Key Points
Platelet granule content is dispensable for maintaining vascular integrity during skin and lung inflammation.
In stark contrast, lack of platelet granule secretion causes increased mortality in experimental stroke due to intracranial hemorrhage.
Abstract
Platelets maintain hemostasis after injury, but also during inflammation. Recent studies have shown that platelets prevent inflammatory bleeding through (hem) immunoreceptor tyrosine-based activation motif-dependent mechanisms irrespective of aggregation during skin and lung inflammation. Although the exact mechanisms underlying this process remain unknown, it was speculated that mediators released from platelet granules might be involved. Maintaining cerebral hemostasis during stroke treatment is of high clinical relevance because hemorrhage may aggravate the disease state and increase mortality. Although it was shown that platelets help maintain hemostasis in the ischemic brain, their exact contribution remains ill defined. Here we show that Unc13d−/−/Nbeal2−/− mice, which lack platelet α- and dense-granule secretion, show no signs of hemorrhage in models of skin or lung inflammation. In stark contrast, lack of platelet granule release resulted in impaired hemostasis in the ischemic brain after transient middle cerebral artery occlusion leading to increased intracranial hemorrhage and mortality. Our results reveal for the first time that platelet granule constituents are essential for maintenance of hemostasis during thrombo-inflammatory brain infarction but not experimental inflammation of the skin or lung, thereby uncovering vascular bed-specific differences in the prevention of inflammatory bleeding.
Introduction
Platelet aggregation is essential for hemostasis but also contributes to ischemic diseases, such as myocardial infarction or stroke.1,2 Platelet activation involves the release of dense and α-granule content, which amplifies the activation response and promotes thrombus formation. Dense granules contain nonprotein molecules such as serotonin and adenosine diphosphate, although platelet α-granules store more than 300 different proteins involved in a variety of processes like platelet adhesion, inflammation, and angiogenesis.3
Stroke is a leading cause of death and disability worldwide.4 Maintaining cerebral hemostasis during stroke treatment is of high clinical relevance because intracerebral hemorrhage (ICH) may dramatically impair clinical outcome. A major limitation in the use of antithrombotic regimen in acute ischemic stroke is their inherent bleeding risk.
Besides limiting posttraumatic hemorrhage, platelets also safeguard hemostasis under acute inflammatory conditions in the skin, lung, and the ischemic brain.5,6 Signaling through platelet (hem) immunoreceptor tyrosine-based activation motif-coupled receptors, GPVI and CLEC-2, is critical in this process,7 but the downstream effector mechanisms remained elusive. During neutrophil infiltration at sites of inflammation, platelet activation via GPVI plays a dual role: inducing neutrophil granule secretion, which causes tissue damage while at the same time preventing inflammatory bleeding.8 Based on these findings, we and others have speculated that platelet granule content might be critical in maintaining hemostasis during inflammation.7,9 Remarkably, mice deficient in platelet α-granules (Nbeal2−/−) or which lack dense-granule secretion and show reduced α-granule secretion (Unc13d−/−), had no increase in ICH following focal cerebral ischemia.10-12
Here, we systematically analyzed inflammatory bleeding in skin, lung, and the acutely ischemic brain of wild-type (WT) and Unc13d−/−/Nbeal2−/− (DKO) mice that lack α- and dense-granule secretion.
Study design
Mice
Reverse passive arthus (rpA) reaction
Anesthetized shaved mice received intradermal injections of anti-bovine serum albumin antibody, followed by IV injection of bovine serum albumin (75 μg/g). Mice were euthanized and skinned 4 hours later. For hemoglobin (Hb) quantification, punch biopsies of inflamed skin areas were homogenized in phosphate-buffered saline and optical density at 405 nm was measured.
Lipopolysaccharide (LPS)-induced inflammation of the lung
Mice were anesthetized and inoculated intranasally with 10 μg LPS. Some 6 hours later, Hb concentration in bronchoalveolar lavage (BAL) was quantified as above.
Transient middle cerebral artery occlusion (tMCAO) was induced as described.13 A thread was advanced through the carotid artery into the middle cerebral artery to reduce cerebral blood flow and removed after 60 minutes to allow reperfusion. Infarct volume was quantified 24 hours later on 2,3,5-triphenyltetrazolium chloride (TTC)-stained brain sections. Global neurologic and motor functions were evaluated using the Bederson score14 and the grip test.15
Platelet depletion/transfusion
Full thrombocytopenia was induced in WT or human interleukin (hIL)-4Rα/GPIbα–Tg mice by IV injection of 2.5 μg/g anti-GPIbα or anti–hIL-4R antibody, respectively. To reduce platelet counts to 40% of WT levels, mice received 0.1 μg/g anti-GPIbα. Platelet counts were checked by flow cytometry.
Results and discussion
DKO mice were born at expected Mendelian ratios, viable and fertile, and showed grossly normal blood parameters with the exception of a macrothrombocytopenia comparable to that seen in Nbeal2−/− mice10 (see supplemental Table 1, available on the Blood Web site). DKO platelets were unable to secrete both their α- and dense-granule content, which resulted in impaired platelet activation and aggregation (supplemental Figures 1 and 2). DKO animals were protected from arterial thrombosis and displayed a pronounced hemostatic defect in the tail-bleeding model (supplemental Figure 3).
DKO mice maintain vascular integrity in the inflamed skin and lung
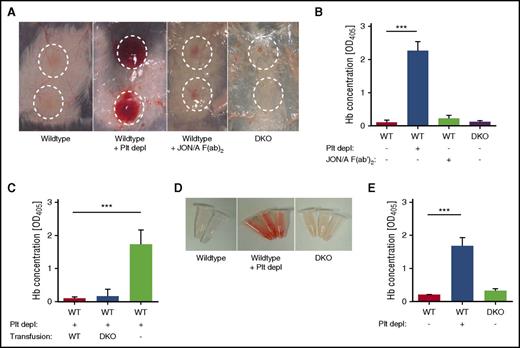
To investigate whether platelet granule secretion maintains vascular integrity during inflammation, DKO animals were subjected to the rpA reaction of the skin and LPS-induced lung inflammation. In agreement with previous studies,6,7 platelet-depleted WT mice showed massive intradermal hemorrhage at the site of inflammation, whereas control mice or mice pretreated with GPIIb/IIIa-blocking JON/A-F(ab)216 displayed edema formation but no bleeding. Strikingly, no bleeding was observed in DKO mice subjected to the rpA reaction (Figure 1A), and this was confirmed by quantification of the Hb content in tissue punch biopsies from inflammatory spots (Figure 1B). Mice with single deficiencies in Nbeal2 or Munc13-4 also showed no bleeding in this model (supplemental Figure 4).
DKO mice maintain vascular integrity during inflammation of the skin and lung. (A-B) WT, platelet-depleted WT, WT treated with JON/A F(ab)2 to block integrin GPIIb/IIIa, and DKO mice were subjected to the rpA reaction to induce local skin inflammation. (A) Representative images. Inflammatory spots are highlighted. (B) Quantification of the Hb content in tissue punch biopsies from inflammatory spots. (C) Quantification of the Hb content in tissue punch biopsies from platelet-depleted hIL-4Rα/GPIbα–Tg mice transfused with WT or DKO platelets or untransfused mice subjected to the rpA reaction. (D-E) WT, platelet-depleted WT, and DKO mice were subjected to LPS-induced lung inflammation. (D) Representative images of BAL 4 hours after LPS application. (E) Quantification of the Hb content in BAL liquid. Results are presented as mean ± SD. n = 4 mice per group, representative of 3 independent experiments. ***P < .001. Plt depl, platelet depleted; SD, standard deviation.
DKO mice maintain vascular integrity during inflammation of the skin and lung. (A-B) WT, platelet-depleted WT, WT treated with JON/A F(ab)2 to block integrin GPIIb/IIIa, and DKO mice were subjected to the rpA reaction to induce local skin inflammation. (A) Representative images. Inflammatory spots are highlighted. (B) Quantification of the Hb content in tissue punch biopsies from inflammatory spots. (C) Quantification of the Hb content in tissue punch biopsies from platelet-depleted hIL-4Rα/GPIbα–Tg mice transfused with WT or DKO platelets or untransfused mice subjected to the rpA reaction. (D-E) WT, platelet-depleted WT, and DKO mice were subjected to LPS-induced lung inflammation. (D) Representative images of BAL 4 hours after LPS application. (E) Quantification of the Hb content in BAL liquid. Results are presented as mean ± SD. n = 4 mice per group, representative of 3 independent experiments. ***P < .001. Plt depl, platelet depleted; SD, standard deviation.
To exclude that global Munc13-4- and Nbeal2-deficiency causes defects in cells other than platelets (eg, neutrophils) thereby altering the inflammatory response, platelet transfusion studies were conducted using hIL-4Rα/GPIbα–Tg mice, in which endogenous platelets can be depleted by antibodies against the hIL-4 receptor.7 Platelet-depleted mice that received WT or DKO platelets maintained vascular integrity during inflammation, whereas nontransfused (thrombocytopenic) mice developed pronounced hemorrhage (Figure 1C).
Intranasal application of LPS causes severe lung inflammation in mice, which in the absence of platelets provokes massive pulmonary hemorrhage.6 Following LPS-induced lung inflammation, the BAL of thrombocytopenic mice showed a high content of red blood cells and Hb, which was markedly reduced in WT and DKO mice (Figure 1D-E).
These results indicate that α- and dense-granule contents are dispensable for maintaining vascular integrity at sites of acute inflammation in skin and lung.
ICH and increased mortality in DKO mice after tMCAO
To determine the impact of combined deficiencies in platelet α- and dense-granule secretion on ischemic brain infarction, we subjected DKO mice to a 60-minute tMCAO, followed by a 24-hour reperfusion period where thrombo-inflammatory infarct growth occurs, thereby mimicking key features of cerebral ischemia/reperfusion injury in humans.17
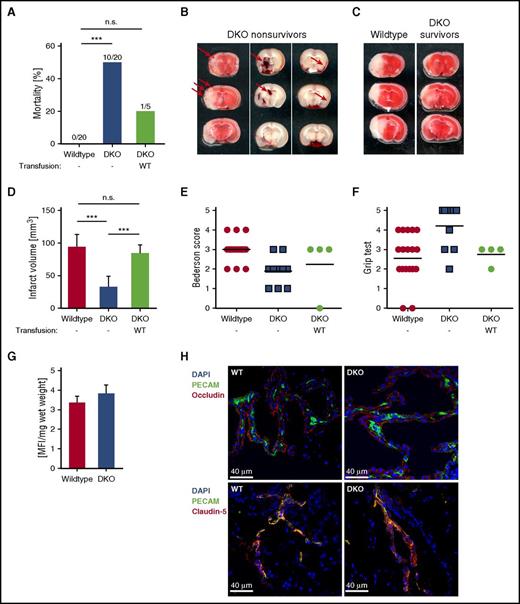
Strikingly, double-deficient mice showed significantly increased mortality (50%) within 24 hours after tMCAO compared with 0% in WT (Figure 2A), and this was due to ICH with microbleedings, intraparenchymal and subarachnoid hemorrhage present in the infarcted areas (Figure 2B). This effect was not due to the reduced platelet count in the DKO mice, as Unc13d−/− mice in which the platelet count had been reduced to 40% showed no ICH after tMCAO (supplemental Figure 5), which is in agreement with previous reports that only platelet counts <10% cause ICH after tMCAO.6,18 Furthermore, ICH in the DKO mice was not due to a defective blood-brain barrier because fluorescein isothiocyanate-dextran leakage and cerebrovascular localization of tight-junction proteins occludin and claudin-5 was indistinguishable between naïve WT and DKO mice (Figure 2G-H). Interestingly, infarct volumes of surviving DKO animals were significantly reduced compared with WT mice (Figure 2C-D). The survivors also showed less neurologic deficits as assessed by the Bederson score14 and the grip test15 (Figure 2E-F). Importantly, transfusion of WT platelets into DKO mice reversed the phenotype by reducing mortality (Figure 2A) and increasing infarct volumes to those seen in WT mice (Figure 2D), thus confirming the critical role of platelets in these processes.10,12
Increased rate of hemorrhage and mortality after tMCAO in DKO mice. (A) Mortality rates 24 hours after tMCAO of WT and DKO mice, as well as DKO mice transfused with WT platelets. (B) Representative images show microbleedings in TTC-stained brain sections (left) or intraparenchymal (middle) and subarachnoid (right) bleedings (arrows) in native brain sections of nonsurviving double-deficient mice after stroke. (C) Representative images of coronal sections stained with TTC 24 hours after tMCAO of WT and surviving DKO mice. Infarcted areas are shown in white. (D) Planimetric analysis was used to quantify the infarct volume. Results are presented as mean ± SD. n = 20 (WT), n = 10 (DKO), and n = 4 (DKO + WT platelets) mice. Bederson score (E) and grip test (F) were used to analyze neurologic outcome in the surviving animals 24 hours after tMCAO. Each symbol represents 1 mouse. n = 20 (WT), n = 10 (DKO), and n = 4 (DKO + WT platelets) mice. (G) Quantification of fluorescein isothiocyanate-dextran extravasation in brains from naïve WT and DKO animals. n = 5 mice per group. (H) Immune fluorescent staining of tight-junction proteins occludin and claudin-5 in brain sections from naïve WT and DKO animals using a 40× objective. Representative images from n = 3 mice per group. Scale bar 40 μm. ***P < .001. DAPI, 4,6 diamidino-2-phenylindole; MFI, mean fluorescence intensity; n.s., not significant; SD, standard deviation.
Increased rate of hemorrhage and mortality after tMCAO in DKO mice. (A) Mortality rates 24 hours after tMCAO of WT and DKO mice, as well as DKO mice transfused with WT platelets. (B) Representative images show microbleedings in TTC-stained brain sections (left) or intraparenchymal (middle) and subarachnoid (right) bleedings (arrows) in native brain sections of nonsurviving double-deficient mice after stroke. (C) Representative images of coronal sections stained with TTC 24 hours after tMCAO of WT and surviving DKO mice. Infarcted areas are shown in white. (D) Planimetric analysis was used to quantify the infarct volume. Results are presented as mean ± SD. n = 20 (WT), n = 10 (DKO), and n = 4 (DKO + WT platelets) mice. Bederson score (E) and grip test (F) were used to analyze neurologic outcome in the surviving animals 24 hours after tMCAO. Each symbol represents 1 mouse. n = 20 (WT), n = 10 (DKO), and n = 4 (DKO + WT platelets) mice. (G) Quantification of fluorescein isothiocyanate-dextran extravasation in brains from naïve WT and DKO animals. n = 5 mice per group. (H) Immune fluorescent staining of tight-junction proteins occludin and claudin-5 in brain sections from naïve WT and DKO animals using a 40× objective. Representative images from n = 3 mice per group. Scale bar 40 μm. ***P < .001. DAPI, 4,6 diamidino-2-phenylindole; MFI, mean fluorescence intensity; n.s., not significant; SD, standard deviation.
Our data demonstrates that although defective α- or dense-granule secretion alone have no effect on intracerebral hemostasis during stroke, the combination of both causes ICH and an increase in mortality to a similar degree as observed after GPIIb/IIIa blockade.13 Recent clinical trials assessing the potential use of GPIIb/IIIa blockers in acute stroke failed due to excess ICH and inefficacy.19,20 Therefore, despite the impressive reduction in infarct volumes in double-deficient mice after tMCAO, pharmacologic interference with α- and dense-granule secretion may not be a promising approach to treat or prevent acute stroke.
In contrast to the ICH observed during stroke after GPIIb/IIIa blockade, integrin β3-deficient mice did not show bleeding after skin inflammation.6 On the other hand, GPVI-deficient mice were protected from ischemic stroke with no signs of ICH, although they displayed hemorrhage at sites of skin or lung inflammation,7,8 clearly indicating that platelets use different pathways to ensure hemostasis in different inflammatory settings and vascular beds. Future studies will be required to identify the mediators involved in maintaining vascular integrity under these different conditions. From our results, we conclude that molecules from both α and dense granules contribute to cerebral hemostasis after tMCAO. Possible candidates could be angiopoietin-1 or serotonin, which were previously identified as platelet-derived factors involved in preventing intra-tumor hemorrhage.5 Furthermore, angiopoietin-1 is necessary for the maintenance of vascular integrity and survival in a mouse model of cerebral malaria.21 In addition, brain endothelial cells possess P2Y2 receptors for nucleotides such as adenosine triphosphate and adenosine diphosphate, which could regulate permeability.22,23 Indeed, platelet-derived nucleotides were shown to act on the endothelial P2Y2 receptor to enable tumor transmigration.24
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jerry Ware for kindly providing the hIL-4Rα/GPIbα–Tg mice, and Vanessa Klaus and Ayesha A. Baig for help with platelet transfer experiments. The authors also thank the microscopy platform of the Bioimaging Center (Rudolf Virchow Center) for providing technical infrastructure.
This study was supported by the Deutsche Forschungsgemeinschaft: SFB688 (B.N., G.S., and D.S.), and research fellowship DE 2654/1-1 (C.D.).
Authorship
Contribution: C.D. performed experiments, analyzed data, and wrote the manuscript; P.K., J.V., M.K.S., S.B., and K.W. performed experiments and analyzed data; D.S. assisted with experimental design and contributed to the writing of the manuscript; and G.S. and B.N. designed and supervised research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for C.D. is Snyder Institute for Chronic Diseases, University of Calgary, Calgary, AB, Canada.
Correspondence: Bernhard Nieswandt, Department of Experimental Biomedicine, University Hospital and Rudolf Virchow Center, University of Würzburg, Josef-Schneider-Str 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal