Abstract
Clinical outcomes for patients undergoing allogeneic hematopoietic stem cell transplantation continue to improve, but chronic graft-versus-host disease (GVHD) remains a common toxicity and major cause of nonrelapse morbidity and mortality. Treatment of chronic GVHD has previously relied primarily on corticosteroids and other broadly immune suppressive agents. However, conventional immune suppressive agents have limited clinical efficacy in chronic GVHD, and prolonged immune suppressive treatments result in additional toxicities that further limit clinical recovery from transplant and return to normal daily function. Recent advances in our understanding of the immune pathology of chronic GVHD offer the possibility that new therapeutic approaches can be directed in more precise ways to target specific immunologic mechanisms and pathways. In this review, we briefly summarize current standard treatment options and present new therapeutic approaches that are supported by preclinical studies and early-phase clinical trials suggesting that these approaches may have clinical utility for treatment or prevention of chronic GVHD. Further evaluation of these new therapeutic options in well-designed prospective multicenter trials are needed to identify the most effective new agents and improve outcomes for patients with chronic GVHD.
Introduction
In all patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT), engrafting lymphocyte progenitors and mature donor lymphocytes encounter a broad array of recipient antigens that are highly immunogenic. Immune responses directed against these alloantigens result in the clinical syndromes of acute and chronic graft-versus-host disease (GVHD). Eventually, a successful transplant outcome requires that the donor immune system develop tolerance to these alloantigens while maintaining the ability to detect and respond effectively to an even broader array of foreign antigens. In patients with hematologic malignancies, donor immune responses directed against tumor cells (graft versus tumor) also help prevent relapse. Many studies in preclinical model systems and in patients undergoing allogeneic HSCT have enhanced our understanding of the various immunologic mechanisms that play key roles in the development of acute and chronic GVHD and graft-versus-tumor responses. While acute GVHD appears to be mediated primarily by mature donor T cells in the allogeneic stem cell product, chronic GVHD is now known to be a more complex immune reaction. Donor-derived effector T cells and B cells both contribute to the immune pathology of chronic GVHD, and regulatory elements within the T- and B-cell lineages play important roles in the development and maintenance of immune tolerance after HSCT. In this context, the immunologic mechanisms that result in chronic GVHD share many features with common autoimmune diseases, where defects in immune tolerance combine with adaptive immune responses targeting autoantigens to produce chronic tissue damage.
Studies in preclinical models have provided important insights into the complex interactions between subsets of effector T cells and B cells and abnormalities of immune regulation that contribute to tissue destruction and fibrosis in chronic GVHD.1,2 Although targeting a single noneffector cell type for the prevention or management of chronic GVHD may seem counterintuitive, the complex nature of these interactions offers many opportunities for specific interventions that have the potential to interrupt critical pathways needed to initiate and maintain these responses. Nonetheless, it is likely that interventions in individual pathways will not benefit all patients, and we do not yet have diagnostic biomarkers that can predict which pathway may represent the most appropriate target for intervention in any given patient. This review will briefly summarize current standard therapies for chronic GVHD and then focus on new therapeutic approaches that are supported by preclinical data and results of early-phase clinical trials. Many of these new approaches target specific immunologic pathways now known to play a role in chronic GVHD.
Standard therapy for chronic GVHD
Despite advances in the understanding of the pathobiology of chronic GVHD, there have been relatively few advances in the clinical management of this disease. To this day, corticosteroids and meticulous organ-specific care remain the mainstay of therapy.3,4 Corticosteroids are profoundly anti-inflammatory and broadly immune suppressive, affecting both innate and adaptive immunity. The addition of a calcineurin inhibitor to corticosteroids does not increase the response rate, but it allows for a reduction in steroid dosing that can reduce the sequelae of chronic corticosteroid therapy. Alternate-day dosing of corticosteroids similarly can reduce toxicity, but this approach is rarely used in clinical practice. The administration of other agents in combination with corticosteroids has not been proven to be beneficial when examined in prospective randomized trials.5 Therapy of corticosteroid-resistant chronic GVHD has relied primarily on broadly immune suppressive agents and specific inhibitors of T-cell signaling (such as tacrolimus and calcineurin inhibitors). Beyond those agents used in the initial therapy of chronic GVHD, the efficacy of second-line agents is limited, with response rates of ∼30% regardless of the agent that is chosen.3 There are no standard second-line therapies for advanced chronic GVHD, and enrollment in clinical trials designed to evaluate the safety and efficacy of new agents and/or combinations is often recommended in this setting.6 Ultimately, an effective approach to the prevention of chronic GVHD will be more useful than strategies to treat established disease.
Depletion of alloreactive T cells for the prevention of chronic GVHD
Mature donor-derived effector T cells are critical mediators of tissue damage in both acute and chronic GVHD. Various approaches that deplete donor T cells from hematopoietic stem cell products reduce the subsequent incidence and severity of chronic GVHD. These include positive selection of CD34 stem cells as well as methods to deplete T cells that express αβ T cell receptors, pan–T-cell antigens such as CD6 or naive T cells.7-11 T-cell depletion in vivo with antithymocyte globulin (ATG) administered in the peritransplant period to promote engraftment and prevent acute GVHD also reduces the incidence of chronic GVHD.12,13 Methods have also been developed to selectively deplete alloreactive T cells in the early posttransplant period. For example, the administration of posttransplant cyclophosphamide (pTCy) selectively depletes alloantigen-activated T cells in vivo and allows engraftment of hematopoietic stem cells from HLA-mismatched donors. Similarly, in vitro treatment of alloantigen-activated donor T cells with a photosensitizer (TH9402) enables selective photodepletion of these cells and allows the transfusion of donor T cells that can enhance T-cell immune reconstitution. When effective depletion of alloreactive T cells is achieved, these approaches appear to significantly reduce the incidence of both acute and chronic GVHD.14-18
B-cell depletion for established chronic GVHD
Studies in preclinical models and in patients after HSCT have suggested a role for donor B cells in the immune pathology of chronic GVHD.19,20 Ratanatharathorn et al21 were the first to note clinical improvement of chronic GVHD symptoms in a patient who received rituximab for immune-mediated thrombocytopenia that occurred after allogeneic HSCT. This provided a strong rationale for the evaluation of B-cell depletion strategies for the treatment or prevention of chronic GVHD, and subsequent reports have documented the effectiveness of this approach as therapy for steroid-refractory chronic GVHD.22-24 In a prospective study, we demonstrated a response rate of 70%, clinical safety, a dramatic steroid-sparing effect, and a meaningful reduction in chronic GVHD symptom score after a single 4-week course of rituximab. Clinical responses were also associated with reductions in titers of antibodies directed against Y-encoded minor histocompatibility antigens.25 In several published studies, there appeared to be a higher response rate in patients with sclerodermatous chronic GVHD. This led to a prospective randomized crossover trial that compared rituximab with imatinib in subjects previously treated with corticosteroids. However, significant clinical responses were noted in only 27% of subjects initially randomized to rituximab. In this study, clinical response to rituximab was predicted by higher levels of CD27+ activated B cells at trial entry.26
Two studies have examined the role of B-cell depletion combined with corticosteroids as initial therapy for chronic GVHD. The Stanford group treated 35 subjects with moderate to severe chronic GVHD using rituximab plus corticosteroids, demonstrating an overall response rate of 77% at 6 months and a complete response rate of 34%. However, by 24 months, the majority of subjects required additional therapy or succumbed to chronic GVHD.27 In contrast to other studies, clinical response was predicted by a naive (CD19+CD38−IgD+CD27−) B-cell phenotype. Pidala et al28 used the second-generation humanized monoclonal antibody ofatumumab, in conjunction with corticosteroids, in a phase 1 study that enrolled 12 subjects. Treatment was well tolerated, but in this small study, only 4 of 12 patients had complete responses after 6 months of therapy.
B-cell depletion for the prevention of chronic GVHD
Several studies have incorporated monoclonal anti–B-cell therapy as part of the conditioning regimen or given in the peritransplant period with the intent of reducing relapse of B-cell malignancies. In the BMT CTN 0701 trial, 65 subjects were given 4 doses of rituximab (1000 mg/m2) in the week prior and following a reduced-intensity HSCT.29 Despite very good depletion of CD20+ B cells and measurable levels of rituximab in the circulation that persisted beyond 6 months, the rate of chronic GVHD was >40% at 1 year and 60% at 2 years. A slightly larger study performed by the German High-Grade Lymphoma Study group prospectively randomized subjects to receive rituximab or no additional therapy after allogeneic HSCT.30 In this study, subjects received two 4-week courses of rituximab, beginning 21 and 175 days after HSCT. Results from this trial are difficult to interpret, because nearly half of the enrolled subjects received ATG. Although there was a reduction in the rate of chronic GVHD in patients randomized to the rituximab arm (33% vs 41%, P = .28), this was not statistically significant.
Two trials have employed B-cell depletion specifically with the goal of preventing chronic GVHD. The Stanford group administered rituximab 2 to 3 months after total lymphoid irradiation-ATG–based HSCT in a study that included 35 patients with B-cell malignancies.31 The cumulative incidence of chronic GVHD was only 20%, with an expected rate using this preparative regimen of ∼30%. Notably, male patients who receive stem cell transplants from female donors frequently develop antibodies to HY antigens, but this did not occur in patients that received rituximab for the prevention of chronic GVHD. In a phase 2 trial at the Dana-Farber Cancer Institute, 65 patients received single doses of rituximab (375 mg/m2) at 3-month intervals for 1 year after peripheral blood HSCT from related or unrelated donors.32 B-cell depletion was very effective and reconstitution of donor B cells was markedly delayed. The overall incidence of chronic GVHD was 48% at 2 years, however the rate of corticosteroid-requiring chronic GVHD was only 31% at 2 years, and was as low as 23% for patients with related donors. Comparison with a contemporaneous cohort of subjects who did not receive rituximab demonstrated a statistically significant decrease in the rate of steroid-requiring chronic GVHD. Non-relapse mortality was reduced, and overall survival improved, suggesting prevention of severe chronic GVHD can improve long-term survival. Given the promising activity of B-cell depletion for the prevention of chronic GVHD, a randomized trial using a second-generation monoclonal agent, obinutuzumab, is about to begin accrual.
One concern over the routine use of B-cell depletion in the post-HSCT setting is the persistent and profound hypogammaglobulinemia that is anticipated. In all studies, treatment with anti-CD20 antibodies results in profound and prolonged B-cell depletion.33 However, despite delayed reconstitution of B-cell immunity after HSCT, excess infections have generally not been noted when immunoglobulin repletion is routinely administered following American Society for Blood and Marrow Transplantation guidelines.34
Targeting the B-cell receptor signaling pathway
Spleen tyrosine kinase (SYK) and Bruton’s tyrosine kinase (BTK) play essential roles in B-cell receptor signaling. Upon engagement of B-cell receptor, SYK is activated and phosphorylates BTK. Both phosphorylated SYK and BTK can activate the downstream phospholipase C–γ2 pathway, whereas SYK can also activate the B-cell linker BLNK.35,36 Inhibition of B-cell signaling by targeting either SYK or BTK has become possible with the development of small-molecule inhibitors for these tyrosine kinases. While BTK is expressed primarily in B cells and other hematopoietic tissues, SYK has a broader expression pattern, including both epithelial and endothelial cells.35 Therefore, targeting these signaling pathways may be associated with broad on-target effects and potential toxicities, such as increased susceptibility to infections (for the SYK inhibitors) and cytopenias (for the BTK inhibitors). Nevertheless, studies in preclinical models suggest that targeting either SYK or BTK may be promising strategies for chronic GVHD therapy.36-38
Fostamatinib, the first SYK inhibitor to undergo advanced-stage clinical evaluation, has been tested extensively in rheumatologic diseases and is currently being tested in established chronic GVHD (#NCT02611063). Preliminary studies of the BTK inhibitor ibrutinib have also been reported.39 In a study of 28 subjects with steroid-refractory chronic GVHD, ibrutinib was given for a median of 4 months. Of 22 evaluable subjects, 11 had a partial response and 1 had a complete response, and the median corticosteroid dose reduction was 35%. Given these encouraging findings, a randomized trial is being planned, and this agent has been granted breakthrough status by the US Food and Drug Administration. The prospective use of ibrutinib in the post-HSCT setting to prevent relapse of chronic lymphocytic leukemia or other B-cell malignancies will additionally inform the role of ibrutinib for the prevention of chronic GVHD.
Although BTK is selectively expressed in B cells, ibrutinib also inhibits the closely related interleukin-2 (IL-2)–inducible kinase (ITK), which is highly expressed in T cells and ordinarily drives a TH2 response. In murine models, inhibition of both BTK and ITK by ibrutinib contributed to the prevention of chronic GVHD.40 In a recent clinical experience with ibrutinib, T cells were noted to be less activated.39 Inhibition of ITK may lead to a theoretical increase in CD8+-mediated graft-versus-leukemia (GVL), but broad inhibition of T cell activation may inhibit GVL as well as GVHD. Close monitoring of both T- and B-cell function as well as relapse will be needed to better understand the immunologic effects of ibrutinib in patients with chronic GVHD.
Prevention of B-cell development
Rather than depletion or inhibition of B-cell activity, preventing the development of pathogenic B cells may also be a useful strategy for the prevention or treatment of chronic GVHD. T follicular helper (TFH) cells migrate to germinal centers, where they promote differentiation of mature B cells and secretion of high-affinity immunoglobulin G antibodies.41 We have demonstrated that levels of circulating TFH cells are decreased in patients with active chronic GVHD, but these TFH cells are activated and have increased functional ability to promote B-cell maturation and immunoglobulin secretion.42 Strategies to inhibit or prevent migration of these cells to lymphoid organs have been effective in preclinical models and may prove useful for chronic GVHD prevention or therapy in the future.43
Inhibition of cytokine receptor–mediated signaling
Ruxolitinib, a selective inhibitor of Janus kinase 1 (JAK1) and JAK2, is an approved agent for the treatment of myelofibrosis.44,45 JAK1 and JAK2 mediate receptor-mediated signaling for a variety of proinflammatory cytokines, including interferon-γ and IL-6, and inhibition of this pathway has been shown to suppress activation and differentiation of dendritic cells as well as T cells. Proinflammatory cytokines are characteristically elevated in both acute and chronic GVHD, and inhibition of JAK1/JAK2 signaling with ruxolitinib has been shown to reduce GVHD in murine models.46-48 This novel approach has been tested in patients with steroid-refractory acute and chronic GVHD with encouraging results in both settings.46,49
CD4+CD25+Foxp3+ regulatory T cells (Tregs) are critical mediators of immune tolerance and are required to prevent fatal autoimmunity in healthy individuals. While Treg comprise only ∼5% to 10% of circulating CD4+ T cells, these cells use a variety of mechanisms to dominantly suppress autoreactivity and control innate and adaptive immune responses.50-55 Treg impairment is associated with loss of tolerance, autoimmunity, and chronic GVHD.56-58 In preclinical models of allogeneic HSCT, adoptive transfer of Tregs can ameliorate GVHD without impairing therapeutic GVL responses.59-61 In clinical studies, adult recipients of HLA-matched T-cell–replete grafts often experience poor reconstitution of Tregs.56,62 Impaired Treg reconstitution appears predictive for subsequent chronic GVHD in some, but not all, studies.57,62-65 Analysis of Treg homeostasis after HSCT has shown that Treg deficiency after HSCT appears to result primarily from decreased thymic production of Tregs. Although there is a compensatory increased homeostatic proliferation of circulating memory Tregs, this is ultimately insufficient to prevent Treg deficiency, due in part to proliferation-induced telomere shortening,66 enhanced death signaling, and apoptosis of rapidly dividing Treg.57,67 Treatment strategies attempting to enhance Treg number and function are therefore attractive for chronic GVHD therapy, offering the possibility of therapeutic immune modulation without generalized immunosuppression.
Facilitating Treg reconstitution for the prevention of chronic GVHD
GVHD prophylaxis regimens after transplantation with unmodified bone marrow or peripheral blood stem cell grafts often use short-term methotrexate plus a calcineurin inhibitor (CNI) such as cyclosporine A or tacrolimus (Tac). Continuous CNI therapy is often maintained for 6 months after transplant. While effective for acute GVHD prophylaxis, such regimens are suboptimal for chronic GVHD prevention, owing partly to the inhibition of Treg during CNI therapy.68 In animal models the use of alternative immune suppressive agents such as mTOR inhibitors (sirolimus/Sir) and mycophenolate mofetil (MMF) are more permissive of Treg reconstitution.68,69 However, when compared with standard of care Tac/methotrexate in a prospective phase 3 randomized trial, Tac/Sir resulted in equivalent incidence of acute and chronic GVHD, survival, and relapse.70 Similarly, the use of MMF plus steroids in the initial treatment of chronic GVHD was also evaluated in a phase 3 trial and did not result in clinical benefit, with a potentially increased mortality in the double-therapy arm.5
Novel acute GVHD prophylaxis regimens can also preferentially facilitate Treg recovery after HSCT. Posttransplantation cyclophosphamide (pTCy) is frequently used in the context of haploidentical bone marrow transplantation and is now being prospectively evaluated in patients with HLA-matched donors. pTCy selectively depletes activated alloreactive T cells while sparing resting T cells and hematopoietic stem cells. Interestingly, the GVHD protective effect of pTCy appears to be Treg dependent due to the differential expression of aldehyde dehydrogenase in Tregs compared with conventional CD4 T cells (Tcon). This difference protects Tregs and facilitates their subsequent expansion.71,72 Similarly, Treg-sparing effects and GVHD prevention have been reported for other novel regimens, including ruxolitinib,46 hypomethylating agents (azacytidine),73 and proteasome inhibitors (bortezomib).74-77 Bortezomib in conjunction with corticosteroids has also been evaluated as primary therapy for chronic GVHD in a phase 2 trial, with high response rates.78 All novel approaches, however, require confirmation of benefit in prospective randomized trials.
Extracorporeal photopheresis (ECP) is an established immunomodulatory therapy with a documented safety record in steroid-refractory chronic GVHD. The procedure involves leukapheresis of patient peripheral blood mononuclear cells followed by exposure of the cellular product to UV-A light in the presence of 8-methoxypsoralen. Ex vivo–treated cells are subsequently reinfused to complete the procedure. While the mechanisms by which ECP results in clinical improvement are not completely known, it is thought that ECP can suppress alloimmune responses in part by altering the maturation and function of dendritic cells and through the induction of Tregs.79 Regardless of the mechanism, responses in chronic GVHD patients have been associated with increased Treg cell counts, increased cellular activation, and inhibition of Tcon.80,81 ECP has been evaluated in a phase 2 prospective randomized trial, with demonstrated efficacy in sclerodermatous chronic GVHD, including a reduction in steroid dose, meaningful decrease in skin sclerosis, and nonblinded investigator assessment of skin complete or partial responses. However, this study did not meet its prespecified primary end point.82
Innovations in ECP are being developed to enhance its immunologic and clinical efficacy. One example is the use of photodepletion with a dibromorhodamine (TH9402) photosensitizer in lieu of 8-methoxypsoralen, which resulted in selective eradication of endogenous proliferating Tcon with concomitant sparing and expansion of Treg. This resulted in a higher level of circulating Tregs in patients receiving TH9402-based phototherapy.14 Further clinical evaluation of this approach is eagerly awaited, but even conventional ECP remains a useful option for patients with steroid-refractory chronic GVHD, especially for patients with severe cutaneous manifestations of disease who have geographic access to this therapy.
Adoptive Treg-cell therapy
Adoptive transfer of highly purified CD4 Tregs is the most direct method for Treg enhancement in vivo. This approach has been investigated by several groups, with and without ex vivo Treg expansion. In patients receiving stem cell transplants from haploidentical donors, Di Ianni et al infused immunomagnetic bead–purified donor Tregs without ex vivo expansion.83 This study evaluated the ability of donor Tregs to permit infusion of relatively large numbers of donor Tcon without invoking excess GVHD. With Treg infusion, the incidence of GVHD was low and immune recovery was enhanced. In patients receiving umbilical cord blood (UCB) transplants, Blazar and colleagues infused ex vivo–expanded third-party UCB Tregs in adult patients receiving double UCB transplants.84,85 These studies demonstrated the technical feasibility of purifying and selectively expanding Tregs from UCB products, and patients who received expanded Tregs had lower GVHD rates than historical controls. Additional innovations currently being developed include Treg fucosylation to improve engraftment and homing of Tregs,86 selective expansion of minor histocompatibility antigen–specific Tregs,87 and the genetic modification of Tregs with alloantigen-specific chimeric antigen receptors.88 These strategies exemplify different approaches that can be applied to generate highly functional Tregs for adoptive therapy, but each approach will need further clinical evaluation. Since Tregs represent a relatively small fraction of circulating lymphocytes, complex methods for cell purification and expansion are needed to manufacture sufficient numbers of Tregs for each patient and ensure that the final product does not also contain effector T cells that may cause GVHD. Additional theoretical concerns include the functional stability of transferred Tregs that may convert to an effector phenotype. Finally, the persistence of adoptively transferred Tregs may be limited if environmental conditions in vivo will not support their survival or further expansion.
Enhancement of CD4 Tregs in vivo
High-dose IL-2 (aldesleukin) is approved for the treatment of advanced malignant melanoma and renal cell carcinoma. However, at low physiologic concentrations, IL-2 is a critical homeostatic cytokine that is required for normal Treg development, expansion, activity, and survival.89-91 In a phase 1 clinical trial, daily administration of IL-2 at a dose of 1 × 106 IU/m2 per day was demonstrated to be safe and well tolerated in patients with steroid-refractory chronic GVHD. Notably, daily IL-2 treatment at this low dose selectively enhanced CD4+ Tregs in vivo and led to clinical responses in 52% of patients.92,93 In a phase 2 study, 35 adult patients with steroid-refractory chronic GVHD received daily IL-2 (1 × 106 IU/m2 per day) for 12 weeks, with a 61% clinical response rate and improvement at sites of chronic GVHD that included liver, skin, gastrointestinal tract, lung, and joint/muscle/fascia.94 As in the phase 1 study, Tregs and natural killer cell counts rose significantly, without change in conventional CD4 Tcon or CD8 T-cell counts. Notably, high Treg:Tcon ratios at baseline and at week 1 were predictive of IL-2 clinical response. Twenty-three patients with clinical benefit continued to receive daily IL-2 for 2 years. Clinical and Treg immune responses persisted, while the Tcon count and Treg:Tcon ratio gradually normalized. In patients with advanced tissue damage, very few complete responses have been observed and partial response rates are only in the 50% to 60% range, despite all patients experiencing increased number of circulating Tregs. The need for daily subcutaneous injections for prolonged periods is also a limitation. Nonetheless, these encouraging results provide a basis for further evaluation of low-dose IL-2 as primary therapy for chronic GVHD, and prospective comparative trials are currently being planned.
Considering the important role of IL-2 in the development and maintenance of Tregs, many efforts are underway to enhance the efficacy and utility of IL-2 therapy to promote immune tolerance. Approaches being evaluated at the Dana-Farber Cancer Institute include combining daily low-dose IL-2 with infusions of highly purified donor-derived Tregs (#NCT01937468). This combined therapy provides healthy donor Tregs to augment endogenous Tregs that may be dysfunctional in patients with chronic GVHD and also provides low-dose IL-2 as a growth factor to promote the proliferation and persistence of adoptively transferred Tregs. Another approach in pediatric and adult patients with chronic GVHD is evaluating whether individual IL-2 dose-escalation schedules can better sustain Treg cell activation in vivo (#NCT02318082). Alternatively, low-dose IL-2 can be combined with immune suppressive agents that facilitate Tregs, such as sirolimus, MMF, or ECP (#NCT02340676). Because of aldesleukin’s short half-life, current GVHD regimens employ daily dosing schedules that are inconvenient and cumbersome for prolonged therapy. Previous reports have described long-acting IL-2 formulations such as IL-2/albumin fusion proteins95 or IL-2/monoclonal antibody combinations96 and genetically engineered IL-2 variants that have greater selectivity for individual components of the trimeric IL-2 receptor expressed on different cell types.97,98 Use of modified IL-2 agents may enhance the ability of patients to continue therapy for prolonged periods and increase the ability of these agents to induce and maintain immune tolerance.
Ultra-low-dose IL-2 has also been tested in healthy volunteers and early after haploidentical (#NCT02226861), related, and unrelated donor transplantation.99,100 IL-2 has been safe and well tolerated and also promotes Tregs despite short periods of therapy in these studies. These observations suggest that low-dose IL-2 may also be useful for the prevention of chronic GVHD in different settings. With early posttransplant therapy as well as with prolonged treatment late after transplant, further studies will be necessary to determine whether GVL responses and pathogen responses are compromised. While early-phase clinical results and preclinical data are promising, additional trials are necessary before these options can be considered for routine clinical use.
Conclusions
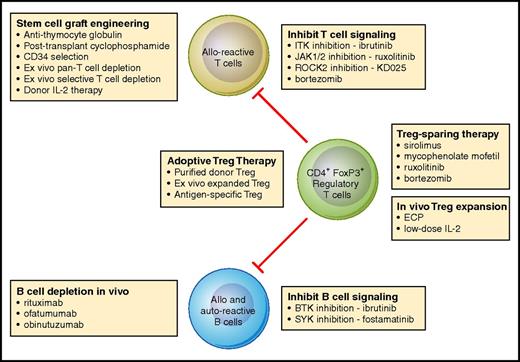
With improved understanding of the immunologic mechanisms that lead to the inability to establish immune tolerance and subsequent development of chronic GVHD, it is now possible to conceive of new therapeutic approaches that target specific immunologic mechanisms. These new approaches have been tested in preclinical models, and several promising therapies have also been evaluated in early-phase clinical trials. Figure 1 provides an overview of current therapies and new agents and identifies the immunologic cells and pathways targeted by different approaches. While results of recent clinical studies are promising, further studies in larger multicenter cohorts of patients are needed to identify the most effective and least toxic regimens. As these agents undergo clinical evaluation, by themselves and in combination, it seems likely that more effective and less toxic therapies will soon be available for the prevention and treatment of chronic GVHD.
Mechanistic interventions for the prevention or treatment of chronic GVHD. Current and new approaches for the prevention or treatment of chronic GVHD primarily target alloreactive donor T cells, allo- and autoreactive B cells, or CD4+FoxP3+ regulatory T cells. As advances in our understanding of the role of each of these cell types in the development of chronic GVHD has advanced in recent years, it is now possible to develop and select for clinical testing a variety of therapeutic interventions that focus on specific mechanistic pathways. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mechanistic interventions for the prevention or treatment of chronic GVHD. Current and new approaches for the prevention or treatment of chronic GVHD primarily target alloreactive donor T cells, allo- and autoreactive B cells, or CD4+FoxP3+ regulatory T cells. As advances in our understanding of the role of each of these cell types in the development of chronic GVHD has advanced in recent years, it is now possible to develop and select for clinical testing a variety of therapeutic interventions that focus on specific mechanistic pathways. Professional illustration by Patrick Lane, ScEYEnce Studios.
Acknowledgments
This study was supported by National Institutes of Health, National Cancer Institute grants P01CA142106, R01CA183559, and R01CA183560.
Authorship
Contribution: All authors wrote and edited the manuscript.
Conflict-of-interest disclosure: J.K. receives research support from Prometheus Labs, Inc. J.R. is a member of the Delinia Inc. scientific advisory board. C.S.C. declares no competing financial interests.
Correspondence: Jerome Ritz, Dana-Farber Cancer Institute, 450 Brookline Ave, M530, Boston, MA 02215; e-mail: jerome_ritz@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal