Key Points
aGVHD after hematopoietic stem cell transplantation is associated with lymphangiogenesis in the intestinal tract and lymph nodes.
Inhibition of lymphangiogenesis by antibodies against VEGFR-3 ameliorated aGVHD.
Abstract
Lymph vessels play a crucial role in immune reactions in health and disease. In oncology the inhibition of lymphangiogenesis is an established therapeutic concept for reducing metastatic spreading of tumor cells. During allogeneic tissue transplantation, the inhibition of lymphangiogenesis has been successfully used to attenuate graft rejection. Despite its critical importance for tumor growth, alloimmune responses, and inflammation, the role of lymphangiogenesis has not been investigated during allogeneic hematopoietic stem cell transplantation (allo-HSCT). We found that acute graft-versus-host disease (aGVHD) is associated with lymphangiogenesis in murine allo-HSCT models as well as in patient intestinal biopsies. Inhibition of aGVHD-associated lymphangiogenesis by monoclonal antibodies against vascular endothelial growth factor receptor 3 (VEGFR-3) ameliorated aGVHD and improved survival in murine models. The administration of anti-VEGFR-3 antibodies did not interfere with hematopoietic engraftment and improved immune reconstitution in allo-HSCT recipients with aGVHD. Anti-VEGFR-3 therapy had no significant impact on growth of malignant lymphoma after allo-HSCT. We conclude that aGVHD is associated with lymphangiogenesis in intestinal lesions and in lymph nodes. Our data show that anti-VEGFR-3 treatment ameliorates lethal aGVHD and identifies the lymphatic vasculature as a novel therapeutic target in the setting of allo-HSCT.
Introduction
It is well known that lymphangiogenesis plays a critical role in tumor metastasis and progression. Vascular endothelial growth factor C (VEGF-C) and its receptor, vascular endothelial growth factor receptor 3 (VEGFR-3), are the most promising therapeutic targets because of their crucial function in regulating lymphangiogenesis.1
Recent data suggest that the lymphatic vasculature is a therapeutic target for modifying allogeneic immune responses. Human studies showed increased lymphangiogenesis during rejection of skin, kidney, and lung allografts.2-4 In animal experiments, it has been shown that the inhibition of lymphangiogenesis mitigates alloimmune responses, leading to better outcome after tracheal, cardiac, islet, and corneal transplantation.5-10
The role of lymphangiogenesis during inflammation outside the setting of organ transplantation is less well established. A current study found that the inhibition of allergic eye disease–associated lymphangiogenesis with anti-VEGFR-3 treatment ameliorated inflammation and resulted in clinical improvement.11 Another group investigated interleukin 10 (IL-10)–deficient mice, which have reduced lymphangiogenesis and spontaneously develop colitis, and found that anti-VEGFR-3 treatment augmented inflammation.12 In line with this observation, it has been demonstrated that the stimulation of lymphatic function ameliorates experimental inflammatory bowel disease.13 Further research is clearly needed before definite conclusions on the role of lymphangiogenesis and its mechanisms of action in different inflammatory conditions can be made.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the most effective immunotherapy for cancer and the only stem cell therapy that is widely used in clinical medicine. The major obstacle to a more favorable therapeutic outcome is acute graft-versus-host disease (aGVHD). The role of lymphangiogenesis and its mechanisms in allo-HSCT and aGVHD are unknown. In this study, we ask whether aGVHD is associated with lymphangiogenesis in experimental models and in patients. We use murine allo-HSCT recipients to test the hypothesis that inhibition of lymphangiogenesis after allo-HSCT could ameliorate aGVHD without having negative effects on engraftment and on antitumor activity.
Materials and methods
Patient materials and podoplanin staining
The protocol for collecting human samples was approved by the institutional ethics committee of the Charité and was in accordance with the Declaration of Helsinki. Intestinal biopsies were collected from patients with suspected intestinal aGVHD after written informed consent was obtained. We performed a search in the biobank of our center to identify intestinal biopsies with aGVHD versus no GVHD taken after allo–stem cell transplantations (SCTs) performed between 2007 and 2016. We identified 12 duodenum biopsies from patients with histological grades III-IV aGVHD and 19 duodenum biopsies from allo-SCT recipients without histological evidence of GVHD. Furthermore, we identified 11 colon biopsies from patients with histological grades III-IV aGVHD and 10 colon biopsies from allo-SCT recipients without GVHD. Formalin-fixed, paraffin-embedded biopsies were assessed for histological GVHD scores by Lerner´s criteria.14 Lymph vessels were stained with a monoclonal mouse anti-human podoplanin antibody (Clone D2-40, DAKO, Hamburg, Germany).15 For quantification, lymph vessels in 10 microscopic high-power fields per sample were counted.
Mice
Female C57BL/6 (H-2b) and 129S2/SVPasCrl (129/SV) (H-2b) mice were purchased from Charles River Laboratories (Sulzfeld, Germany). Female BALB/c (H-2d) mice were purchased from Janvier Laboratory (Saint-Berthevin, France). Animals used in allo-HSCT experiments were 10 to 12 weeks old and housed in the Charité University Hospital Animal Facility under pathogen-free controlled conditions and a 12-hour light/dark cycle. All experiments were approved by the Regional Ethics Committee for Animal Research.
Conditioning regimen and allo-HSCT protocol
The allo-HSCT models are described in more detail elsewhere.16 In short, on 5 consecutive days, 20 mg/kg/day of busulfan (Sigma Aldrich, St. Louis, MO) was given by intraperitoneal injection (IP). Additionally, on day −4 + −5, 100 mg/kg/day of cyclophosphamide monohydrate (Sigma Aldrich) was given IP. For allo-HSCT, C57BL/6 animals received 1.5 × 107 bone marrow (BM) cells and 2 × 106 splenic T cells from LP/J or 129S2/SVPasCrl donor mice by tail vein injection on day 0.
GVHD monitoring
Allo-HSCT recipients were individually scored for five clinical parameters (posture, activity, fur, skin, and weight loss) on a scale from 0 to 2. Clinical GVHD score was assessed by summation of these parameters. Animals were sacrificed when exceeding a score of 6. Survival was monitored daily.
Antibody injections
After allo-HSCT on day 0, mice received IP injections of mF4-31c1 antibody (Eli Lilly & Co., Indianapolis, IN) or rat-IgG control antibody (Sigma-Aldrich) at a dose of 1 mg/mouse/shot. Mice were injected every second day until day +16 post–allo-HSCT or until organ harvesting.
Tumor experiments
For tumor experiments, recipient mice were conditioned with total body irradiation from a 137Cs source as a split dose (800 cGy for BALB/c and 1300 cGy for C57BL/6). BALB/c recipients received 5 × 106 BM cells and 3 × 105 splenic T cells from C57BL/6 donor mice. C57BL/6 mice received 1.5 × 107 BM cells and 3 × 105 splenic T cells from BALB/c donor mice. Additionally, 5 × 105 A20 (BALB/c) or EL4 (C57BL/6) tumor cells stably expressing firefly luciferase were injected intravenously on day 0. Allo-HSCT recipients were injected with 1 mg/mouse of mF4-31c1 or control antibody every second day from day 0 until day +16 post–allo-HSCT. Tumor growth was measured by bioluminescence imaging in an IVIS Lumina II system (PerkinElmer, Waltham, MA). Average radiance (measured in photons per second per centimeter squared per steradian) was determined using the Living Image 3.1 software (PerkinElmer).
Preparation of single-cell suspensions of lymph nodes
Lymph nodes were put in ice-cold Hank’s balanced salt solution (Thermo Scientific, Waltham, MA) and cut into small pieces, transferred into a 15-mL falcon, and centrifuged. The supernatant was carefully taken and used for lymphocyte staining. The pellet was further processed with a 0.6% collagenase II (Worthington, Lakewood, NJ)/0.4% DNase (Sigma) solution to isolate endothelial cells. All single-cell suspensions were collected in magnetic-activated cell sorting buffer (phosphate-buffered saline, 0.5 mM EDTA, 0.5% bovine serum albumin) before flow cytometry staining.
Statistics
Survival data were analyzed using the Kaplan-Meier method and compared with the Mantel-Cox log-rank test. For statistical analysis of all other data, the Student unpaired t test and Mann-Whitney U test were used. Values are presented as mean ± SEM; values of P ≤ .05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA).
Flow cytometry staining and histologic staining are described in the supplemental Methods and materials, available on the Blood Web site.
Results
Acute GVHD is associated with lymphangiogenesis during experimental GVHD
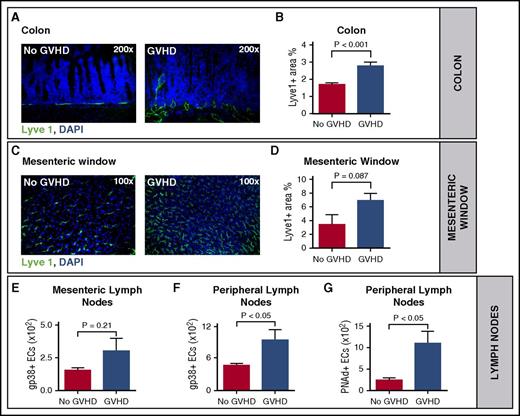
To determine the importance of lymphangiogenesis during aGVHD, we first used a major histocompatibility complex–matched, minor histocompatibility antigen–mismatched murine allo-HSCT model.16 We quantified lymph vessel density in aGVHD target organs (colon and liver) by immunofluorescence microscopy after staining with antibodies against the lymph vessel marker Lyve1. In the colon, we found a significant higher lymph vessel density in allo-HSCT recipients during aGVHD in comparison with syngeneic controls (syn-HSCT) (Figure 1A-B). To exclude confounding Lyve1 expression by myeloid cells, we performed double staining in colon tissue from control antibody and anti-VEGFR-3 antibody-treated allo-HSCT recipients using CD11b or F4/80 (supplemental Figure 1). We found no colocalization of F4/80 or CD11b and the lymph vessel marker Lyve1, suggesting that Lyve1 expression was restricted to lymphatic vessels in colon tissue.
Acute GVHD is associated with lymphangiogenesis during experimental GVHD. (A) Representative images of increased lymphangiogenesis in the colon during GVHD (right) versus no GVHD (left) on day +15 after HSCT. Colon sections of HSCT recipients with GVHD and without GVHD were stained with Lyve1 antibody (green) and counterstained with the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI). (B) Quantification of the Lyve1 positive area in the colon on day +15 after HSCT (n = 5 per group). (C) Lymph vessels in the mesenteric window during GVHD (right) versus no GVHD (left) on day +15 after HSCT. Isolated mesenteric windows of HSCT recipients with GVHD versus no GVHD were stained with Lyve1 antibody (green) and counterstained with DAPI. (D) Quantification of the Lyve1 positive area in the mesenteric window on day +15 after HSCT (n = 4, no GVHD; n = 5, GVHD). (E-F) Quantification of lymphatic endothelial cells (gp38+ ECs) via fluorescence-activated cell sorter (FACS). Endothelial cells were isolated from mesenteric (n = 6 per group) (E) and peripheral (n = 3 per group) (F) lymph nodes of HSCT recipients with GVHD versus no GVHD on day +7 after HSCT. (G) Quantification of peripheral lymph node addressin (PNAd)–positive endothelial cells in peripheral lymph nodes of HSCT recipients with GVHD versus no GVHD (n = 3 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. (A-E) LP/J → C57BL/6 (1.5 × 107 BM cells, 2 × 106 splenic T cells). (F-G) C57BL/6 → BALB/c (5 × 106 BM cells, 1 × 106 splenic T cells).
Acute GVHD is associated with lymphangiogenesis during experimental GVHD. (A) Representative images of increased lymphangiogenesis in the colon during GVHD (right) versus no GVHD (left) on day +15 after HSCT. Colon sections of HSCT recipients with GVHD and without GVHD were stained with Lyve1 antibody (green) and counterstained with the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI). (B) Quantification of the Lyve1 positive area in the colon on day +15 after HSCT (n = 5 per group). (C) Lymph vessels in the mesenteric window during GVHD (right) versus no GVHD (left) on day +15 after HSCT. Isolated mesenteric windows of HSCT recipients with GVHD versus no GVHD were stained with Lyve1 antibody (green) and counterstained with DAPI. (D) Quantification of the Lyve1 positive area in the mesenteric window on day +15 after HSCT (n = 4, no GVHD; n = 5, GVHD). (E-F) Quantification of lymphatic endothelial cells (gp38+ ECs) via fluorescence-activated cell sorter (FACS). Endothelial cells were isolated from mesenteric (n = 6 per group) (E) and peripheral (n = 3 per group) (F) lymph nodes of HSCT recipients with GVHD versus no GVHD on day +7 after HSCT. (G) Quantification of peripheral lymph node addressin (PNAd)–positive endothelial cells in peripheral lymph nodes of HSCT recipients with GVHD versus no GVHD (n = 3 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. (A-E) LP/J → C57BL/6 (1.5 × 107 BM cells, 2 × 106 splenic T cells). (F-G) C57BL/6 → BALB/c (5 × 106 BM cells, 1 × 106 splenic T cells).
In the liver, we also found a significantly elevated Lyve1 area during aGVHD (supplemental Figure 2). However, as Lyve1 expression in liver tissue is not restricted to lymph vessels and its expression pattern in liver blood sinusoids differs under disease conditions such as inflammation, a quantification of lymph vessels by Lyve1 staining in liver tissue during aGVHD may not be reliable.17,18
Next, we were interested in the mesentery, which contains a high amount of lymph vessels and intestinal draining lymph nodes. In mesenteric windows we found a tendency toward a higher Lyve1 area during aGVHD versus syngeneic controls without GVHD, which did not reach statistical significance (Figure 1C-D).
Furthermore, we checked the mRNA expression levels of VEGF-C in syngeneic and allogeneic transplanted mice in the GVHD target organs liver and colon (supplemental Figure 3). At the maximum of aGVHD (day +15) we didn’t see a significant increase of VEGF-C mRNA expression in the liver or colon.
In addition, we quantified the number of lymphatic endothelial cells in lymph nodes from HSCT recipients with aGVHD versus no GVHD by flow cytometry using an antibody against podoplanin (gp38).19 We found a nonsignificant trend toward an increased number of gp38- positive endothelial cells in mesenteric lymph nodes of allo-HSCT recipients with aGVHD in comparison with syn-HSCT recipients without GVHD (Figure 1E). In peripheral lymph nodes we found significantly more gp38+ lymphatic endothelial cells during aGVHD in comparison with no GVHD (Figure 1F). Peripheral lymph node addressin (PNAd), also termed MECA79, is expressed on high endothelial venules of lymphoid tissue, including peripheral and mesenteric lymph nodes.20 The homing of lymphocytes to peripheral lymph nodes is initiated by an adhesive interaction between l-selectin on lymphocytes and PNAd. As is shown in Figure 1G, the number of PNAd-positive endothelial cells was significantly higher in allo-HSCT recipients with aGVHD in comparison with syn-HSCT recipients without GVHD.
We conclude that aGVHD is associated with lymphangiogenesis in colon and peripheral lymph nodes.
Lymph vessels are increased in intestinal lesions during GVHD in humans
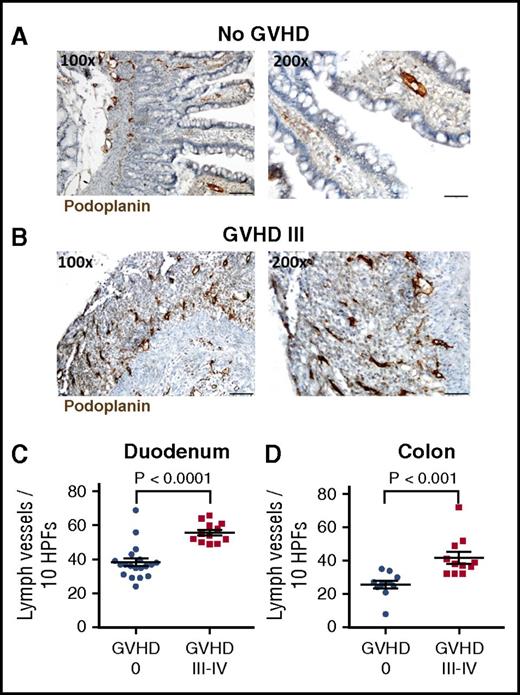
To review our findings in the clinical situation, we examined lymph vessel density in small intestinal biopsies from patients during aGVHD versus no GVHD. We stained tissue sections against the lymph vessel marker podoplanin and found increased staining in high- grade GVHD. Figure 2 demonstrates typical examples of antipodoplanin staining in duodenal biopsies. Figure 2A shows villous duodenal tissue with othotopic lymphatic vascular architecture at the mucosal and submucosal levels. In ulcerative lesions of severe GVHD (Lerner grade III), we found a highly increased density of lymph vessels (Figure 2B) focused at the former mucosal level as a consequence of regenerative vascular proliferation corresponding to granulation tissue. We quantified lymph vessels of duodenum and colon biopsies taken from patients after allo-SCT without GVHD (GVHD 0) and patients with histological grades III-IV aGVHD (Figure 2C-D). In duodenum as well as in colon biopsies, we found significantly elevated numbers of lymph vessels in patients with aGVHD III-IV in comparison with patients without GVHD. Comparing murine tissue sections with the human samples (supplemental Figure 4), we found similar results: lymphangiogenesis prevalently occurred in degenerated mucosal structures and ulceration.
Lymph vessels are increased in intestinal lesions during GVHD in humans. Representative images of lymph vessels in the duodenum of patients without intestinal GVHD (A) and with grade III intestinal GVHD (B). Sections were stained with podoplanin antibody (brown), which is specifically expressed by lymphatic endothelial cells. Destructive mucosal lesions during severe GVHD are associated with lymphatic vascular proliferation. (C-D) Quantification of lymph vessels in duodenum (C) and colon (D) biopsies from patients after allo-SCT without GVHD (GVHD 0) and patients with histological aGVHD grades III-IV. Number of lymph vessels in 10 high-power fields (HPFs) was determined. Bars in ×100 magnification, 100 µm; and in ×200 magnification, 50 µm; n = 12-19 (C); n = 10-11 (D). Error bars indicate mean ± SEM; significance was tested by the Mann-Whitney U test.
Lymph vessels are increased in intestinal lesions during GVHD in humans. Representative images of lymph vessels in the duodenum of patients without intestinal GVHD (A) and with grade III intestinal GVHD (B). Sections were stained with podoplanin antibody (brown), which is specifically expressed by lymphatic endothelial cells. Destructive mucosal lesions during severe GVHD are associated with lymphatic vascular proliferation. (C-D) Quantification of lymph vessels in duodenum (C) and colon (D) biopsies from patients after allo-SCT without GVHD (GVHD 0) and patients with histological aGVHD grades III-IV. Number of lymph vessels in 10 high-power fields (HPFs) was determined. Bars in ×100 magnification, 100 µm; and in ×200 magnification, 50 µm; n = 12-19 (C); n = 10-11 (D). Error bars indicate mean ± SEM; significance was tested by the Mann-Whitney U test.
We conclude that severe intestinal GVHD in humans is associated with increased lymphangiogenesis in destructive mucosal lesions, which correlates to the murine situation.
Anti-VEGFR-3 treatment results in inhibition of GVHD-associated lymphangiogenesis
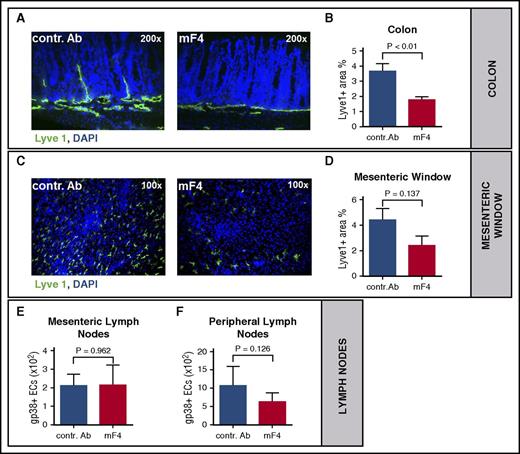
To selectively inhibit lymphangiogenesis during GVHD, we used the anti-VEGFR-3 monoclonal antibody mF4-31c1, which specifically antagonizes the binding of VEGF-C to VEGFR-3.21,22 We first confirmed by flow cytometry that immune cells, such as T cells, B cells, granulocytes, dendritic cells and monocytes do not express VEGFR-3 in different wild type mice strains as well as in allo-HSCT recipients with aGVHD (data not shown). In the LP/J → C57BL/6 model we injected mF4-31c1 or control antibody at a dose of 1mg per mouse every second day from day 0 and harvested organs for immunofluorescent staining as well as flow cytometry. Quantification of Lyve1 positive area showed a significant reduction of lymph vessel density in the colon of mF4-31c1-treated allo-HSCT recipients vs control antibody treated allo-HSCT recipients (Figure 3A-B). In the mesentery we found a non-significant trend toward a lower lymph vessel density in mF4-31c1-treated allo-HSCT recipients vs control antibody treated allo-HSCT recipients during aGVHD (Figure 3C-D). We next analyzed mesenteric lymph nodes and found no significant differences in lymph vessel density between the groups (Figure 3E). The amount of lymph vessel specific endothelial cells in peripheral lymph nodes of mF4-31c1 treated allo-HSCT recipients was moderately reduced (Figure 3F), again without reaching statistical significance. To exclude the possibility that anti-VEGFR-3 therapy has any effects on hemangiogenesis, we stained colon and liver sections from control antibody and mF4-31c1 treated allo-HSCT recipients with aGVHD against the blood vessel marker CD31 and found no significant differences in blood vessel density (supplemental Figure 5) between the GVHD vs no GVHD cohorts.
Anti-VEGFR-3 treatment results in inhibition of GVHD-associated lymphangiogenesis. Allo-HSCT recipients received intraperitoneal injections of 1 mg anti-VEGFR-3 antibody (mF4-31c1) or control antibody every second day from day 0 to day +10 or day +14: LP/J or 129/SV → C57BL/6, conditioning with Bu/Cy, allo-HSCT with 1.5 × 107 BM cells, 2 × 106 T cells. (A) Visualization of the reduction of lymphangiogenesis after anti-VEGFR-3 treatment in the colon on day +15 after allo-HSCT. Colon sections of mice treated with control antibody or anti-VEGFR-3 antibody (mF4-31c1) were stained with Lyve1 antibody (green) and counterstained with DAPI. (B) Quantification of Lyve1 positive area in the colon after control antibody or mF4-31c1 treatment (n = 4 per group). (C) Representative images of lymph vessels in the mesenteric window of mF4-31c1 antibody versus control antibody-treated allo-HSCT recipients. Mesenteric windows were taken on day +11 after HSCT and stained against Lyve1 (green) and counterstained with DAPI. (D) Quantitative analysis of Lyve1 positive area of mesenteric windows from control antibody and mF4-31c1– treated allo-HSCT recipients on day +11 post–allo-HSCT (n = 4 per group). (E-F) Quantification of lymphatic endothelial cells (gp38+ ECs) in mesenteric (E) and peripheral (F) lymph nodes via FACS. Endothelial cells were isolated from mesenteric and peripheral lymph nodes of the control antibody and mF4-31c1–treated allo-HSCT recipients on day +11 (n = 5 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. contr. Ab, control antibody.
Anti-VEGFR-3 treatment results in inhibition of GVHD-associated lymphangiogenesis. Allo-HSCT recipients received intraperitoneal injections of 1 mg anti-VEGFR-3 antibody (mF4-31c1) or control antibody every second day from day 0 to day +10 or day +14: LP/J or 129/SV → C57BL/6, conditioning with Bu/Cy, allo-HSCT with 1.5 × 107 BM cells, 2 × 106 T cells. (A) Visualization of the reduction of lymphangiogenesis after anti-VEGFR-3 treatment in the colon on day +15 after allo-HSCT. Colon sections of mice treated with control antibody or anti-VEGFR-3 antibody (mF4-31c1) were stained with Lyve1 antibody (green) and counterstained with DAPI. (B) Quantification of Lyve1 positive area in the colon after control antibody or mF4-31c1 treatment (n = 4 per group). (C) Representative images of lymph vessels in the mesenteric window of mF4-31c1 antibody versus control antibody-treated allo-HSCT recipients. Mesenteric windows were taken on day +11 after HSCT and stained against Lyve1 (green) and counterstained with DAPI. (D) Quantitative analysis of Lyve1 positive area of mesenteric windows from control antibody and mF4-31c1– treated allo-HSCT recipients on day +11 post–allo-HSCT (n = 4 per group). (E-F) Quantification of lymphatic endothelial cells (gp38+ ECs) in mesenteric (E) and peripheral (F) lymph nodes via FACS. Endothelial cells were isolated from mesenteric and peripheral lymph nodes of the control antibody and mF4-31c1–treated allo-HSCT recipients on day +11 (n = 5 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. contr. Ab, control antibody.
We conclude that the anti-VEGFR-3 antibody mF4-31c1 effectively inhibits GVHD-associated lymphangiogenesis in colon.
