It is frustrating that after more than 3 decades, we still don’t know the optimal graft-versus-host disease (GVHD) prophylaxis regimen. In this issue of Blood, Kanakry and coworkers report on posttransplantation cyclophosphamide (PTCy) as a single-agent GVHD prophylaxis in HLA-matched bone marrow transplantation (BMT) and include an analysis of how much additional immunosuppressive therapy the patients eventually required.1
Kanakry and coworkers applied posttransplantation cyclophosphamide as a single-agent GVHD prophylaxis in HLA-matched related or unrelated bone marrow transplantation and analyzed how much additional immunosuppressive therapy the patients eventually required.
Kanakry and coworkers applied posttransplantation cyclophosphamide as a single-agent GVHD prophylaxis in HLA-matched related or unrelated bone marrow transplantation and analyzed how much additional immunosuppressive therapy the patients eventually required.
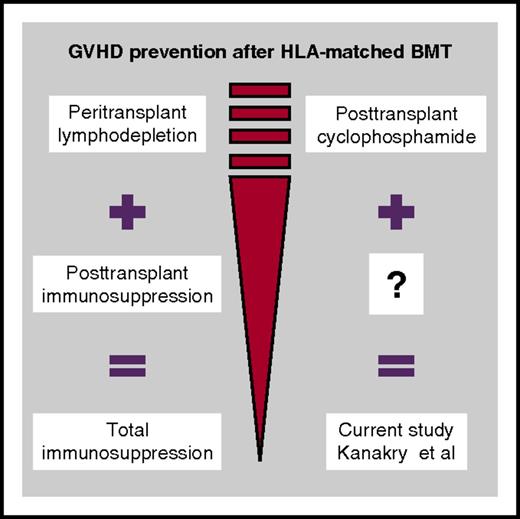
The strategies for GVHD prophylaxis involve one-off measures (eg, ex vivo or in vivo T-cell depletion, short-course methotrexate), which are performed during the peritransplant period with the goal of depleting alloreactive donor T cells and immunosuppressive drugs that are given continuously for several months posttransplant with the goal of preventing activation of T cells (see figure). The studies in the late 1980s showed that a dual attack is better; the combination of short-course methotrexate early after transplant with long-term administration of cyclosporine has demonstrated superiority over single-agent use.2 New drugs and technologies for both early lymphodepletion (eg, anti–T-cell globulin [ATG] thymoglobulin or ATG-Fresenius, alemtuzumab, CD34+ selection, TCRαβ+ selective T-cell depletion) and long-term immunosuppression (eg, mammalian target of rapamycin inhibitors) have been developed during the last few decades and have been tested alone or in various combinations. Novel concepts include measures to restore immunity or promote immunotolerance (eg, add-back of unspecific, virus/leukemia-specific, or regulatory T cells).
The Johns Hopkins University team pioneered the use of PTCy as an alternative GVHD prevention strategy. The benefit of PTCy over other strategies has been clearly demonstrated in the haploidentical setting.3 Vigorous T-cell depletion can abrogate GVHD; however, it is associated with a dramatic increase of infections and relapses and finally, worse outcome. The remarkable efficacy of PTCy in the setting of haploidentical transplantation is based on its strong anti-GVHD potential through the selective depletion of proliferating alloreactive donor lymphocytes, combined with the preservation of resting memory T cells, which lead in rapid immune reconstitution and low infection rates. Kanakry and colleagues hypothesize that PTCy may also be effective after HLA-matched transplantation, and they started in 2004 to use PTCy as the sole immunosuppression prophylaxis after sibling and unrelated BMT. In this issue of Blood, Kanakry and colleagues assess the longitudinal requirement for additional immunosuppressive therapy in 339 patients treated with this transplantation platform. Their study population includes the typical patients seen in a transplant unit, including mainly middle-aged acute leukemia patients treated with myeloablative regimens, with nearly one third of them not in morphologically complete remission at the time of transplant. Approximately 50% of related-donor and 30% of unrelated-donor transplanted patients never required additional immunosuppression beyond PTCy. The remaining patients received additional immunosuppressive therapy mainly for acute and late acute GVHD events, which was typically initiated at 2 to 4 months after transplantation, was limited to 1 or 2 agents (mainly corticosteroids and calcineurin inhibitors), and lasted typically for 4.5 to 5 months. Remarkably, the incidence of chronic GVHD was very low, ranging between 7% after related-donor transplant and 10% to 21% after unrelated-donor transplant (depending on the study cohort). Relapse rates were as expected, according to the Disease Risk Index stratification.
Are these results strong enough to change our current GVHD prophylaxis practice in HLA-matched related or unrelated transplantation, as happened with haploidentical transplants in which the PTCy concept revolutionized the field? No. It is particularly important to understand that these data apply to bone marrow recipients only, whereas patients receiving growth factor–mobilized blood, which is the popular graft source for allogeneic transplantation, require pharmacological immunosuppression beyond PTCy.4 Why does PTCy in HLA-matched transplantation show less remarkable results, as in the haploidentical setting? On the basis of the existent theoretical background, the selective T-cell depletion effect of PTCy can be induced only in the setting of HLA-mismatched transplantation, in which cross-reactive T cells in the donor inoculum enter a proliferating phase within 3 to 4 days after infusion to the patient in a manner mimicking the in vitro mixed-lymphocyte reaction process. In the HLA-matched setting, donor alloreactive T cells recognize foreign minor antigens presented in the context of “self”-HLA in a manner analogous to immune recognition of a virus-infected cell. The burst of proliferation of alloreactive cells does not occur so fast, and therefore administration of PTCy 3 to 4 days after transplant probably does not produce the same selective allodepletion effect as it does in the setting of haplotransplants.
Is PTCy better than ATG or ex vivo T-cell depletion? This cannot be answered with the current study. The immunosuppression-free survival rates reported here cannot be compared with the ones reported in the ATG/alemtuzumab studies, which contain mainly recipients of peripheral-blood stem cells.5-8 However, it is fair to say that the data presented here do suggest that control of GVHD, and especially of chronic GVHD, can be achieved in bone marrow–transplanted patients with PTCy given either alone or with the addition of modest amounts of pharmacological immunosuppression. These results compare favorably with the 41% to 53% expected incidence of chronic GVHD after sibling or unrelated T cell–replete BMT with the use of a standard methotrexate/cyclosporine combination.9 Why is cyclophosphamide more efficient than methotrexate in the HLA-matched setting? Regulatory T cells are resistant to cyclophosphamide because of their high aldehyde–dehydrogenase levels, and thus PTCy may, in addition to lymphodepletion, promote immunotolerance through preservation of the regulatory T cells.
Are we making progress in GVHD prophylaxis? Yes. PTCy is a safe, easy, and inexpensive GVHD prophylaxis regimen. Though its role seems to have been established in the haplotransplant setting, much work is needed to find its role in HLA-matched transplantation. The primary endpoint of this study was the burden of immunosuppression administered after transplant with bone marrow from a HLA-matched donor as the graft source. The little immunosuppression required after PTCy does not equal a true clinical benefit. Well-contacted large studies should analyze in more detail how PTCy influences immune recovery, infection rates, relapses, GVHD, survival, and immunosuppression-free survival in different transplant settings and compare that with current GVHD strategies in order to establish the value, the optimal timing, and manner of administration of this immunomodulating manipulation in the HLA-matched transplantation. There is still a long way to go to make transplantations safer and more efficient. The article in this issue of Blood prompts us to rethink: How much immunosuppression do we really need?
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal