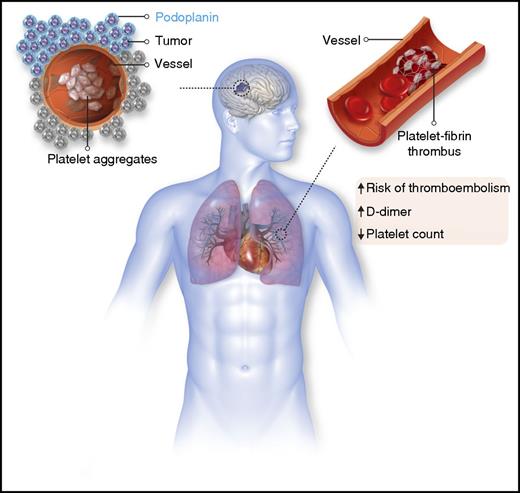
In this issue of Blood, Riedl et al evaluate the link between tumor podoplanin expression and the prothrombotic state inherent to glioma and find that podoplanin expression is associated with altered markers of coagulation activation and an increased risk of venous thromboembolism.1
Podoplanin expression in glioma and its association with venous thromboembolism.
Podoplanin expression in glioma and its association with venous thromboembolism.
The care of patients with glioma is frequently influenced by the development of venous thromboembolism. After decades of investigation, the mechanism by which malignant brain tumors influence coagulation remains speculative at best. In the 1970s, it was theorized that thrombosis in brain tumors was largely the result of spatial disruption causing “a derangement of normal hypothalamic control of anticoagulation.”2 Much attention has focused on the role of tissue factor in mediating the hypercoagulable state in glioma. Tissue factor is overexpressed in glioma, but the association between tumor expression or circulating activity and venous thromboembolism in glioma is debated.3 Building on emerging data focusing on platelet activation as central to cancer-associated thrombosis, Riedl et al now provide evidence that increased podoplanin expression in glioma cells coincides with the development of venous thromboembolism.
Podoplanin is a transmembrane sialoglycoprotein expressed in normal tissue, including lymphatic endothelial cells, and is thought to play a critical role in the embryonic division of lymphatic and vascular systems.4 Aberrant expression of podoplanin was initially identified in a mouse colonic adenocarcinoma cell line and later documented in a wide range of tumors, including colon, lung, ovary, testicular seminoma, and brain.5 Interestingly, the initial description of podoplanin expression in tumor cells was tied to its potential role in mediating thrombosis in malignancy.5 Purified podoplanin induced platelet aggregation while inhibitory antibodies blocked tumor cell–mediated platelet aggregation.5,6 Podoplanin directly activates platelets by functioning as a ligand for C-type lectin receptor type 2 (CLEC-2) on platelets, and inhibition of CLEC-2 not only attenuates tumor-induced platelet aggregation but also limits the generation of distant tumor metastases.7 The role of podoplanin and CLEC-2 appears to extend beyond tumor cells, as platelet accumulation following inferior vena cava ligation is significantly reduced in CLEC-2–deficient mice8 and pathologic alterations of podoplanin–CLEC-2 axis were recently implicated in other thrombotic conditions, including inflammation9 and atherothrombosis.10
The current study by Riedl et al takes the podoplanin hypothesis into the clinic and establishes an association between high expression of podoplanin in glioma patients and the eventual development of venous thromboembolism. The tumor biopsy specimens that stained strongest for podoplanin also contained an increased number of intravascular platelet aggregates (see figure). To confirm systemic hypercoagulability in the patients, the researchers demonstrated that high tumor podoplanin expression correlated with laboratory evidence of coagulation activation, including elevated D-dimer levels. Exposure of platelets to a glioblastoma cell line derived from a patient in the study resulted in podoplanin-dependent platelet aggregation. Collectively, these data provide promising insight into potential pathologic role of podoplanin in mediating venous thromboembolism in glioma.
While these investigations point to the central role of podoplanin in leading to a hypercoagulable state in glioma, there are several unresolved issues. How does increased tumor expression of podoplanin result in thrombosis in distant vascular beds? Establishing that podoplanin circulates in the plasma or that venous thromboembolism in these patients is due to CLEC-2–dependent platelet activation will be key to supporting the underlying hypothesis, especially considering the soluble P-selectin levels did not correlate with tumor podoplanin expression. Confirmatory studies in other cohorts will also be important, considering that the link between podoplanin expression and venous thromboembolism depended to some extent on which statistical model was used to test the null hypothesis.
How might these data lead to improving the care of patients with glioma? Notably, inhibition of the podoplanin–CLEC-2 interaction does not appear to substantively impact bleeding in animal models.4 Developing a small-molecule inhibitor to target podoplanin could lead to the ideal antithrombotic, one that prevents pathologic thrombosis without causing hemorrhagic complications. This is especially relevant to a cancer population that experiences a high rate of intracranial hemorrhage with the currently available anticoagulants.
Conflict-of-interest disclosure: The author declares no competing financial interests.