Anti-VEGFR-3 treatment ameliorates lethal GVHD
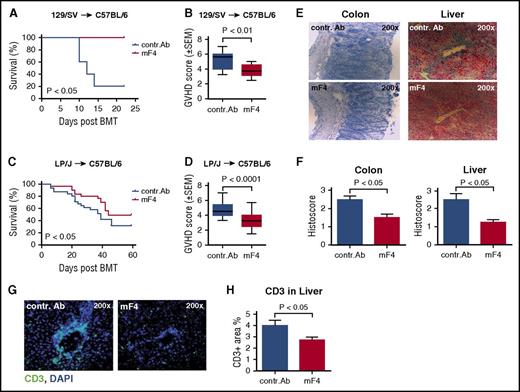
To analyze the effect of lymphangiogenesis inhibition on the severity of aGVHD, we performed experiments with two different well-characterized minor-mismatch allo-HSCT models: 129/SV→C57BL/6 and LP/J→C57BL/6.16 We injected the anti-VEGFR-3 antibody mF4-31c1 at a dose of 1 mg per recipient every second day from day 0 until day +16 after allo-HSCT. In both models, the GVHD-related mortality was significantly reduced in mF4-31c1–treated allo-HSCT recipients in comparison with control antibody-treated allo-HSCT recipients (Figure 4A,C). Furthermore, clinical GVHD scores were significantly lower in mF4-31c1–treated allo-HSCT recipients versus control antibody-treated allo-HSCT recipients in both models (Figure 4B,D). The more prominent effect of anti-VEGFR-3 treatment on survival in the 129/SV→C57BL/6 model is likely due to faster GVHD-related mortality in this model in comparison with the LP/J→C57BL/6 model.
Anti-VEGFR-3 treatment ameliorates lethal GVHD. Allo-HSCT recipients received intraperitoneal injections of 1 mg anti-VEGFR-3 antibody (mF4-31c1) or control antibody every second day from day 0 to day +16. LP/J or 129/SV → C57BL/6, conditioning with Bu/Cy; allo-HSCT with 1.5 × 107 BM cells, 2 × 106 T cells. (A) Survival curve of control antibody and mF4-31c1 treated allo-HSCT recipients using the 129/SV → C57BL/6 model (n = 5 per group), data from one representative experiment are shown, analysis with the log-rank test. (B) GVHD scores of control antibody and mF4-31c1–treated allo-HSCT recipients on day +14 using the 129/SV → C57BL/6 model. Data from one representative experiment are shown (n = 5 per group). (C) Survival curve in the LP/J → C57BL/6 model. Combined data from three independent experiments are presented (n = 28 per group). Analysis was done with the log-rank test. (D) GVHD scores of control antibody versus mF4-31c1–treated allo-HSCT recipients in the LP/J → C57BL/6 model on day +18 after HSCT. Combined data from three independent experiments are presented (n = 28 per group), analysis with the log-rank test. (E) Representative images of histopathology in the colon and liver. Organs were taken on day +11 after HSCT; histological staining was performed with hematoxylin and eosin (n = 4 per group). (F) Histopathological scores of colon and liver sections from control antibody and mF4-31c1–treated allo-HSCT recipients on day +11 after HSCT; scoring was done according to Lerner criteria (n = 4 per group). (G) Representative images of CD3+ cell infiltration in the liver. Organs were taken on day +11, liver sections were stained with CD3 antibody (green) and counterstained with DAPI. (H) Quantification of CD3 positive area in the liver of control antibody or mF4-31c1–treated mice (n = 4 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. BMT, bone marrow transplantation.
Anti-VEGFR-3 treatment ameliorates lethal GVHD. Allo-HSCT recipients received intraperitoneal injections of 1 mg anti-VEGFR-3 antibody (mF4-31c1) or control antibody every second day from day 0 to day +16. LP/J or 129/SV → C57BL/6, conditioning with Bu/Cy; allo-HSCT with 1.5 × 107 BM cells, 2 × 106 T cells. (A) Survival curve of control antibody and mF4-31c1 treated allo-HSCT recipients using the 129/SV → C57BL/6 model (n = 5 per group), data from one representative experiment are shown, analysis with the log-rank test. (B) GVHD scores of control antibody and mF4-31c1–treated allo-HSCT recipients on day +14 using the 129/SV → C57BL/6 model. Data from one representative experiment are shown (n = 5 per group). (C) Survival curve in the LP/J → C57BL/6 model. Combined data from three independent experiments are presented (n = 28 per group). Analysis was done with the log-rank test. (D) GVHD scores of control antibody versus mF4-31c1–treated allo-HSCT recipients in the LP/J → C57BL/6 model on day +18 after HSCT. Combined data from three independent experiments are presented (n = 28 per group), analysis with the log-rank test. (E) Representative images of histopathology in the colon and liver. Organs were taken on day +11 after HSCT; histological staining was performed with hematoxylin and eosin (n = 4 per group). (F) Histopathological scores of colon and liver sections from control antibody and mF4-31c1–treated allo-HSCT recipients on day +11 after HSCT; scoring was done according to Lerner criteria (n = 4 per group). (G) Representative images of CD3+ cell infiltration in the liver. Organs were taken on day +11, liver sections were stained with CD3 antibody (green) and counterstained with DAPI. (H) Quantification of CD3 positive area in the liver of control antibody or mF4-31c1–treated mice (n = 4 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. BMT, bone marrow transplantation.
Next, we analyzed histological GVHD scores in mF4-31c1–treated allo-HSCT recipients versus control antibody-treated allo-HSCT recipients. Figure 4E visualizes profoundly diminished target organ GVHD in the liver and colon as a result of anti-VEGFR-3 treatment. Quantification of histological scoring demonstrates significantly less GVHD-associated target organ damage in the liver and colon of mF4-31c1–treated allo-HSCT recipients versus control antibody-treated allo-HSCT recipients (Figure 4F). Also, the numbers of tissue-infiltrating CD3+ T cells in GVHD target organs were diminished as a result of the inhibition of lymphangiogenesis by mF4-31c1 treatment (Figure 4G-H).
Taken together, the inhibition of lymphangiogenesis by anti-VEGFR-3 treatment reduces GVHD-associated target organ damage and mortality after allo-HSCT.
Effect of anti-VEGFR-3 treatment on hematopoietic reconstitution after allo-HSCT
In anti-VEGFR-1/anti-VEGFR-2–treated allo-HSCT recipients, we have previously found an inhibition of hematopoietic engraftment.23 Therefore, we were specifically interested in the effect of anti-VEGFR-3 antibody treatment on hematopoietic reconstitution. In the 129/SV → C57BL/6 model, recipients received mF4-31c1 antibody or control antibody at a dose of 1 mg every second day from day 0 to day +10. On day +11, allo-HSCT recipients were sacrificed, and cells from the blood, bone marrow, and spleen were analyzed via flow cytometry. As shown in Figure 5A-B, treatment with mF4-31c1 does not inhibit donor cell engraftment in blood or bone marrow.
Effect of anti-VEGFR-3 treatment on hematopoietic reconstitution after allo-HSCT. Allo-HSCT recipients received intraperitoneal injections of 1 mg anti-VEGFR-3 antibody (mF4-31c1) or control antibody every second day from day 0 to day +10; 129/SV → C57BL/6, conditioning with Bu/Cy, allo-HSCT with 1.5 × 107 BM cells, 2 × 106 T cells. On day +11, allo-HSCT recipients were sacrificed, and organs were harvested for cell isolation and FACS analysis. (A-B) Chimerism was analyzed by staining of bone marrow cells (A) and blood cells (B) against Ly9.1 and H2kb (n = 4 per group). (C-J) Cell counts of immune cells in the blood of control antibody versus mF4-31c1–treated animals measured by flow cytometry (n = 4 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. TReg, regulatory T-cells.
Effect of anti-VEGFR-3 treatment on hematopoietic reconstitution after allo-HSCT. Allo-HSCT recipients received intraperitoneal injections of 1 mg anti-VEGFR-3 antibody (mF4-31c1) or control antibody every second day from day 0 to day +10; 129/SV → C57BL/6, conditioning with Bu/Cy, allo-HSCT with 1.5 × 107 BM cells, 2 × 106 T cells. On day +11, allo-HSCT recipients were sacrificed, and organs were harvested for cell isolation and FACS analysis. (A-B) Chimerism was analyzed by staining of bone marrow cells (A) and blood cells (B) against Ly9.1 and H2kb (n = 4 per group). (C-J) Cell counts of immune cells in the blood of control antibody versus mF4-31c1–treated animals measured by flow cytometry (n = 4 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. TReg, regulatory T-cells.
In mF4-31c1–treated allo-HSCT recipients versus control antibody-treated allo-HSCT recipients, we found significantly higher numbers of total donor leukocytes in the peripheral blood (Figure 5C). The higher number of leukocytes in anti-VEGFR-3–treated allo-HSCT recipients was due to elevated numbers of different donor cell subpopulations, including myeloid cells such as granulocytes (Figure 5D), monocytes (Figure 5E), dendritic cells (Figure 5F), B-lymphocytes (Figure 5G), and T-lymphocytes (Figure 5H-J). Regarding T-lymphocytes, we found higher numbers of CD4+ (Figure 5H), CD8+ (Figure 5I), and FoxP3+ regulatory T cells (Figure 5J) in mF4-31c1–treated allo-HSCT recipients versus control antibody-treated allo-HSCT recipients.
Next, we checked the effect of anti-VEGFR-3 therapy in the syngeneic situation using the same conditioning and antibody treatment. In mF4-31c1–treated syn-HSCT recipients, we found no significant differences in any of the analyzed cell populations (supplemental Figure 6). Because our data from syn-transplanted animals did not show the same result upon anti-VEGFR-3 treatment, we speculate that improved immune reconstitution after allo-HSCT was due to reduced bone marrow GVHD.24
Effect of anti-VEGFR-3 treatment on tumor-associated mortality and tumor growth post–allo-HSCT
To investigate whether the inhibition of lymphangiogenesis influences tumor growth, we performed tumor experiments in the C57BL/6 → BALB/c model with the A20 B-cell lymphoma cell line as well as in the BALB/c → C57BL/6 model with the EL4 T-cell lymphoma cell line (Figures 6A and 7A).25 To enable monitoring of tumor growth by in vivo bioluminescent signal intensity measurement, we used luciferase-expressing tumor cells, which were injected intravenously on day 0. Allo-HSCT recipients received the anti-VEGFR-3 antibody mF4-31c1 or control antibody at a dose of 1 mg per mouse every second day from day 0 until day +16.
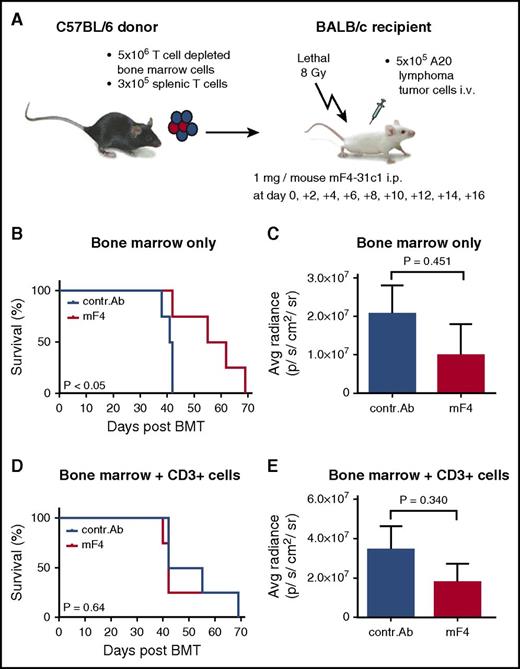
Effect of anti-VEGFR-3 treatment on tumor-associated mortality and tumor growth post–allo-HSCT in the C57BL/6 → BALB/c model. BALB/c allo-HSCT recipients were injected IV with 5 × 106 C57BL/6 bone marrow cells and 5 × 105 A20-TGL tumor cells. One milligram per mouse of control antibody or mF4-31c1 antibody was injected every second day from day 0 to day +16. (A) Schematic representation of the C57BL/6 → BALB/c GVHD model used for tumor experiments. (B-C) Allo-HSCT with bone marrow only; no T cells were given. (B) Survival curve of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Data from one representative experiment are shown; analysis was done with the log-rank test. (C) Average radiance data of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). (D-E) Allo-HSCT with bone marrow and additional 3 × 105 donor splenic T cells. (D) Survival curve of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Data from one representative experiment are shown; analysis was done with the log-rank test. (E) Average radiance data of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. Avg, average.
Effect of anti-VEGFR-3 treatment on tumor-associated mortality and tumor growth post–allo-HSCT in the C57BL/6 → BALB/c model. BALB/c allo-HSCT recipients were injected IV with 5 × 106 C57BL/6 bone marrow cells and 5 × 105 A20-TGL tumor cells. One milligram per mouse of control antibody or mF4-31c1 antibody was injected every second day from day 0 to day +16. (A) Schematic representation of the C57BL/6 → BALB/c GVHD model used for tumor experiments. (B-C) Allo-HSCT with bone marrow only; no T cells were given. (B) Survival curve of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Data from one representative experiment are shown; analysis was done with the log-rank test. (C) Average radiance data of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). (D-E) Allo-HSCT with bone marrow and additional 3 × 105 donor splenic T cells. (D) Survival curve of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Data from one representative experiment are shown; analysis was done with the log-rank test. (E) Average radiance data of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test. Avg, average.
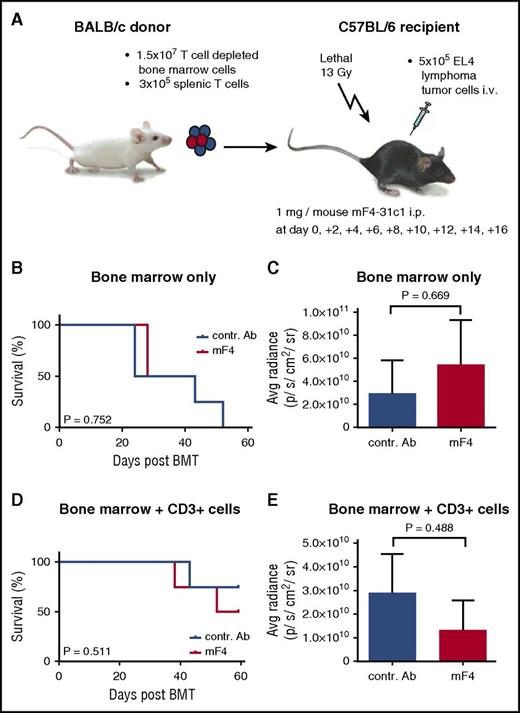
Effect of anti-VEGFR-3 treatment on tumor-associated mortality and tumor growth post–allo-HSCT in the BALB/c → C57BL/6 model. C57BL/6 allo-HSCT recipients received 1.5 × 107 BALB/c bone marrow cells and 5 × 105 EL4-TGL tumor cells. One milligram per mouse of control antibody or mF4-31c1 antibody was injected every second day from day 0 to day +16. (A) Schematic representation of the BALB/c → C57BL/6 GVHD model used for tumor experiments. (B-C) Allo-HSCT with bone marrow only; no T cells were given. (B) Survival curve of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Data from one representative experiment are shown, analysis with the log-rank test. (C) Average radiance data of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). (D-E) Allo-HSCT with bone marrow and additional 3 × 105 donor splenic T cells. (D) Survival curve of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Data from one representative experiment are shown, analysis with the log-rank test. (E) Average radiance data of control antibody and mF4-31c1– treated allo-HSCT recipients (n = 4 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test.
Effect of anti-VEGFR-3 treatment on tumor-associated mortality and tumor growth post–allo-HSCT in the BALB/c → C57BL/6 model. C57BL/6 allo-HSCT recipients received 1.5 × 107 BALB/c bone marrow cells and 5 × 105 EL4-TGL tumor cells. One milligram per mouse of control antibody or mF4-31c1 antibody was injected every second day from day 0 to day +16. (A) Schematic representation of the BALB/c → C57BL/6 GVHD model used for tumor experiments. (B-C) Allo-HSCT with bone marrow only; no T cells were given. (B) Survival curve of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Data from one representative experiment are shown, analysis with the log-rank test. (C) Average radiance data of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). (D-E) Allo-HSCT with bone marrow and additional 3 × 105 donor splenic T cells. (D) Survival curve of control antibody and mF4-31c1–treated allo-HSCT recipients (n = 4 per group). Data from one representative experiment are shown, analysis with the log-rank test. (E) Average radiance data of control antibody and mF4-31c1– treated allo-HSCT recipients (n = 4 per group). Error bars indicate mean ± SEM; significance was tested with an unpaired Student t test.
First, we investigated the effect of anti-VEGFR-3 treatment on A20 tumor growth in BALB/c allo-HSCT recipients transplanted with C57BL/6 T-cell–depleted bone marrow or bone marrow with additional T cells. We found that mF4-31c1 treatment resulted in a significant reduction of mortality (P < .05) in the bone marrow–only group (Figure 6B), but we saw no difference in survival when mice were transplanted with bone marrow and T cells (Figure 6D). In this experiment, all animals that died had a high tumor load, making it very likely that deaths were tumor associated. At day +41 we quantified tumor growth in bioluminescence in vivo imaging using the IVIS system. In both settings, with or without transplanting additional T cells, we found no significant differences of average radiance (photons per second per centimeter squared per steradian) between anti-VEGFR-3 and control antibody-treated allo-HSCT recipients (Figure 6C,E).
In addition, we investigated the effect of anti-VEGFR-3 treatment on tumor growth in another model using EL4 lymphoma cells (Figure 7A). C57BL/6 allo-HSCT recipients were transplanted with BALB/c T-cell–depleted bone marrow without additional T cells. In this model, almost all animals showed tumor engraftment, but we found no significant effects of mF4-31c1 treatment on mortality (P > .05) (Figure 7B). At day +35 we quantified tumor growth in bioluminescence in vivo imaging and found no significant differences in average radiance (photons per second per centimeter squared per steradian) between mF4-31c1–treated and control antibody-treated allo-HSCT recipients (Figure 7C). When we added allogeneic T cells, we saw no significant effects of mF4-31c1 on tumor growth or survival (Figure 7D-E).
We conclude that anti-VEGFR-3 treatment did not have a significant influence on malignant lymphoma growth in allo-HSCT recipients in our models.
Discussion
To our knowledge, this is the first study investigating the role of lymphangiogenesis during allo-HSCT. We found that aGVHD is associated with enhanced lymphangiogenesis. Our observation positively connects to results from D’Alessio et al, who found an increased lymph vessel density in patients suffering from Crohn’s disease and ulcerative colitis.13 Our data are also in line with a series of previous publications on lymphangiogenesis during rejection of allogeneic tissue transplants, including kidney,3 pancreatic islet,7 skin,2 cornea,8-10 heart,6 lung,4 and trachea.5 Alitalo proposed a model of how lymphangiogenesis regulates graft survival during solid organ transplantation26 : Lymphatic vessel activation occurs in response to VEGF-C, which is produced by inflammatory cells. Lymphangiogenesis enhances the flow of lymph-containing soluble antigens and activated antigen-presenting cells to lymph nodes.27 The higher number of antigens and antigen-presenting cells in lymph nodes and spleen consequently lead to enhanced allogeneic T-cell responses and rejection of the graft. This model is supported by preclinical data from Cursiefen’s group, showing that lymphatic vessels primarily mediate corneal rejection and demonstrating that antilymphangiogenic therapy leads to better graft survival.8,28 The therapeutic efficacy of blocking lymphangiogenesis to prolong allograft survival was confirmed and extended by others in experimental models of islet, tracheal, cardiac, and corneal transplantation.5-7,9,10,29 Our finding that therapy with the anti-VEGFR-3 antibody mF4-31c1 ameliorates aGVHD adds further evidence to the hypothesis that the inhibition of lymphangiogenesis can attenuate allogeneic immune responses.
There are several possible mechanisms that may be involved in anti-VEGFR-3 effects on aGVHD. The main mechanism with which anti-VEGFR-3 treatment mediates its beneficial effect on aGVHD is likely to be the inhibition of lymphangiogenesis in GVHD target organs and in draining lymph nodes. In this article, we demonstrate increased lymph vessel density during severe aGVHD. On the basis of the previously mentioned experimental data from solid organ transplantation describing that higher lymph vessel density correlates with increased lymph flow and increased antigen presentation,8,26 we speculate that this effect contributes to alloactivation during aGVHD. By inhibiting lymphangiogenesis, we were able to reduce lymph vessel density during aGVHD, possibly leading to reduced lymph flow and diminished immune activation during aGVHD.
An alternative mechanism, which may have contributed to ameliorated aGVHD, is the impact of anti-VEGFR-3 therapy on the permeability of the lymphatic endothelium. The group of Silvio Danese demonstrated that the activation of VEGFR-3 on lymphatic endothelial cells leads to loosening of cell-cell contacts and a reduced lymphatic endothelial barrier.30 Therefore, anti-VEGFR-3 treatment could reduce the permeability of lymph vessels in target organs impeding the diapedesis of inflammatory leukocytes.
Another mechanism could be VEGF-C binding to VEGFR-2, which has been described previously.31 Upon blocking the binding of VEGF-C to VEGFR-3, VEGF-C may alternatively have increased binding to VEGFR-2. However, our data argue against an important role of VEGFR-2 during aGVHD,23 making this possibility less likely.
It is reported in the literature that VEGFR-3 is able to influence blood vessel angiogenesis not only in embryogenesis but also in adulthood.32 Our data suggest that the inhibition of VEGFR-3 is independent of hemangiogenesis and has no impact on blood vessels.
Finally, VEGFR-3 has been described as being expressed on small subsets of macrophages and dendritic cells.33,34 Therefore, we cannot exclude the possibility that the anti-VEGFR-3 antibody had off-target effects outside the lymphatic vasculature that may have contributed to the observed efficacy. However, this possibility appears less likely because the specificity of the antimurine VEGFR-3 blocking antibody, which we have used in our experiments, to bind to lymphatic vasculature was previously demonstrated.22 In addition, we found no considerable expression of VEGFR-3 on immune cells during aGVHD.
In our experimental models as well as in patient biopsies, we found that aGVHD is associated with increased lymph vessel density in the intestinal tract. In the murine models, we used anti-VEGFR-3 antibodies and observed the most effective reduction of lymph vessel density in the colon, whereas the reduction of lymph vessel density in lymph nodes and the mesentery was less prominent. This may be due to target organ tropism of GVHD. However, tissue heterogeneity of the lymphatic vascular system, which has been described in detail recently,35 could also be involved. Depending on the organ and the associated physiologic and pathologic condition, lymph vessels show tissue-specific functional specialization. For example, lymph vessels in the intestinal tract and the mesentery show specialized features for fat absorption and metabolism, a different junctional organization than skin dermal capillary vessels, and display a set of differentially expressed genes, in comparison with dermal lymphatic endothelial cells. Nevertheless, our murine data showing lymphangiogenesis in destructive mucosal lesions in the colon strongly correlate with the pathogenic situation in the patient, in which we see most prominent lymphangiogenesis in mucosal ulcerations.
In tumor-bearing allo-HSCT recipients, we found no significant differences in malignant lymphoma growth between anti-VEGFR-3 antibody and control antibody-treated animals. These data contrast with the critical role, which the lymphatic system has, of spreading of solid tumor cells.36 Increased intratumoral lymph vessel density correlates positively with metastases and has been used to predict survival in cancer patients.37 Furthermore, overexpression of VEGF-C was found to promote lymph node metastasis,38 breast cancer,39 mammary cancer,40 and salivary gland cancer.41
We found that anti-VEGFR-3 treatment did not interfere with hematopoietic engraftment, which is an important safety issue for therapies in the setting of HSCT. This observation contrasts with our previous finding that VEGFR-1 and VEGFR-2 antibodies lead to inhibition of hematopoietic engraftment. These results may be explained by the fact that VEGFR-1 and VEGFR-2 are expressed on hematopoietic precursor cells.42 Furthermore, the activation of VEGFR-1 and VEGFR-2 has been demonstrated in the reconstitution of hematopoiesis and engraftment.43,44 Notably, we demonstrated improved reconstitution of lymphatic cells and myeloid cells in peripheral blood of allo-HSCT recipients who were treated with anti-VEGFR-3 antibodies. After syngeneic HSCT, anti-VEGFR-3 treatment did not improve immune reconstitution, suggesting that the observed beneficial effect on hematopoietic reconstitution is due to reduced GVHD in the bone marrow.24
In summary, we present novel evidence that aGVHD is associated with lymphangiogenesis in intestinal lesions and in lymph nodes. Our data show that anti-VEGFR-3 treatment ameliorates lethal aGVHD and identifies the lymphatic vasculature as a novel therapeutic target in the setting of allo-HSCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (PE1450/3-1), the Deutsche Krebshilfe (110466), the DKMS Stiftung Leben Spenden (DKMS-SLS-MHG-2016-02), the Else Kröner-Fresenius-Stiftung (2010_A104), the José Carreras Leukämie-Stiftung (R11/04; 11R2016), the Monika Kutzner Stiftung, the Stefan-Morsch-Stiftung (2013.06.29), and the Wilhelm Sander-Stiftung (2010.039.1; 2014.150.1).
Authorship
Contribution: S.M., Y.S., and O.P. designed the study; S.M., Y.S., M.K., K.R., S.C., and J.M. performed GVHD experiments; S.M., C.G., and M.K. performed tumor experiments; S.M. and Y.S. analyzed results; S.E. analyzed patient biopsies; S.M. and O.P. wrote the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Sarah Mertlitz, Hematology, Oncology, and Tumor Immunology, Charité University Medicine Berlin, Augustenburger Platz 1, 13353 Berlin, Germany; e-mail: sarah.mertlitz@charite.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal