Key Points
Nonneutralizing antibodies against FVIII are detected in untreated or minimally treated patients with hemophilia A.
The presence of nonneutralizing antibodies is associated with a substantially increased risk of inhibitor development.
Abstract
The development of anti-factor VIII (FVIII) neutralizing antibodies (inhibitors) is the major complication in hemophilia A. Nonneutralizing antibodies (NNAs) have been detected in hemophilia patients and also in unaffected individuals. The aim of this study was to assess the prevalence of NNAs and to evaluate whether their presence is associated with the development of inhibitors in a cohort of previously untreated or minimally treated patients with hemophilia A; plasma samples of 237 patients with severe hemophilia A enrolled in the SIPPET trial were collected before any exposure to FVIII concentrates and analyzed for the presence of anti-FVIII NNAs. Patients were observed for the development of neutralizing antibodies. NNAs were found in 18 (7.6%) of 237 patients at screening, and there was a clear age gradient. Of those with NNAs, 7 patients subsequently developed an inhibitor for a cumulative incidence of 45.4% (95% confidence interval [CI], 19.5% to 71.3%); among the 219 patients without NNAs, 64 (29%) developed an inhibitor (cumulative incidence, 34.0%; 95% CI, 27.1%-40.9%). In Cox regression analyses, patients with NNAs at screening had an 83% higher incidence of inhibitor development than patients without NNAs (hazard ratio [HR], 1.83; 95% CI, 0.84-3.99). For high-titer inhibitors, the incidence rate had an almost threefold increase (HR, 2.74; 95% CI, 1.23-6.12). These associations did not materially change after adjustment. The presence of anti-FVIII NNAs in patients with severe hemophilia A who were not previously exposed to FVIII concentrates is associated with an increased incidence of inhibitors.
Introduction
The development of alloantibody neutralizing factor VIII (FVIII) coagulant activity (inhibitors) represents the main complication in treatment of hemophilia A. It occurs in approximately one third of previously untreated patients and causes substantial morbidity and mortality for patients and increased costs for the health care system.1-3 What causes the development of inhibitors is not fully understood, but some risk factors have been identified.4-9
FVIII inhibitors consist of a polyclonal population of antibodies that are targeted to multiple antigenic sites within the A2, A3, and C2 domains of the protein.10 In addition to inhibitors, anti-FVIII antibodies are present in healthy individuals and in patients with hemophilia A without exerting coagulant inhibitory activity. Several laboratory platforms for detecting total FVIII-binding antibodies have been developed, based upon immunoblotting assays, fluorescence immune assays, and enzyme-linked immunosorbent assays (ELISAs).11-31 These assays have helped identify nonneutralizing antibodies (NNAs) directed toward nonfunctional FVIII epitopes that escape detection in functional assays. Their prevalence is approximately 2% to 3% in healthy individuals,16,18,21,24 but estimates in patients who have hemophilia with different degrees of severity vary widely from 12% to 54%.13-22,24,25,29-31 Although NNAs and inhibitors cannot be distinguished on the basis of their isotypes, clonality, and epitopes,32 recent data published by Hofbauer et al16 indicate that anti-FVIII immunoglobulin G (IgG) with inhibitory activity has an up to 100-fold higher affinity for FVIII than IgG without inhibitory activity. On the basis of cross-reactivity studies, it has also been suggested that FVIII inhibitors in hemophilia A patients originate from the expansion of a natural anti-FVIII clone of B lymphocytes that exists before any treatment with FVIII and secretes anti-FVIII antibodies similar to the natural antibodies found in healthy individuals. Furthermore, FVIII inhibitors seem to be produced by a B-cell clone that has undergone an antigen-driven affinity maturation and hypermutation of the V region.33 With this as background, it is not known whether NNAs are present before any exposure to FVIII concentrates or whether they have any relationship to subsequent inhibitor development, although Boylan et al15 have suggested that NNAs could be an early sign of subsequent inhibitor development. Currently available data on NNAs stem from large but heterogeneous cohorts of patients who have had multiple transfusions, some with previous inhibitors that were then eradicated, and from others during immune tolerance induction for inhibitor eradication. The majority of studies had cross-sectional designs and could not assess temporal relationships. In particular, none of the previous studies were performed on plasma samples collected before any exposure to FVIII concentrate.11-31
The aim of this study was to determine the prevalence and significance of anti-FVIII NNAs in patients enrolled in the prospective randomized SIPPET study who were screened for neutralizing and nonneutralizing anti-FVIII antibodies before any treatment with FVIII concentrates.
Patients and methods
Patients
This was a cohort study performed within the framework of the SIPPET randomized trial.34 Patients younger than age 6 years affected with severe hemophilia A who had never been exposed to FVIII concentrates, had not been exposed or had been only minimally exposed (less than 5 times) to blood components (whole blood, fresh-frozen plasma, packed red cells, platelets, or cryoprecipitate), and were inhibitor negative by modified Bethesda assay were included and were randomly assigned to treatment with a single plasma-derived FVIII (pdFVIII) or recombinant FVIII (rFVIII) concentrate. Patients were observed and monitored at scheduled time points for the occurrence of FVIII inhibitors.34 Data were collected on the patient’s family history of hemophilia and inhibitors, age at screening, country of enrollment, FVIII gene mutations, and FVIII sources (for more details, see Peyvandi et al34 ). FVIII antigen levels (measured at screening by Asserachrom VIIIC:Ag; Diagnostica Stago, Asnières sur Seine, France) were categorized as <1% and ≥1%. Minimally treated patients were defined as those exposed to blood components less than 5 times before screening, whereas previously untreated patients were those never exposed to blood components. Screening was defined as the time of inclusion in the SIPPET trial before any exposure to FVIII concentrate. At this time, a blood sample was collected, patients were screened for the presence of inhibitory antibodies by using the Bethesda assay with the Nijmegen modification, and samples were stored for future determination of the presence of NNAs by using an ELISA. Plasma samples collected at screening were available for 237 patients and were tested for anti-FVIII NNAs.
Approval for this study was obtained from the medical ethics committee at each study center. Parents or guardians of all children provided written informed consent.
Inhibitor testing
Inhibitor testing at screening and follow-up was performed centrally at the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy, by using the Bethesda assay with the Nijmegen modification.35 In case inhibitors occurred during follow-up, inhibitor levels were confirmed on a second sample within 14 days after the first positivity, and patients were observed for 6 months to establish whether the inhibitor was transient or persistent.
NNA testing
ELISA plates (96 wells, Maxisorp, Nunc; St. Louis, MO) were coated overnight at 4°C with 1.2 μg/mL of full-length rFVIII Advate (Baxter Healthcare Corporation, Westlake Village, CA) previously dialyzed against phosphate-buffered saline (PBS). The plates were then blocked for 2 hours at room temperature with PBS containing 5% skim milk powder (Merck, Kenilworth, NJ). After blocking, the plates were washed 3 times with PBS containing 0.1% Tween-20 (Sigma and Merck, Kenilworth, NJ). The same washing procedure was performed between the different incubation steps. Samples were diluted in PBS, 1% skim milk powder (Merck, Kenilworth, NJ), and 0.01% Tween-20 (Sigma and Merck, Kenilworth, NJ) and were added to the coated plates and incubated for 2 hours at room temperature. After a washing step, the plates were incubated for 1 hour at room temperature with horseradish peroxidase anti-human IgG (GE Healthcare, Little Chalfont, United Kingdom ) and diluted 1:2000 in PBS, 1% skim milk powder, and 0.01% Tween-20. Then, o-phenylenediamine dihydrochloride substrate (Sigma and Merck, Kenilworth, NJ) was added, and the reaction was stopped by adding 3 mol/L H2SO4. Absorbance was read at 492 nm and was corrected for background at 620 nm.
Each plasma sample was analyzed twice in different assay runs. In case of a discrepancy when values deviated from the range of interassay variability (CV = 25%), a third assay was performed. The mean of 2 assay results or of the 2 closest values in the case of 3 assays was used. In the first run, all plasma samples were diluted 1:10, and in the second run, samples with high IgG concentration were further diluted to have an absorbance value that fell within the linear portion of the standard curve. In each assay, a positive control sample (plasma with a high-titer inhibitor) diluted 1:40 and a negative control sample (normal pool plasma) diluted 1:10 were also used.
Total IgG from the plasma of a patient with hemophilia A and a high-titer inhibitor (600 Bethesda units [BU]) was purified on a Protein G-Sepharose column (GE Healthcare, Little Chalfont, United Kingdom). Specific anti-FVIII IgG was then isolated by using affinity chromatography on Affi-Gel 10 affinity media (Bio-Rad, Hercules, CA) coupled with rFVIII (Advate) and 4000 IU anti-FVIII IgG per 2 mL of slurry gel. The affinity purified anti-FVIII–specific IgGs were checked for purity by means of sodium dodecyl sulfate-poly acrylamide gel electrophoresis and used in an ELISA to construct the standard curve. The cutoff for positive anti-FVIII NNAs was set at 1.64 µg/mL of specific anti-FVIII IgG, corresponding to 100% specificity and 96% of sensitivity in the receiver operating characteristic curve constructed by using the results of anti-FVIII IgG measured in 107 healthy individuals and 101 patients with hemophilia A; positive FVIII inhibitor was detected by using the Bethesda assay (mean, 103.9 BU; median, 14.5 BU; range, 0.5-1400 BU).
Statistical analysis
Prevalence was estimated as a proportion with confidence intervals (CIs) obtained by the exact binomial method of Clopper-Pearson. We used odds ratios and 95% CIs to assess putative determinants of NNAs. Analysis by age at screening was performed by stratifying patients into 5 age categories. Independent sample Student t test was used to compare age between the 5 groups.
Kaplan-Meier survival analyses were performed to assess the cumulative incidence for all inhibitors and for high-titer inhibitors by NNA serotype. The incidence rates were compared by using Cox regression survival analyses, taking into account FVIII gene mutations (categorized as null vs non-null mutation) as covariates,34 FVIII antigen levels, self-reported family history for the inhibitor, trial treatment arm (pdFVIII or rFVIII), age at screening (in months), and country of enrollment. Adjustments in multivariable Cox models were each made individually, because there were too few events to include all variables in a single multivariable model. CIs were derived from this model. Because 2 deaths occurred during the trial,34 we performed a sensitivity analysis assuming that both patients had developed a high-titer inhibitor at the truncated follow-up instead of dying. Statistical analyses were performed by using SPSS, version 23.0 (IBM Corporation, Armonk, NY).
Results
Characteristics of the study cohort
The mean age at screening was 18.3 months (median, 13 months; range, 0-67 months). FVIII gene causative mutations were detected in 94.1% of the patients (n = 223): 185 (83%) were carrying a null mutation (large deletions, nonsense mutations, inversions, or frameshifts). In 237 patients, 107 (45.1%) had a positive family history of hemophilia, and 23 (21.5%) reported a positive family history of inhibitors. In all, 121 patients (51.1%) were randomly assigned to treatment with pdFVIII and 116 (48.9%) to treatment with rFVIII. Of 237 patients observed for a mean of 27 exposure days (median, 23 days; range, 1-50 days), 71 developed an FVIII inhibitor (30.0%), which in 48 patients (67.6%) was at high titer.
Determinants of NNAs toward FVIII
NNAs were detected at screening in 18 (7.6%) of 237 patients (95% CI, 4.9%-11.7%). Table 1 shows the general characteristics of NNA-positive and NNA-negative patients. Patients with NNAs were older than those without (mean, 27.4 months; median, 24 months; range, 2-59 months vs mean, 17.5 months; median, 12 months; range, 0-67 months, respectively; P = .007), and NNA prevalence clearly increased with age (Table 2). Non-null mutations, measurable FVIII antigen levels in plasma, and a positive family history of inhibitors were also associated with a higher prevalence of NNAs at screening (Table 2). Previous exposure to blood components was associated with a reduced prevalence (odds ratio, 0.49; 95% CI, 0.17-1.43). CIs were wide for all of these associations because of the limited sample size, and none changed after adjustment for age.
General characteristics and prevalence of patients with NNAs in the study cohort
| Characteristic . | NNA negative . | NNA positive . | Crude OR . | 95% CI . | P . | Age-adjusted OR . | 95% CI . | P . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | |||||||
| Age at screening (months) | ||||||||||
| Mean | 17.5 | 27.4 | ||||||||
| Median | 12 | 24 | ||||||||
| Maximum | 67 | 59 | ||||||||
| Minimum | 0 | 2 | ||||||||
| FVIII mutation | ||||||||||
| Null | 173 | 93.5 | 12 | 6.5 | 1.0 | 1.0 | ||||
| Non-null | 33 | 86.8 | 5 | 13.2 | 2.18 | 0.72-6.61 | .167 | 2.29 | 0.74-7.10 | .153 |
| FVIII antigen levels, % | ||||||||||
| <1 | 195 | 92.9 | 15 | 7.1 | 1.0 | 1.0 | ||||
| ≥1 | 21 | 87.5 | 3 | 12.5 | 1.86 | 0.50-6.94 | .358 | 1.71 | 0.45-6.50 | .434 |
| Family history of inhibitors | ||||||||||
| No | 69 | 95.8 | 3 | 4.2 | 1.0 | 1.0 | ||||
| Yes | 21 | 91.3 | 2 | 8.7 | 2.19 | 0.34-14.00 | .407 | 2.93 | 0.41-20.95 | .284 |
| Previous exposure to blood components (<5) | ||||||||||
| No | 123 | 90.4 | 13 | 9.6 | 1.0 | 1.0 | ||||
| Yes | 96 | 95.0 | 5 | 5.0 | 0.49 | 0.17-1.43 | .193 | 0.41 | 0.14-1.22 | |
| Characteristic . | NNA negative . | NNA positive . | Crude OR . | 95% CI . | P . | Age-adjusted OR . | 95% CI . | P . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | |||||||
| Age at screening (months) | ||||||||||
| Mean | 17.5 | 27.4 | ||||||||
| Median | 12 | 24 | ||||||||
| Maximum | 67 | 59 | ||||||||
| Minimum | 0 | 2 | ||||||||
| FVIII mutation | ||||||||||
| Null | 173 | 93.5 | 12 | 6.5 | 1.0 | 1.0 | ||||
| Non-null | 33 | 86.8 | 5 | 13.2 | 2.18 | 0.72-6.61 | .167 | 2.29 | 0.74-7.10 | .153 |
| FVIII antigen levels, % | ||||||||||
| <1 | 195 | 92.9 | 15 | 7.1 | 1.0 | 1.0 | ||||
| ≥1 | 21 | 87.5 | 3 | 12.5 | 1.86 | 0.50-6.94 | .358 | 1.71 | 0.45-6.50 | .434 |
| Family history of inhibitors | ||||||||||
| No | 69 | 95.8 | 3 | 4.2 | 1.0 | 1.0 | ||||
| Yes | 21 | 91.3 | 2 | 8.7 | 2.19 | 0.34-14.00 | .407 | 2.93 | 0.41-20.95 | .284 |
| Previous exposure to blood components (<5) | ||||||||||
| No | 123 | 90.4 | 13 | 9.6 | 1.0 | 1.0 | ||||
| Yes | 96 | 95.0 | 5 | 5.0 | 0.49 | 0.17-1.43 | .193 | 0.41 | 0.14-1.22 | |
Crude and age-adjusted odds ratio (OR) for the presence of NNAs according to potential determinants.
Distribution of NNAs over each age category and the corresponding risk estimates for the presence of NNAs
| . | Patients . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Age at screening (months) . | Total . | NNA-negative . | NNA-positive . | OR . | 95% CI . | P* . | ||
| No. . | % . | No. . | % . | |||||
| All | 237 | 219 | 92.4 | 18 | 7.6 | |||
| ≥40 | 25 | 20 | 80.0 | 5 | 20.0 | 6.50 | 1.43-29.52 | .018 |
| 30-39 | 23 | 20 | 87.0 | 3 | 13.0 | 3.90 | 0.73-20.80 | .101 |
| 20-29 | 41 | 38 | 92.7 | 3 | 7.3 | 2.05 | 0.40-10.65 | .319 |
| 10-19 | 67 | 63 | 94.0 | 4 | 6.0 | 1.65 | 0.36-7.65 | .331 |
| 0-9 | 81 | 78 | 96.3 | 3 | 3.7 | 1.0 | Ref | |
| . | Patients . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Age at screening (months) . | Total . | NNA-negative . | NNA-positive . | OR . | 95% CI . | P* . | ||
| No. . | % . | No. . | % . | |||||
| All | 237 | 219 | 92.4 | 18 | 7.6 | |||
| ≥40 | 25 | 20 | 80.0 | 5 | 20.0 | 6.50 | 1.43-29.52 | .018 |
| 30-39 | 23 | 20 | 87.0 | 3 | 13.0 | 3.90 | 0.73-20.80 | .101 |
| 20-29 | 41 | 38 | 92.7 | 3 | 7.3 | 2.05 | 0.40-10.65 | .319 |
| 10-19 | 67 | 63 | 94.0 | 4 | 6.0 | 1.65 | 0.36-7.65 | .331 |
| 0-9 | 81 | 78 | 96.3 | 3 | 3.7 | 1.0 | Ref | |
P value is the comparison of each age category to the reference (Ref).
NNAs and inhibitor development
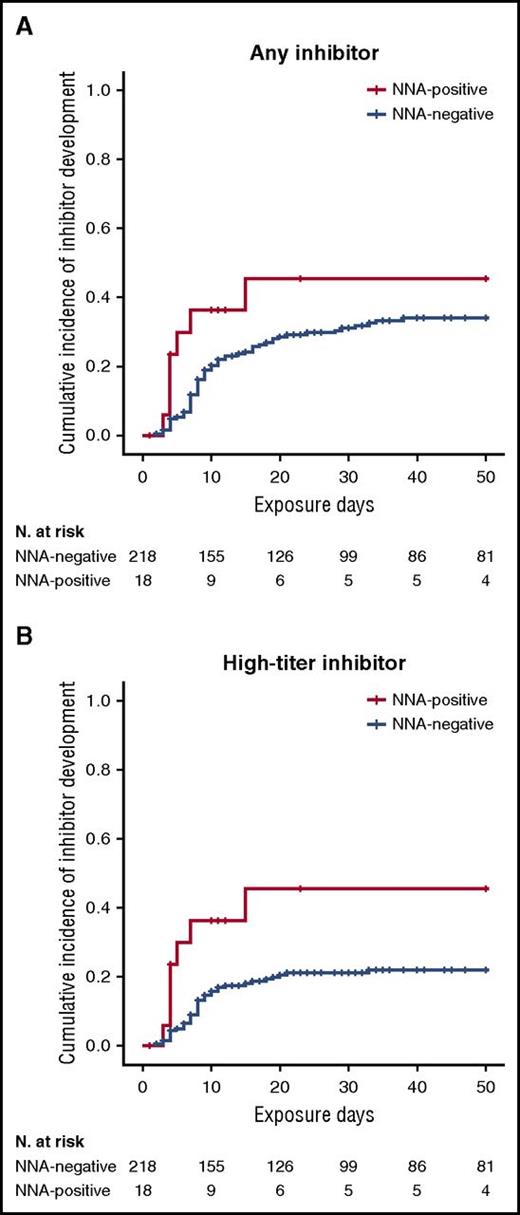
Among patients who were positive for NNAs at screening (n = 18), 7 subsequently developed an inhibitor for a cumulative incidence of 45.4% (95% CI, 19.5%-71.3%); among patients who were negative for NNAs (n = 219), 64 developed an inhibitor for a cumulative incidence of 34.0% (95% CI, 27.1%-40.9%). In the NNA-positive group, all inhibitors were high-titer, whereas in the NNA-negative group, 41 of 64 inhibitors were high-titer for a cumulative incidence of 21.9% (95% CI, 15.8%-28.0%). Importantly, no inhibitor among NNA-positive patients was transient compared with 26.6% (17 of 64 patients) of inhibitors in the NNA-negative group, which were transient and disappeared within 6 months.
Figure 1 shows the Kaplan-Meier plots for all inhibitors and for high-titer inhibitors by the presence or absence of NNAs at screening. In univariable Cox regression analyses, the presence of NNAs was associated with an 83% higher incidence of inhibitors than was the absence of NNAs (hazard ratio [HR], 1.83; 95% CI, 0.84-3.99). For high-titer inhibitors, there was an almost threefold increase in the rate (HR, 2.74; 95% CI, 1.23-6.12). In adjusted models, the effect of NNAs on inhibitor development became stronger if anything (Table 3).
Kaplan-Meier survival curves for inhibitor development by NNA presence. Cumulative incidence for (A) all inhibitors and (B) high-titer inhibitors.
Kaplan-Meier survival curves for inhibitor development by NNA presence. Cumulative incidence for (A) all inhibitors and (B) high-titer inhibitors.
Cox regression analysis for inhibitor development by NNA presence
| Adjustment variable . | All inhibitors . | High-titer inhibitor . | ||
|---|---|---|---|---|
| HR . | 95% CI . | HR . | 95% CI . | |
| Crude unadjusted model | 1.83 | 0.84-3.99 | 2.74 | 1.23-6.12 |
| Age at screening | 1.91 | 0.85-4.26 | 2.75 | 1.20-6.32 |
| Mutation | 2.33 | 1.05-5.15 | 3.53 | 1.56-8.00 |
| FVIII antigen level | 1.73 | 0.79-3.78 | 2.95 | 1.32-6.60 |
| Countries of enrollment | 2.36 | 1.03-5.42 | 3.91 | 1.62-9.44 |
| Familial history of inhibitor | 1.96 | 0.89-4.29 | 3.03 | 1.35-6.81 |
| Treatment arm | 1.73 | 0.79-3.78 | 2.61 | 1.17-5.84 |
| Exposure to blood components | 1.75 | 0.80-3.86 | 2.75 | 1.22-6.22 |
| Mutation, countries of enrollment | 3.62 | 1.49-9.42 | 5.76 | 2.17-15.26 |
| Adjustment variable . | All inhibitors . | High-titer inhibitor . | ||
|---|---|---|---|---|
| HR . | 95% CI . | HR . | 95% CI . | |
| Crude unadjusted model | 1.83 | 0.84-3.99 | 2.74 | 1.23-6.12 |
| Age at screening | 1.91 | 0.85-4.26 | 2.75 | 1.20-6.32 |
| Mutation | 2.33 | 1.05-5.15 | 3.53 | 1.56-8.00 |
| FVIII antigen level | 1.73 | 0.79-3.78 | 2.95 | 1.32-6.60 |
| Countries of enrollment | 2.36 | 1.03-5.42 | 3.91 | 1.62-9.44 |
| Familial history of inhibitor | 1.96 | 0.89-4.29 | 3.03 | 1.35-6.81 |
| Treatment arm | 1.73 | 0.79-3.78 | 2.61 | 1.17-5.84 |
| Exposure to blood components | 1.75 | 0.80-3.86 | 2.75 | 1.22-6.22 |
| Mutation, countries of enrollment | 3.62 | 1.49-9.42 | 5.76 | 2.17-15.26 |
HR compares the risk of inhibitor development among those with NNA at screening vs those without. Adjustments in multivariable Cox models were each performed individually.
Because of the stringency of our cutoff value for nonneutralizing anti-FVIII antibody positivity, we also analyzed data by using a lower cutoff of 1.035 µg/mL, corresponding to the highest value of the Younden index obtained from the receiver operating characteristic curve (99% sensitivity and 98% specificity), and results did not change: all inhibitors by NNA positivity had an HR of 1.69 (95% CI, 0.97-2.96) and high-titer inhibitors had an HR of 2.33 (95% CI, 1.25-4.34). Two deaths occurred during the trial, both among NNA-negative patients. We performed a sensitivity analysis assuming that both patients had developed a high-titer inhibitor at the truncated follow-up instead of dying. We recalculated the crude HR by using the presence of NNAs, and the results did not change. The HR by the presence of NNAs was 1.78 (95% CI, 0.81-3.87) for all inhibitors and 2.62 (95% CI, 1.18-5.84) for high-titer inhibitors. Severe nonfatal adverse events included 9 episodes of intracranial bleeding and 2 episodes of gastrointestinal bleeds, all of which occurred in the NNA-negative group. Per protocol, these adverse events were not considered as reasons to withdraw from the study, so patients continued to be treated and completed the study. Further sensitivity analyses were performed for patients who were censored before reaching the predefined end point of 3 years follow-up or 50 exposure days, and no major deviations from the overall findings were found.
Discussion
We studied a cohort of 237 patients with severe hemophilia A who were previously untreated and minimally exposed to blood components and thereby at risk for FVIII inhibitor development after exposure to FVIII concentrates. A sensitive ELISA was used to detect NNAs at screening before any exposure to FVIII, which were present in 7.6% of patients. Age, non-null mutation, FVIII antigen levels, and family history of inhibitors seemed to influence the prevalence of NNAs. Importantly, the presence of NNAs was associated with an increased risk of inhibitor development, particularly for high-titer inhibitors, with a nearly threefold increased incidence rate.
In previous reports, NNA prevalence ranged from 2% to 3% in healthy individuals16,18,21,24 and from 12.2% to 53.8% in patients with hemophilia A.13-22,24,25,29,30 Several factors, particularly that all previous studies predominantly included patients with multiple transfusions, explain this large variation and the gaps in knowledge about their potential clinical role. For instance, the intron 22 inversion of FVIII mutation was associated with an increased frequency of NNAs in patients who had had multiple transfusions in 1 study29 that was not confirmed in another study.20 Among patients who had had multiple transfusions, those with NNAs were older than those with no NNAs in 1 study20 but not in others.13,30
Given that the increasing prevalence with age can be projected to be 20% at the age of more than 40 months, NNAs can be considered an age-related phenomenon in patients previously untreated and minimally exposed to blood components, which may reflect maturation of the immune system, and in particular of the adaptive immune system, in growing children.36 This finding is also supported by the increase in natural IgGs during the first years that remain stable with aging.37
We found a higher prevalence of NNAs at screening in untreated patients with non-null mutations than in patients with null mutations. One may hypothesize that patients with minor gene variations have residual nonfunctional plasma levels of FVIII antigen that elicit an immune response before exposure to concentrates. This view is supported by the association between detectable FVIII antigen and NNAs. Measurable FVIII antigen in plasma,38 potential contamination with maternal blood during delivery,39 and the presence of milk fat globule epidermal growth factor-FVIII (a glycoprotein with a strong homology with circulating FVIII in breast milk) might all be examples of early exposure.40 The presence of NNAs before any specific treatment may be the expression of a natural anti-FVIII B-cell clone that exists independently of any environmental FVIII exposure.41
Whereas some findings, particularly those regarding determinants of NNAs, had considerable statistical uncertainty and should be confirmed in a larger cohort, the association of NNAs at screening with subsequent inhibitor development was robust.
In our study, the presence of NNAs at screening in children not previously treated with any FVIII concentrate was associated with an almost threefold increased risk of development of high-titer inhibitors. In autoimmune diseases, such as systemic lupus erythematosus or rheumatoid arthritis, the appearance of autoantibodies often precedes the clinical onset of disease.42-44 One hypothetical explanation is that the autoantibody response needs to mature before it acquires distinct characteristics, such as specificity of antigen recognition or an increase or shift in antigen recognition.42-45 Similarly, we found that detectable inhibitors, and mostly high-titer inhibitors, can be preceded by NNAs.
Furthermore, the effect of NNAs on inhibitor development did not change its direction when adjusted for other variables, but the HR increased after adjustment for mutation. This can be explained by the inverse relations of null mutation with NNA presence on the one hand and inhibitor development on the other.
This study has some limitations. The follow-up of patients started from screening and not from birth, and therefore patients who died early were missed. Given the low fatality rate of hemophilia and the implausibility of a differential death rate by NNAs, this is an unlikely source of bias.
In conclusion, the development of anti-FVIII inhibitors is an event with multiple causes and although several factors have been identified,4-9,34,46-50 none of them reliably predict the risk of inhibitor for an individual patient. The identification of NNAs as an additional marker for inhibitor development needs to be validated in further studies, and once confirmed, it could be useful for measuring NNAs immediately at diagnosis or before any exposure to FVIII products. This may contribute to improved prediction scores, which in turn may lead to individualized interventions tailored to reduce the risk of inhibitor formation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the SIPPET investigators for their help with patient recruitment and data collection (the full list of investigators is provided in the supplemental Appendix available on the Blood Web site). We also thank Luigi Flaminio Ghilardini (Universitá degli Studi di Milano, Milan) for his help with figures and tables.
This work was supported by research grants from Fellowship Project Hemophilia Bayer Awards (A.C., 2014), from “Changing possibilities in hemophilia – Italian Academy 2015” (A.C., 2015), from CSL Behring Professor Heimburger Award (A.C., 2016), and from Aspire-Pfizer (A.C., 2016).
Authorship
Contribution: A.C., F.R.R., and F.P. designed the study, interpreted data, and wrote the manuscript; C.V. developed and validated the enzyme-linked immunosorbent assay and performed the laboratory work; I.G. collected data and performed the literature search; R.P. performed data analysis and contributed to writing the manuscript; P.M.M. helped revise the manuscript; and all authors critically revised the work, provided substantial input, and gave final approval to the version to be published.
The funders had no role in study design; protocol preparation; patient recruitment; data collection, handling, analysis, and interpretation; or writing of the report.
Conflict-of-interest disclosure: R.P. has received travel support from Pfizer. P.M.M. has received honoraria, participated in advisory boards, and served as a speaker for Baxalta, Bayer AG, Grifols, Kedrion Biopharma, Novo Nordisk, Biotest Pharmaceuticals, and LFB. F.P. has received honoraria and served as a speaker for Bayer AG, Biotest Pharmaceuticals, CSL Behring, Grifols, Novo Nordisk, and Sobi, has served as a consultant for Kedrion Biopharma, LFB, and Octapharma, and has received research funding from Alexion Pharmaceuticals, Biotest Pharmaceuticals, Kedrion Biopharma, and Novo Nordisk (to Fondazione Luigi Villa), and is a member of the scientific advisory board for Ablynx. The remaining authors declare no competing financial interests.
Correspondence: Flora Peyvandi, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico Ca'Granda Ospedale Maggiore Policlinico, Fondazione Luigi Villa, and Department of Pathophysiology and Transplantation, Università di Milano, via Pace 9, 20122 Milan, Italy; e-mail: flora.peyvandi@unimi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal