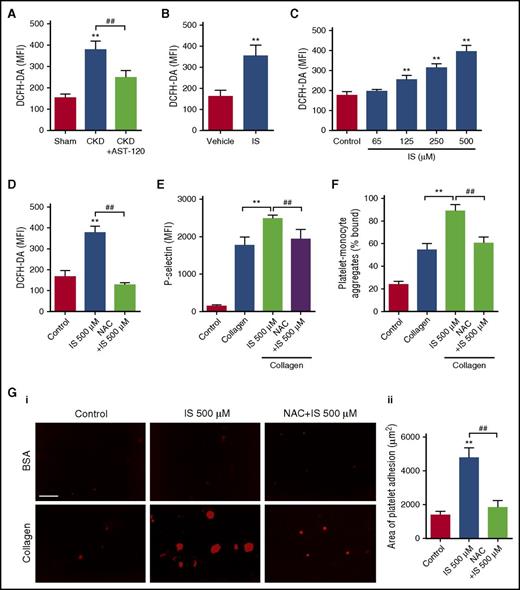
To reveal the mechanism underlying the effect of IS on platelet activation, we detected the ROS production in platelets after IS treatment by flow cytometry. As shown in Figure 3A, a higher level of ROS was detected in platelets of CKD mice compared with the sham group, whereas AST-120 administration significantly inhibited ROS overproduction in the platelets of CKD mice. Then, we found that IS treatment not only increased ROS production in the platelets in vivo, but also dose-dependently increased ROS production in the platelets in vitro (Figure 3B-C). In contrast, pretreatment with N-acetylcysteine (NAC), a ROS scavenger, almost completely inhibited IS-induced ROS production and P-selectin expression in the platelets (Figure 3D-E; supplemental Figure 3A), as well as the increase of platelet-monocyte aggregates formation (Figure 3F; supplemental Figure 3B). Accordingly, NAC pretreatment remarkably attenuated IS-induced thrombus formation in vitro (Figure 3G). These findings indicate that ROS overproduction is involved in IS-induced platelet hyperactivation.
ROS overproduction is involved in IS-induced platelet hyperactivation. (A) Flow cytometric analysis of ROS levels in platelets of CKD mice after AST-120 treatment by DCFH-DA staining (n = 6). Data are mean ± SD. **P < .01 vs sham group; ##P < .01 vs CKD group, ANOVA. (B) Flow cytometric analysis of ROS levels in platelets of IS-treated mice (n = 6). Data are mean ± SD. **P < .01 vs vehicle group, Student t test. (C) Flow cytometric analysis of ROS levels in platelets after being treated with indicated concentrations of IS (n = 6). Data are mean ± SD. **P < .01 vs control group, ANOVA. (D-G) Platelets were pretreated with 5 mM NAC for 15 minutes and then stimulated with 500 μM IS for another 15 minutes. (D) Flow cytometric analysis of ROS levels in platelets (n = 6). (E) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen (n = 6). (F) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in response to 2 μg/mL collagen in vitro (n = 6). (G) Representative images of thrombus formation on a collagen-coated flow chamber under shear-flow conditions (i). Scale bar, 50 μm. Quantification of the area of platelet adhesion for each group (n = 6) (ii). (D-G) Data are mean ± SD. **P < .01 vs control or collagen group; ##P < .01 vs IS 500 μM group, ANOVA. DCFH-DA, dichlorodihy-drofluorescein diacetate.
ROS overproduction is involved in IS-induced platelet hyperactivation. (A) Flow cytometric analysis of ROS levels in platelets of CKD mice after AST-120 treatment by DCFH-DA staining (n = 6). Data are mean ± SD. **P < .01 vs sham group; ##P < .01 vs CKD group, ANOVA. (B) Flow cytometric analysis of ROS levels in platelets of IS-treated mice (n = 6). Data are mean ± SD. **P < .01 vs vehicle group, Student t test. (C) Flow cytometric analysis of ROS levels in platelets after being treated with indicated concentrations of IS (n = 6). Data are mean ± SD. **P < .01 vs control group, ANOVA. (D-G) Platelets were pretreated with 5 mM NAC for 15 minutes and then stimulated with 500 μM IS for another 15 minutes. (D) Flow cytometric analysis of ROS levels in platelets (n = 6). (E) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen (n = 6). (F) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in response to 2 μg/mL collagen in vitro (n = 6). (G) Representative images of thrombus formation on a collagen-coated flow chamber under shear-flow conditions (i). Scale bar, 50 μm. Quantification of the area of platelet adhesion for each group (n = 6) (ii). (D-G) Data are mean ± SD. **P < .01 vs control or collagen group; ##P < .01 vs IS 500 μM group, ANOVA. DCFH-DA, dichlorodihy-drofluorescein diacetate.
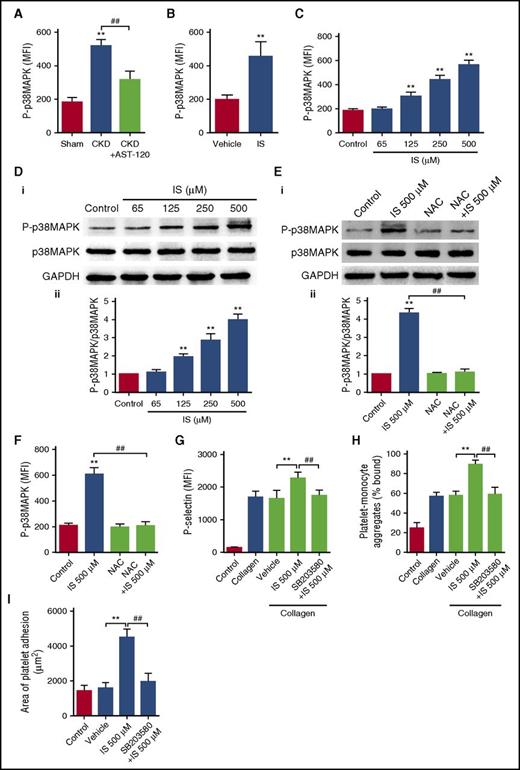
Subsequently, we observed that p38MAPK, the main signal pathway downstream of ROS,26 was significantly activated in the platelets in both CKD and IS-treated mice, whereas AST-120 administration significantly inhibited p38MAPK activation in the platelets of CKD mice (Figure 4A-B). In vitro, IS treatment dose-dependently stimulated the phosphorylation of p38MAPK in the platelets (Figure 4C-D), and IS-induced p38MAPK activation was significantly inhibited by NAC pretreatment (Figure 4E-F). Moreover, pretreatment with SB203580, an inhibitor of p38MAPK, could suppress IS-induced p38MAPK activation, platelet hyperactivation, and thrombus formation in a way similar to the effects of NAC (Figure 4G-I; supplemental Figure 4). Collectively, these results suggest that ROS/p38MAPK signaling plays a key role in IS-induced platelet hyperactivation.
IS increases platelet activity via ROS-induced activation of p38MAPK. (A) Flow cytometric analysis of the expression of P-p38MAPK in platelets of CKD mice after AST-120 treatment (n = 6). Data are mean ± SD. **P < .01 vs sham group; ##P < .01 vs CKD group, ANOVA. (B) Flow cytometric analysis of the expression of P-p38MAPK in platelets of IS-treated mice (n = 6). Data are mean ± SD. **P < .01 vs vehicle group, Student t test. (C-D) Platelets were incubated with indicated concentrations of IS for 15 minutes. (C) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (D) Western blot analysis of the expression of P-p38MAPK and p38MAPK in platelets (i). Quantification of the ratio of P-p38MAPK/p38MAPK for each group (n = 3) (ii). (E-I) Platelets were pretreated with 5 mM NAC or 20 μM SB203580 for 15 minutes and then stimulated with 500 μM IS for another 15 minutes. (E) Western blot analysis of the expression of P-p38MAPK and p38MAPK in platelets (i). Quantification of the ratio of P-p38MAPK/p38MAPK for each group (n = 3) (ii). (F) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (G) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen (n = 6). (H) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in response to 2 μg/mL collagen in vitro (n = 6). (I) Quantification of the area of platelet adhesion for each group (n = 6). (C-I) Data are mean ± SD. **P < .01 vs control or vehicle group; ##P < .01 vs IS 500 μM group, ANOVA.
IS increases platelet activity via ROS-induced activation of p38MAPK. (A) Flow cytometric analysis of the expression of P-p38MAPK in platelets of CKD mice after AST-120 treatment (n = 6). Data are mean ± SD. **P < .01 vs sham group; ##P < .01 vs CKD group, ANOVA. (B) Flow cytometric analysis of the expression of P-p38MAPK in platelets of IS-treated mice (n = 6). Data are mean ± SD. **P < .01 vs vehicle group, Student t test. (C-D) Platelets were incubated with indicated concentrations of IS for 15 minutes. (C) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (D) Western blot analysis of the expression of P-p38MAPK and p38MAPK in platelets (i). Quantification of the ratio of P-p38MAPK/p38MAPK for each group (n = 3) (ii). (E-I) Platelets were pretreated with 5 mM NAC or 20 μM SB203580 for 15 minutes and then stimulated with 500 μM IS for another 15 minutes. (E) Western blot analysis of the expression of P-p38MAPK and p38MAPK in platelets (i). Quantification of the ratio of P-p38MAPK/p38MAPK for each group (n = 3) (ii). (F) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (G) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen (n = 6). (H) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in response to 2 μg/mL collagen in vitro (n = 6). (I) Quantification of the area of platelet adhesion for each group (n = 6). (C-I) Data are mean ± SD. **P < .01 vs control or vehicle group; ##P < .01 vs IS 500 μM group, ANOVA.
Klotho counteracts IS-induced platelet hyperactivation via inhibiting ROS/p38MAPK signaling
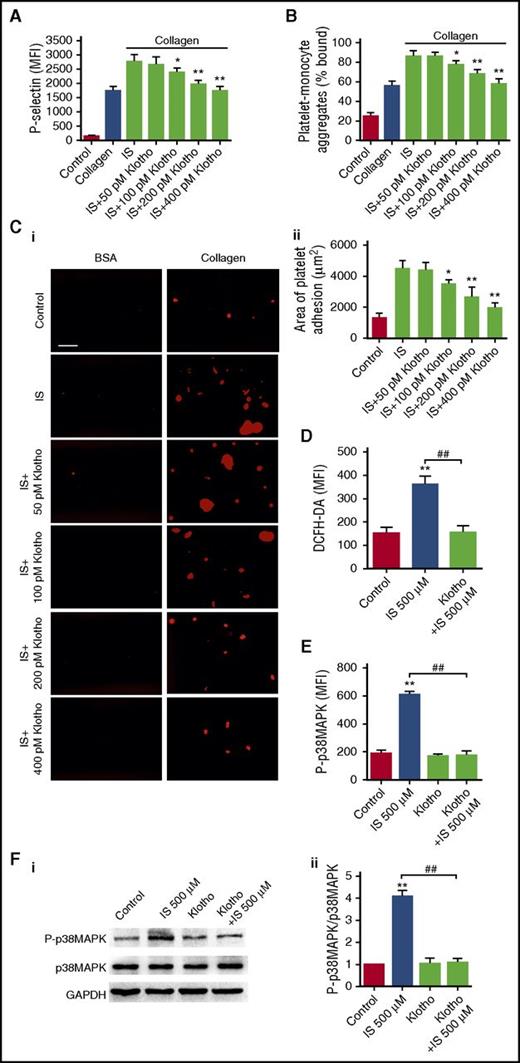
Klotho is a humoral antioxidant protein predominantly expressed in renal tubular epithelial cells.27,28 Previously, we have shown that Klotho could protect against IS-induced endothelial dysfunction and myocardial hypertrophy.19,20 Thus, we next investigated the effect of Klotho on IS-induced platelet hyperactivation. As shown in Figure 5A-C, Klotho dose-dependently inhibited IS-induced platelet hyperactivation and thrombus formation, similar to the actions of NAC and SB203580. Although Klotho administration only slightly reduced serum levels of IS in CKD mice (supplemental Figure 5), Klotho pretreatment significantly attenuated IS-induced ROS production and p38MAPK activation (Figure 5D-F), suggesting that Klotho has a distinct ability to counteract IS-induced platelet hyperactivation via inhibiting ROS/p38MAPK signaling.
Klotho counteracts IS-induced platelet hyperactivation via inhibiting ROS/p38MAPK signaling. (A-C) Platelets were pretreated with indicated concentrations of Klotho and then stimulated with 500 μM IS. (A) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen after Klotho pretreatment (n = 6). (B) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in response to 2 μg/mL collagen after Klotho pretreatment in vitro (n = 6). (C) Representative images of thrombus formation on a collagen-coated flow chamber under shear-flow conditions (i). Scale bar, 50 μm. Quantification of the area of platelet adhesion for each group (n = 6) (ii). (A-C) Data are mean ± SD. *P < .05, **P < .01 vs IS group, ANOVA. (D-F) Platelets were pretreated with 400 pM Klotho and then stimulated with 500 μM IS. (D) Flow cytometric analysis of ROS levels in platelets (n = 6). (E) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (F) Western blot analysis of the expression of P-p38MAPK and p38MAPK in platelets (i). Quantification of the ratio of P-p38MAPK/p38MAPK for each group (n = 3) (ii). (D-F) Data are mean ± SD. **P < .01 vs control group, ##P < .01 vs IS 500 μM group, ANOVA. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Klotho counteracts IS-induced platelet hyperactivation via inhibiting ROS/p38MAPK signaling. (A-C) Platelets were pretreated with indicated concentrations of Klotho and then stimulated with 500 μM IS. (A) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen after Klotho pretreatment (n = 6). (B) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in response to 2 μg/mL collagen after Klotho pretreatment in vitro (n = 6). (C) Representative images of thrombus formation on a collagen-coated flow chamber under shear-flow conditions (i). Scale bar, 50 μm. Quantification of the area of platelet adhesion for each group (n = 6) (ii). (A-C) Data are mean ± SD. *P < .05, **P < .01 vs IS group, ANOVA. (D-F) Platelets were pretreated with 400 pM Klotho and then stimulated with 500 μM IS. (D) Flow cytometric analysis of ROS levels in platelets (n = 6). (E) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (F) Western blot analysis of the expression of P-p38MAPK and p38MAPK in platelets (i). Quantification of the ratio of P-p38MAPK/p38MAPK for each group (n = 3) (ii). (D-F) Data are mean ± SD. **P < .01 vs control group, ##P < .01 vs IS 500 μM group, ANOVA. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Klotho reduction aggravates IS-induced platelet hyperactivation
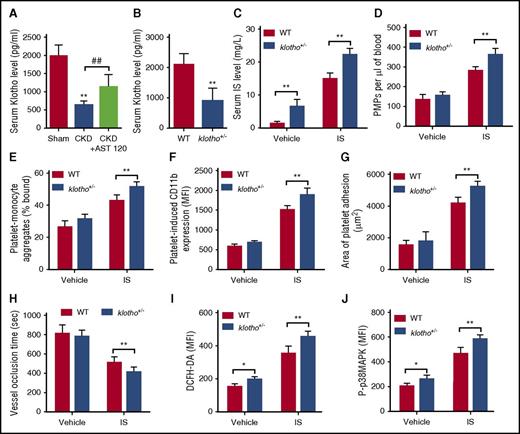
In contrast to the remarkable increase in serum IS, the serum level of Klotho decreased significantly in CKD mice, whereas AST-120 administration partially recovered the serum Klotho level (Figure 6A). Regarding the opposite relationship between IS and Klotho, we further investigated the in vivo influence of Klotho reduction on IS-induced platelet hyperactivation. The klotho+/− mice with a low level of Klotho (Figure 6B), similar to that in CKD mice, were treated with IS for 8 weeks. Compared with WT mice, klotho+/− mice had a higher level of serum IS, and IS-treatment further increased the serum IS level in klotho+/− mice (Figure 6C). Meanwhile, klotho+/− mice displayed even higher levels of PMPs, platelet-monocyte aggregates, and platelet-induced monocyte activation in the circulation (Figure 6D-F). Moreover, IS administration was much easier to induce thrombus formation in klotho+/− mice (Figure 6G-H). Meanwhile, Klotho reduction enhanced IS-induced ROS production and p38MAPK activation in the platelets (Figure 6I-J). These data indicate that, during the progression of CKD, Klotho reduction may aggravate IS-induced platelet hyperactivation.
Klotho reduction aggravates IS-induced platelet hyperactivation. (A) Serum Klotho levels in CKD mice after AST-120 treatment (n = 8). Data are mean ± SD. **P < .01 vs sham group; ##P < .01 vs CKD group, ANOVA. (B) Serum Klotho levels in klotho+/− mice (n = 8). Data are mean ± SD. **P < .01 vs WT group, Student t test. (C-J) WT and klotho+/− mice were IP injected with IS (100 mg/kg per day) for 8 weeks. (C) Serum IS levels were measured in WT and klotho+/− mice after IS treatment (n = 8). (D) Flow cytometric analysis of the concentration of PMPs in whole blood (n = 6). (E) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in whole blood in vivo (n = 6). (F) Flow cytometric analysis of platelet-induced CD11b expression in platelet-associated monocytes (n = 6). (G) Quantification of the area of platelet adhesion for each group (n = 6). (H) The time for carotid artery occlusion by thrombus for each group (n = 6). (I) Flow cytometric analysis of ROS levels in platelets (n = 6). (J) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (C-J) Data are mean ± SD. *P < .05, **P < .01 vs WT group, Student t test.
Klotho reduction aggravates IS-induced platelet hyperactivation. (A) Serum Klotho levels in CKD mice after AST-120 treatment (n = 8). Data are mean ± SD. **P < .01 vs sham group; ##P < .01 vs CKD group, ANOVA. (B) Serum Klotho levels in klotho+/− mice (n = 8). Data are mean ± SD. **P < .01 vs WT group, Student t test. (C-J) WT and klotho+/− mice were IP injected with IS (100 mg/kg per day) for 8 weeks. (C) Serum IS levels were measured in WT and klotho+/− mice after IS treatment (n = 8). (D) Flow cytometric analysis of the concentration of PMPs in whole blood (n = 6). (E) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in whole blood in vivo (n = 6). (F) Flow cytometric analysis of platelet-induced CD11b expression in platelet-associated monocytes (n = 6). (G) Quantification of the area of platelet adhesion for each group (n = 6). (H) The time for carotid artery occlusion by thrombus for each group (n = 6). (I) Flow cytometric analysis of ROS levels in platelets (n = 6). (J) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (C-J) Data are mean ± SD. *P < .05, **P < .01 vs WT group, Student t test.
Klotho administration attenuates IS-induced thrombosis and atherosclerosis in apoE−/− mice
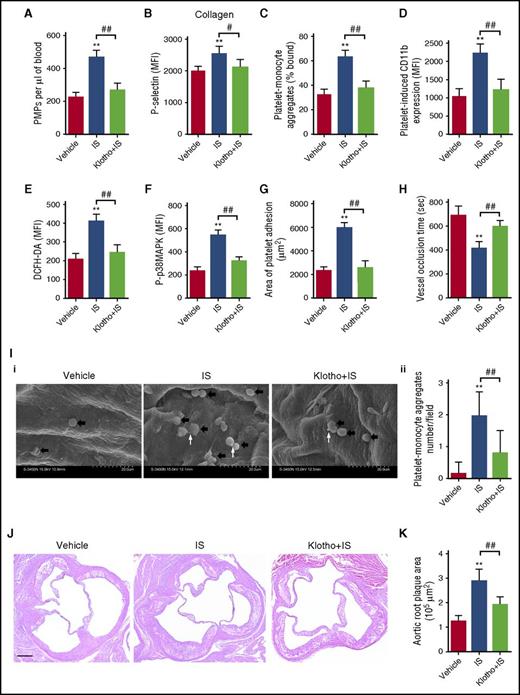
To further evaluate the pathological significance of IS-induced platelet hyperactivity and the inhibitive effect of Klotho against IS, we administrated IS to apoE−/− mice treated with or without exogenous Klotho protein (supplemental Figure 6). As shown in Figure 7A-D, IS administration significantly increased platelet activities in apoE−/− mice, whereas Klotho treatment markedly reduced IS-induced platelet hyperactivity. Meanwhile, Klotho treatment significantly attenuated IS-induced ROS production and p38MAPK activation in the platelets of apoE−/− mice (Figure 7E-F). In addition, long-term IS administration significantly promoted thrombus formation in apoE−/− mice (Figure 7G-H), along with evident platelet-leukocyte aggregates adhesion to atherosclerotic endothelium (Figure 7I), whereas Klotho treatment significantly attenuated IS-induced thrombus formation (Figure 7G-I). Furthermore, we also observed that IS administration accelerated the development of atherosclerosis in apoE−/− mice, and exogenous Klotho treatment was able to alleviate the atherosclerotic lesions formation (Figure 7J-K). Simultaneously, we found similar pathological changes in platelet activities, thrombosis, and atherosclerosis in apoE−/− mice with CKD, and exogenous Klotho treatment could ameliorate thrombosis and atherosclerosis in apoE−/− mice with CKD (supplemental Figure 7).
Klotho administration attenuates IS-induced thrombosis and atherosclerosis in apoE−/−mice. (A) Flow cytometric analysis of the concentration of PMPs in whole blood (n = 6). (B) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen (n = 6). (C) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in whole blood in vivo (n = 6). (D) Flow cytometric analysis of platelet-induced CD11b expression in platelet-associated monocytes (n = 6). (E) Flow cytometric analysis of ROS levels in platelets (n = 6). (F) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (G) Quantification of the area of platelet adhesion for each group (n = 6). (H) The time for carotid artery occlusion by thrombus for each group (n = 6). (I) Representative images of platelet-monocyte aggregates adhesion on atherosclerotic endothelium as seen by SEM. Black arrows indicate leukocytes adhered on atherosclerotic endothelium. White arrows indicate platelets associated with leukocytes (i). Quantification of the number of platelet-monocyte aggregates for each group (n = 20 fields) (ii). (B-I) Data are mean ± SD. **P < .01 vs vehicle group, ##P < .01 vs IS group, ANOVA. (J) Representative cross-sections (hematoxylin and eosin staining) of the aortic sinus. Scale bar, 100 μm. (K) Quantification of the atherosclerotic plaque area in aortic root (n = 6). Data are mean ± SD. **P < .01 vs vehicle group, ##P < .01 vs IS group, ANOVA.
Klotho administration attenuates IS-induced thrombosis and atherosclerosis in apoE−/−mice. (A) Flow cytometric analysis of the concentration of PMPs in whole blood (n = 6). (B) Flow cytometric analysis of P-selectin expression in platelets in response to 2 μg/mL collagen (n = 6). (C) Flow cytometric analysis of the percentage of platelet-monocyte aggregates in whole blood in vivo (n = 6). (D) Flow cytometric analysis of platelet-induced CD11b expression in platelet-associated monocytes (n = 6). (E) Flow cytometric analysis of ROS levels in platelets (n = 6). (F) Flow cytometric analysis of the expression of P-p38MAPK in platelets (n = 6). (G) Quantification of the area of platelet adhesion for each group (n = 6). (H) The time for carotid artery occlusion by thrombus for each group (n = 6). (I) Representative images of platelet-monocyte aggregates adhesion on atherosclerotic endothelium as seen by SEM. Black arrows indicate leukocytes adhered on atherosclerotic endothelium. White arrows indicate platelets associated with leukocytes (i). Quantification of the number of platelet-monocyte aggregates for each group (n = 20 fields) (ii). (B-I) Data are mean ± SD. **P < .01 vs vehicle group, ##P < .01 vs IS group, ANOVA. (J) Representative cross-sections (hematoxylin and eosin staining) of the aortic sinus. Scale bar, 100 μm. (K) Quantification of the atherosclerotic plaque area in aortic root (n = 6). Data are mean ± SD. **P < .01 vs vehicle group, ##P < .01 vs IS group, ANOVA.
Discussion
The high incidence of thrombosis increases the risk for CKD patients. So far, there is still a lack of deep understanding about the etiology of CKD-associated thrombosis. In this study, we show for the first time that uremic solute IS contributes to the pathogenesis of CKD-associated thrombosis by enhancing platelet activity. Meanwhile, we also find that Klotho protein has the ability to counteract IS-induced platelet hyperactivity and thrombosis in CKD mice, which provides a potential new avenue for the treatment of CKD-associated thrombosis.
Platelets that are produced by megakaryocytes play crucial roles in various physiological and pathological events, including hemostasis and thrombosis.29 Although platelet dysfunction has been reported in CKD patients,30-32 the comprehensive changes in platelet activities in uremic environment and the related mechanisms are not fully recognized. Here, by using a CKD mouse model, we found that the activities of platelets were significantly enhanced with the development of CKD, manifested by elevated response to collagen and thrombin, increased levels of PMPs, and platelet-monocyte aggregates in the blood. Subsequently, we found that IS, a typical uremic toxin, which increases significantly in the blood with the impairment of renal function, had a distinct ability to enhance platelet activity both in vitro and in vivo. A high tendency of thrombus formation was accompanied with platelet hyperactivity in CKD mice and IS-treated mice, indicating that platelet hyperactivity induced by IS plays an important role in the pathogenesis of CKD-associated thrombosis.
As known, a typical characteristic of the CKD environment is high oxidative stress, a state mainly resulting from the accumulation of metabolism products. As an important protein-bound uremic solute that cannot be effectively eliminated from the body by routine dialysis, IS has a strong pro-oxidative property. It has been reported that IS impairs the functions of various cells and tissues by enhancing oxidative stress.19,33-36 Therefore, we next investigated whether oxidative stress is involved in IS-induced platelet hyperactivation. As expected, IS displayed a distinct ability to promote ROS production in platelets both in vitro and in vivo, whereas NAC, a ROS scavenger, could inhibit IS-induced platelet hyperactivation. Further, we found that p38MAPK activation, a main signal pathway downstream of ROS, mediated the effect of IS on platelet activation.
Apart from oxidative stress, IS is a potent endogenous agonist for aryl hydrocarbon receptor that plays important roles in platelet activation.37,38 Notably, the endothelium is a crucial modulator of platelet reactivity.39 We and others have found that IS can induce endothelial dysfunction via inducing oxidative stress,20,34,35 which may also make contributions to the development of thrombosis in CKD patients.
In our study, we applied several different approaches to systematically study the changes in the activities of platelets. Interestingly, we did not find a significant increase in the expression level of P-selectin, a widely used marker of platelet activation, in the resting platelets in CKD- and IS-treated mice, compared with that in the sham control group. Nevertheless, a significantly higher increase in P-selectin expression was detected in IS-treated platelets in response to collagen and thrombin in vitro. In contrast, marked increases in the circulating PMPs and platelet-monocyte aggregates were detected in the blood in CKD mice. It has been reported that activated platelets will degranulate rapidly and lose P-selectin expression in vivo, and continue to exist in circulation,40 which may explain the reason why P-selectin expression cannot directly reflect the platelet activation in CKD mice. Consistent with other studies,41,42 our results suggest that PMPs and platelet-monocyte aggregates may be more sensitive markers of platelet activation in CKD patients.
Besides thrombosis, platelets also participate in the formation and development of atherosclerotic lesions.43-45 In clinic, CKD is often associated with atherosclerosis.4 By using apoE−/− mice, a CKD-associated atherosclerosis mouse model was created for experimental study.46,47 In this study, we found that long-term administration with IS promoted atherosclerosis formation in apoE−/− mice, accompanied by increases in platelet activities, indicating that IS-induced platelet hyperactivation may be also involved in CKD-associated atherosclerosis.
Klotho is an aging suppressor protein and functions as a hormone.27 Klotho has a distinct ability to inhibit ROS generation and scavenge the overproduced ROS, thereby conferring oxidative stress resistance.28 Klotho is predominantly expressed in renal tubular cells.27 In contrast to the increase of serum IS, there is a significant decrease in Klotho during the progression of CKD.19,48 Interestingly, it has been reported that IS can decrease Klotho expression in the kidneys via DNA methylation.49 On the other hand, previous studies, including our own, have shown that Klotho has protective effects against IS-induced endothelial dysfunction and myocardial hypertrophy via inhibiting oxidative stress.19,20 In this study, we found that Klotho treatment remarkably attenuated IS-induced platelet hyperactivity both in vitro and in vivo through inhibiting IS-induced activation of ROS/p38MAPK signaling. Moreover, we observed that Klotho insufficiency might aggravate IS-induced platelet hyperactivation by using klotho+/− mice, and supplement with exogenous Klotho protein is an effective way to inhibit IS-induced platelet hyperactivation and thrombosis in CKD mice. Recently, some studies have reported that Klotho deficiency might contribute to the development of atherosclerosis and atherothrombosis.50-52 In our study, we showed that exogenous Klotho administration not only significantly attenuated IS-induced thrombosis, but also retarded the acceleration of atherosclerosis in apoE−/− mice with CKD. There are multiple targets of Klotho.27,53,54 As reported, Klotho is an endogenous inhibitor of insulin-like growth factor 1 receptor (IGF-1R), whereas IGF-1 can potentiate platelet activation via activating IGF-1R signaling.27,55 Therefore, the regulation of IGF-1R signaling may be also involved in the inhibitory effect of Klotho on IS-induced platelet activation. Our findings, together with the results of previous studies, demonstrate that there may exist a reciprocal inhibition between IS and Klotho in the pathological process of CKD and its complications.
In conclusion, this study not only discloses the mechanism of platelet hyperactivity in CKD environment, but also provides new insights into the pathogenesis and treatment of CKD-associated thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yang Liu and Haiying Ran for technical support for FACS, Mengyang Deng for assistance with animal experiments, Qing Zhou for assistance with histology analysis, Quanfang Wei for technical assistance in SEM, and Weibo Zhao for advice on mouse platelet activity assay.
This work was supported by grants from the National Natural Science Fund of China (81270290, 81500087, and 81573084), the Program for Scientific and Technological Innovation Leader of Chongqing (CSTCKJCXLJRC06), and the project for Chongqing Basic Science and Advanced Technology Research (cstc2015jcyjBX0028).
Authorship
Contribution: K.Y. performed experiments, analyzed data, and wrote the paper; C.D. performed experiments, analyzed data, and participated in manuscript preparation; X.W., F.L., F.C., M.S., and M.C. contributed to animal experiments and data analysis; Y.X., S.W., S.C., M.H., and T.H. contributed to the in vitro experiments and image scoring; Y.S. contributed to the initial experimental design and discussed the manuscript; and J.W. and J.Z. conceived and supervised the study, analyzed the data, and wrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jinghong Zhao, Department of Nephrology, Xinqiao Hospital, Third Military Medical University, Xinqiao St 183, Chongqing 400037, China; e-mail: zhaojh@tmmu.edu.cn; and Junping Wang, Chongqing Engineering Research Center for Nanomedicine, Institute of Combined Injury, Third Military Medical University, Chongqing 400038, China; e-mail: wangjunping@tmmu.edu.cn.
References
Author notes
K.Y. and C.D. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal