In this issue of Blood, Fajgenbaum et al propose much needed consensus criteria for the diagnosis of idiopathic multicentric Castleman disease (iMCD).1
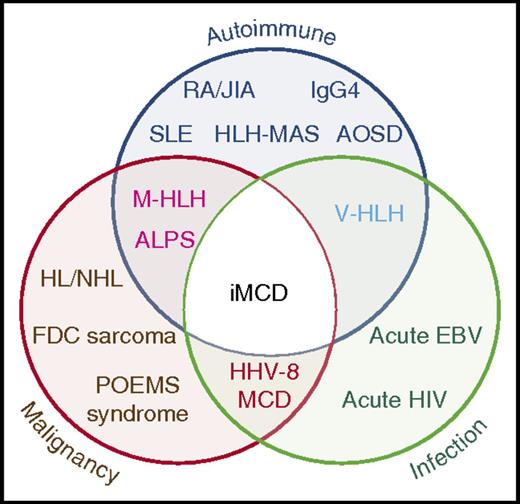
Venn diagram showing overlap between iMCD and other entities with similar clinical and pathologic presentations which must be excluded before making the diagnosis ALPS, autoimmune lymphoproliferative syndrome; AOSD, adult-onset Still disease; EBV, Epstein-Barr virus; FDC, follicular dendritic cell; HL, Hodgkin lymphoma; HLH-MAS, hemophagocytic lymphohistiocytosis- macrophage activation syndrome; IgG4, IgG4-related disease; JIA, juvenile idiopathic arthritis; M-HLH, malignancy-associated hemophagocytic lymphohistiocytosis; NHL, non-Hodgkin lymphoma; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal paraprotein, skin changes; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; V-HLH, viral hemophagocytic lymphohistiocytosis. See Figure 1 in the article by Fajgenbaum et al that begins on page 1646.
Venn diagram showing overlap between iMCD and other entities with similar clinical and pathologic presentations which must be excluded before making the diagnosis ALPS, autoimmune lymphoproliferative syndrome; AOSD, adult-onset Still disease; EBV, Epstein-Barr virus; FDC, follicular dendritic cell; HL, Hodgkin lymphoma; HLH-MAS, hemophagocytic lymphohistiocytosis- macrophage activation syndrome; IgG4, IgG4-related disease; JIA, juvenile idiopathic arthritis; M-HLH, malignancy-associated hemophagocytic lymphohistiocytosis; NHL, non-Hodgkin lymphoma; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal paraprotein, skin changes; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; V-HLH, viral hemophagocytic lymphohistiocytosis. See Figure 1 in the article by Fajgenbaum et al that begins on page 1646.
In collaboration with an international cohort of clinicians and pathologists who reviewed more than 240 cases, the authors concurred on a set of evidence-based criteria, thereby paving the way for a uniform approach to managing this enigmatic condition. In 1956, Castleman et al2 described clinicopathologic features of 13 asymptomatic patients who presented with a mediastinal mass. This form of unicentric CD is a distinct entity, readily diagnosed on pathologic examination and, in most cases, treated effectively by excision of the mass.
The multicentric form has been more problematic in terms of both its clinical definition and its pathology; this form has been described as plasma cell, plasmablastic, hyaline vascular, transitional, or mixed. Variants of MCD such as TAFRO syndrome (which includes a constellation of features such as thrombocytopenia, anasarca/ascites, reticulin fibrosis in bone marrow, renal dysfunction, and organomegaly) have also been reported.3 The multicentric form of CD is associated with constitutional symptoms, largely related to increased cytokines, particularly interleukin-6 (IL-6), laboratory abnormalities, multifocal lymphadenopathy and, in some cases, hepatosplenomegaly. With the identification of human herpesvirus 8 (HHV-8), it became clear that a subset of cases of MCD were caused by HHV-8, which produces a viral IL-6 similar to its human counterpart. These cases occur most commonly but not exclusively in HIV-positive individuals and can readily be identified in paraffin sections with commercial antibodies to HHV-8 latency-associated nuclear antigen encoded by ORF73.
Unfortunately, the criteria for pathologic and clinical diagnosis of the significant number of patients with HHV-8–negative or iMCD are ill defined, and there is considerable overlap with many autoimmune, neoplastic, and infectious diseases (see figure). Pathologic features are commonly those of a nonspecific polytypic plasmacytosis admixed with variably prominent hypervascular or regressed germinal centers, with or without prominent dendritic histiocytes. For consensus, in addition to Major Criteria for the diagnosis (characteristic pathology and multicentric lymphadenopathy), at least 2 of 11 Minor Criteria with at least 1 laboratory abnormality are required. Most importantly, the article emphasizes the need to exclude infectious, neoplastic, and autoimmune diseases that mimic iMCD.
The Minor Criteria, whether clinical such as constitutional symptoms or laboratory such as elevated C-reactive protein and anemia, are nonspecific and must be applied in the context of the pathologic findings. It is expected that the list may be refined in the next phase of the study, during which the criteria will be analyzed and validated through the international registry, which the Castleman Disease Collaborative Network and University of Pennsylvania launched in 2016. Although not all may agree with the criteria proposed or their weight in reaching a diagnosis, this proposal, based on review of a large number of cases by an international panel of experts, provides a platform for more uniform consensus diagnosis. Accurate and timely diagnosis of patients with iMCD is increasingly critical now that there are effective targeted therapies, such as the chimeric anti-IL-6 antibody siltuximab.4
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal