In this issue of Blood, Gayle et al provide evidence that apilimod, a potent and selective phosphatidylinositol-3-phosphate 5 kinase (PIKfyve) inhibitor, induces significant cytotoxicity at clinically achievable concentrations in preclinical models of B-cell non-Hodgkin lymphoma (NHL) via inhibition of the autophagy flux and perturbation of lysosome homeostasis.1
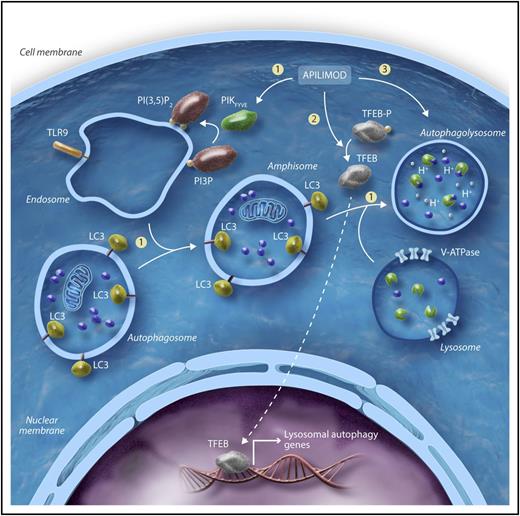
Schematic representation of the proposed mechanism of action. By binding to and inhibiting PIKfyve function, apilimod blocks the formation of PI(3,5)P2 (1). Apilimod mediated-TFEB dephosphorylation promotes TFEB nuclear translocation and transcription of its target gene, CLCN7 (2). In addition to CLCN7, OSTM1 and SNX10 are 2 other key genes upregulated with apilimod treatment. Both CLCN7 and OSTM1 encode anion exchange transporters of pivotal importance for lysosome homeostasis, whereas SNX10 plays an important role in regulating endolysosomal trafficking (3). Apilimod-mediated impairment of lysosomal homeostasis (upregulation of CLCN7, OSTM1) in the setting of endolysosomal membrane traffic dysfunction (upregulation of SNX10) may induce tumor cell stress, ultimately leading to lymphoma cell death. LC3, light chain 3; TFEB-p, transcription factor EB-phosphorylated; TLR9, Toll-like receptor 9; V-ATPase, vacuolar-type H+ ATPase. Professional illustration by Somersault18:24.
Schematic representation of the proposed mechanism of action. By binding to and inhibiting PIKfyve function, apilimod blocks the formation of PI(3,5)P2 (1). Apilimod mediated-TFEB dephosphorylation promotes TFEB nuclear translocation and transcription of its target gene, CLCN7 (2). In addition to CLCN7, OSTM1 and SNX10 are 2 other key genes upregulated with apilimod treatment. Both CLCN7 and OSTM1 encode anion exchange transporters of pivotal importance for lysosome homeostasis, whereas SNX10 plays an important role in regulating endolysosomal trafficking (3). Apilimod-mediated impairment of lysosomal homeostasis (upregulation of CLCN7, OSTM1) in the setting of endolysosomal membrane traffic dysfunction (upregulation of SNX10) may induce tumor cell stress, ultimately leading to lymphoma cell death. LC3, light chain 3; TFEB-p, transcription factor EB-phosphorylated; TLR9, Toll-like receptor 9; V-ATPase, vacuolar-type H+ ATPase. Professional illustration by Somersault18:24.
Autophagy is a highly conserved sequential catabolic process that occurs at basal levels in healthy cells and allows them to sequester and degrade faulty proteins and damaged constituents in autophagolysosomes.2 Degradation ultimately occurs by exposing the cargo to the catalytic activity of lysosomal proteases (cathepsins). Accumulating evidence supports the role of enhanced autophagy during the neoplastic transformation process, as well as in the progression of already established neoplasms, by promoting cell survival under adverse conditions such as hypoxia and nutrient deprivation through the recycling of metabolic precursors and elimination of cellular debris.3 In support of the role of autophagy in cancer, genetic and pharmacologic inhibition of this process in different tumor types, including NHL, promotes tumor cell death, suggesting that the administration of autophagy inhibitors may be beneficial to cancer patients.4,5 Chloroquine and hydroxychloroquine are 2 autophagy flux inhibitors tested in early phase clinical trials in solid tumors and hematologic malignancies; however, low potency and off-target effects have limited their clinical development, highlighting the need for more selective and potent inhibitors of autophagy.6
PIKfyve is an endosomal lipid kinase that phosphorylates P1(3)P to yield phosphatidylinositol 3,5-bisphosphate (PI[3,5]P2). PIKfyve-mediated PI(3,5)P2 signaling has been shown to play a critical role in multiple biological processes, including autophagy, by regulating endosomal membrane trafficking.7
Apilimod mesylate is an orally bioavailable small molecule initially developed as an interleukin-12 (IL-12) and IL-23 inhibitor and evaluated in clinical trials in patients with inflammatory diseases.8 In this issue of Blood, Gayle et al identified apilimod among the most potent drugs in a library of clinically relevant compounds using a high-throughput screening assay. After the initial screen, the antiproliferative activity of apilimod was tested in cell lines derived from different tumor types. B-cell NHL cells were identified as the most broadly sensitive. Interestingly, apilimod at low nanomolar concentrations induced significant cell death in mantle cell lymphoma, germinal center, activated B-cell, and myc-driven diffuse large B-cell lymphomas (DLBCL). Cytotoxic activity of apilimod appeared to be selective against malignant B cells, as a variety of normal cells including B cells and tissues from healthy donors were highly resistant to apilimod. Furthermore, apilimod showed significant antitumor activity in xenograft as well as syngeneic mouse lymphoma models. Using capture mass spectrometry and a genetic approach, the authors showed PIKfyve to be the critical target for apilimod-mediated B-cell NHL cell death. Their data show that treatment of lymphoma cell lines with apilimod induces p62 and LC3-II accumulation that is further increased by cotreatment with rapamycin, an autophagy inducer, thus suggesting blockage of the autophagy flux. Treatment with apilimod was associated with enlargement of the lysosomal compartment and increase of pro- (inactive) cathepsin levels without lysosomal membrane permeabilization and mature (active) cathepsin accumulation in the cytosol, indicating a noncanonical mechanism of cell death at the lysosomal level. Interestingly, the authors showed that, as a consequence of PIKfyve inhibition, transcription factor EB (TFEB), which is highly expressed in B-cell NHL cells, was translocated to the nucleus in its unphosphorylated/active form where it promoted the transcription of its target gene CLCN7. A genome-wide CRISPR screen identified OSTM1 and SNX10 as key genes involved in apilimod-mediated cell death. Both CLCN7 and OSTM1 encode a Cl−/H+ exchanger important for lysosomal acidification, whereas SNX10 plays an important role in regulating endolysosomal trafficking. Consistent with the role of these genes in apilimod-mediated cell death, CRISPR knockout of TFEB, CLCN7, OSTM1, and SNX10 conferred resistance to apilimod. These results indicate that defects in the acidification of the lysosomal compartment as well as impaired endolysosomal membrane trafficking are important in apilimod-mediated cell death via reduced degradation of the lysosomal cargo (see figure).
Overall, apilimod is an exciting compound that produces significant lymphoma cell death at clinically achievable concentrations. Importantly, its safety and clinical activity are now being evaluated in a phase 1 dose escalation study in patients with relapsed/refractory B-cell NHL (NCT02594384). Regardless, several questions remain that will require further investigation: (1) If this agent is shown to be well tolerated, is there a role for combinations of apilimod with chemotherapy in B-cell NHL? It has been shown that autophagy-addicted tumor cells are more susceptible to chemotherapy and radiation when autophagy is inhibited, providing rationale for such combination studies. Interestingly, Gayle et al report significant activity of apilimod in myc-driven DLBCL cell lines. These preliminary results cannot be directly translated to DLBCL patients, of course, but are certainly intriguing considering the particularly aggressive nature and the poor prognosis associated with myc-driven diseases. It is interesting to note that myc overexpression via amplification or translocation induces cytoprotective autophagy via the PERK/eIF2α/ATF4 pathway in lymphoma, and inhibition of autophagy in the same model leads to myc-dependent cell death.9 Although further studies are warranted, the fact that myc-driven lymphoma could potentially exploit this pathway to escape stressful conditions provides the rationale for a dual approach with apilimod and chemotherapy specifically in myc-driven lymphomas. (2) What is the effect of apilimod on immune function? Although the authors provide preliminary evidence showing lack of apilimod cytotoxicity on a variety of normal cells and tissues, no data are provided on the effect of autophagy inhibition on the function of immune cells. The role of autophagy in the maintenance of normal stem cells, in the activation and proliferation of B and T cells, and in the function of antigen-presenting cells is well documented. For example, tumor-bearing autophagy-deficient mice are unable to mount an effective antitumor response due to the inability to efficiently present tumor antigens and to a defective T-cell–mediated antitumor immune response.10 Although these aspects can be preliminarily assessed in the phase 1 clinical trial, additional studies will be required to investigate how these concepts apply to patients with cancer treated with autophagy inhibitors in the presence or absence of immunogenic chemotherapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.