Key Points
Activating and inhibitory antibodies to the LFA-1 integrin inhibit the α4β1 integrin.
Inhibition occurs by intracellular signaling resulting from integrin phosphorylations.
Abstract
Binding of intercellular adhesion molecule-1 to the β2-integrin leukocyte function associated antigen-1 (LFA-1) is known to induce cross-talk to the α4β1 integrin. Using different LFA-1 monoclonal antibodies, we have been able to study the requirement and mechanism of action for the cross-talk in considerable detail. LFA-1-activating antibodies and those inhibitory antibodies that signal to α4β1 induce phosphorylation of Thr-758 on the β2-chain, which is followed by binding of 14-3-3 proteins and signaling through the G protein exchange factor Tiam1. This results in dephosphorylation of Thr-788/789 on the β1-chain of α4β1 and loss of binding to its ligand vascular cell adhesion molecule-1. The results show that with LFA-1 antibodies, we can activate LFA-1 and inhibit α4β1, inhibit both LFA-1 and α4β1, inhibit LFA-1 but not α4β1, or not affect LFA-1 or α4β1. These findings are important for the understanding of integrin regulation and for the interpretation of the effect of integrin antibodies and their use in clinical applications.
Introduction
To conduct effective immune surveillance, the host has to be able to recruit leukocytes to specific sites of infection and inflammation. Leukocytes adhere to the vascular endothelium in a sequence of events, including the capture of circulating leukocytes and subsequent leukocyte rolling, arrest, firm adhesion, and transendothelial migration. These processes are tightly regulated and mediated by the selectin and integrin adhesion receptors and their ligands.1-3
Integrins are heterodimeric transmembrane receptors. The family of leukocyte-specific β2 integrins consists of 4 members. They have a common β2-chain (CD18) and 1 of 4 α-chains: αL (CD11a), αM (CD11b), αX (CD11c), and αD (CD11d). The leukocyte function-associated antigen-1 heterodimer (LFA-1, αLβ2, CD11a/CD18) is primarily expressed in lymphocytes and binds to intercellular adhesion molecules (ICAMs).4,5 In addition, leukocytes express β1 integrins, among them α4β1 (very late antigen 4). α4β1 binds to the endothelial protein vascular cell adhesion molecule-1 (VCAM-1), but also to extracellular matrix proteins such as fibronectin.1
Integrins can communicate in 2 directions across the plasma membrane. Outside-in signaling occurs by integrin ligand binding, whereas inside-out signaling is activated by ligand binding to nonintegrin receptors, such as chemokine receptors or the T-cell receptor (TCR), which convey signals to integrins through intracellular pathways. This results in changes in integrin activity. Integrins can exist in at least 3 different conformations: inactive bent, extended, and extended open.6 The integrin cytoplasmic domains are short and devoid of catalytic activity. However, integrin function is regulated by changes in phosphorylation and protein interactions in the cytoplasmic domains.7-10
LFA-1 and α4β1 mediate distinct steps in the adhesion cascade. α4β1 mediates rolling and adhesion strengthening, whereas LFA-1 is needed for firm adhesion and migration.1 Importantly, integrins have the ability to modulate their adhesive properties within seconds after chemokine stimulation.11 We and others have previously shown that TCR activation, chemokines, and ligands to LFA-1 regulate α4β1 activity in T cells.12,13 Changes in both the phosphorylation status of β1 and β2 chains and the β-chain binding proteins mediate this cross-talk.13 The signaling pathway from the phosphorylated Thr-758 in the β2 integrin, followed by 14-3-3 binding, recruitment of T-cell lymphoma invasion and metastasis 1 (Tiam1) protein, and upregulation of Ras-related C3 botulinum toxin substrate 1 (Rac1) was described earlier.14-16 This leads to dephosphorylation of β1 Thr-788/789, reduced β1-14-3-3 binding, increased filamin binding to β1, and reduced adhesion of cells to VCAM-1.13
We have now studied the effect of LFA-1-specific antibodies on cross-talk to α4β1. We show that activating and some blocking LFA-1 antibodies inhibit α4β1-binding to VCAM-1. Other LFA-1 antibodies may inhibit LFA-1 but do not affect α4β1. The cross-talk is a result of intracellular signaling through phospholipases (phospholipase C β [PLCβ] and PLCγ) and protein kinase C (PKC), resulting in phosphorylation of Thr-758 on β2, signaling through Tiam1, decrease of β1 phosphorylation, and inhibition of α4β1 adhesion to VCAM-1. To the best of our knowledge, this is the first time it has been shown that antibodies to a leukocyte integrin can regulate another integrin in different ways as a result of intracellular signaling.
Methods
Reagents and antibodies
VCAM-1-Fc, ICAM-1-Fc, fibronectin, and stromal cell-derived factor 1α (SDF-1α) were from R&D Systems (Minneapolis, MN). A polyclonal rabbit antiserum against the β2-chain phosphorylated on Thr-758 was produced by GenicBio Ltd. (Shanghai, China). CBR LFA-1/2, CBR LFA-1/7, MEM83, and TS2/4 were from T.A. Springer (Boston Children’s Hospital, Boston, MA). Other antibodies were 7E4 and R2E7B,17 MEM48, MEM148, anti-phospho-β1 Thr-788/789, 12G10-488 (Abcam, Cambridge, United Kingdom), TS1/22, TS1/18, anti-α4 (Thermo Scientific, Waltham, MA), MHM23 (Dako, Glostrup, Denmark), MHM24 (DSHB, Iowa City, IA), anti-CD3, anti-CD25-PE (ImmunoTools, Friesoythe, Germany), anti-β1 (MAB13, BD Biosciences, San Jose, CA), anti-PLCβ3, anti-phospho-PLCβ3 Ser-1105, anti-phospho-PLCγ1 Tyr-783 (Cell Signaling, Danvers, MA), anti-talin 8d4, anti-actin (Sigma-Aldrich, St Louis, MO), anti-PLCγ1, anti-14-3-3 (Santa Cruz Biotechnology, Dallas, TX), blocking antibodies to α4 (2B4, R&D Systems) and α5 (P1D6, Millipore), horseradish peroxidase–linked Abs against mouse and rabbit IgG (GE Healthcare, Little Chalfont, United Kingdom). Fab fragments of the 7E4 antibody were produced by papain cleavage (10 µg papain/mg antibody; 37°C for 8 h) (Sigma-Aldrich) and purified by Protein G/A sepharose (PGS/PAS, GE Healthcare). Other reagents include Tiam1 (NSC23766, 100 μM) and ras-related C3 botulinum toxin substrate 1 (Rac1) (EHT1864, 10 μM) inhibitors (Biotechne, Minneapolis, MN), Cytochalasin D (Sigma-Aldrich).
Cell cultures, immunoprecipitation, and immunoblotting
The human T-cell lymphoma cell line Jβ2.7, which lacks CD11 chains,18 was grown in RPMI1640 medium supplemented with 10% FBS, 2 mM l-glutamine, and 100 U/mL penicillin-streptomycin. Jβ2.7 expressing wt LFA-1 has been described.13 Naive T cells were purified from buffy coats obtained from the Finnish Red Cross Blood Transfusion Service (Helsinki, Finland) by Ficoll-Hypaque gradient centrifugation and nylon-wool columns. T blasts were induced by ImmunoCult human CD3/CD28/CD2 T-cell activator and 30 IU/mL IL-2 in ImmunoCult-XF T Cell Expansion Medium (Stemcell Technologies, Grenoble, France) and used after 96 hours. Jβ2.7 or Jβ2.7/LFA-1 cells or T blasts were incubated with different LFA-1 antibodies for 15 minutes. Cells were washed in cold PBS, lysed on ice in 2% radioimmune precipitation assay buffer14 with protease and phosphatase inhibitors (Roche, Basel, Switzerland). Lysates were centrifuged at 13,400 rpm for 60 minutes at 4°C. For co-immunoprecipitation, cells were treated with CBR LFA-1/2 or TS2/4 and lysed. Prewashed PGS beads were added for 3 hours at 4°C. The unbound fraction was mixed with α4 antibody for 1 hour, and PGS beads for another 2 hours at 4°C. Beads were washed 4 times with 1% radioimmune precipitation assay buffer.14 Equal amounts of protein were analyzed by SDS-PAGE and immunoblotting and detected with ECL (Pierce Biotechnology, IL).
Cell adhesion and migration assays
For the static adhesion assay, culture wells were coated with 6 µg/mL ICAM-1 or 10 µg/mL fibronectin, blocked with 2% BSA, and 10 000 Jβ2.7/LFA-1-cells or T blasts were allowed to adhere for 30 minutes with or without pre-incubation with SDF-1α (50 ng/mL) or indicated antibodies (10 µg/mL except 30 μg/mL 7E4) for 15 minutes. Loose cells were washed off by placing the plate upside-down in PBS for 1 hour, and adhered cells counted from 10 screens using an EVOS microscope (Life Technologies, Carlsbad, CA). Flow adhesion assays were performed in VCAM-1-coated (10 µg/mL) flow chamber channels (μ-SlideVI0.4; Ibidi GmbH, Martinsried, Germany), using the multiphaser NE-1000 syringe pump (shear flow rate, 0.3 dynes/cm2; New Era Pump Systems, Inc., Farmingdale, NY). Attached cells were counted from 6 separate screens. The migration assay was performed over 5 µm pore-size Transwell membranes coated with fibronectin or BSA (5 µg/mL). Cells were incubated with α4 or α5 blocking antibody for 10 minutes and an activating (CBR LFA-1/2) or neutral (TS2/4) LFA-1 antibody for 15 minutes. Cells were allowed to migrate toward SDF-1α (25 ng/mL) at 37°C for 1 hour. Migrated cells were collected and counted.
Small interfering RNA and Rac1 pulldown
Tiam1 (200 nM; sc-36669) or control siRNA (Santa Cruz Biotechnology) was electroporated into cells with the Neon electroporator (Thermo Fisher Scientific).14 The active Rac-1 pulldown kit was from Thermo Scientific.
Immunofluorescence
Cells were treated with SDF-1α or antibodies for 15 minutes or left untreated. To cluster α4β1, cells were treated with soluble VCAM-1 (5 μg/mL) and an anti-VCAM-1 antibody (ImmunoTools). Cells were seeded on poly-L-lysine and fixed in 4% paraformaldehyde. Cells were stained with antibodies for 1 hour, and Alexa fluor secondary antibodies 488 or 633 or TRITC-phalloidin, washed in PBS, mounted with Prolong Hold Antifade reagent (Thermo Scientific), and analyzed with a Leica TCS SP5 MP confocal microscope (Wetzlar, Germany) and Leica Application Suite, using a HCX APO 63x/1,30 Corr (glycerol) CS 21 objective in room temperature.
Quantifications
Quantifications were done using ImageJ (v1.50b) software. Fluorescence intensity was quantified as corrected total cell fluorescence. Statistical analyses were performed in Excel with unpaired Student t test. Mean standard deviations are included in figures.
Results
LFA-1-specific antibodies affect the activity of α4β1
We have previously shown that activation of LFA-1 through the TCR receptor or chemokine receptors, induces a signaling pathway, leading to the phosphorylation of Thr-758 on the β2-chain, 14-3-3 and Tiam1 binding,14-16,19 and downregulation of α4β1 binding to VCAM-1.13 We have now found that activating and inhibitory antibodies to LFA-1 can regulate this signaling pathway. We collected antibodies to both β2 and αL, which have been described to activate, inhibit, or have no effect on LFA-1 ligand binding (Table 1). We first tested their ability to specifically bind LFA-1 on the human Jurkat cell line, Jβ2.7/LFA-1, expressing LFA-1, and, as a control on Jβ2.7 cells, lacking LFA-1 on their cell surface. Antibodies bound to LFA-1 on Jβ2.7/LFA-1 cells, but there was no binding to Jβ2.7 cells, as shown by flow cytometry (supplemental Figure 1, available on the Blood Web site). We next assessed the ability of these antibodies to activate or inhibit binding to the LFA-1 ligand ICAM-1 in a static adhesion assay. Jβ2.7/LFA-1 cells (Figure 1A) or CD25-positive T blasts (Figure 1B) were incubated with SDF-1α or the indicated activating antibodies, or incubated with SDF1-α together with blocking or neutral antibodies and ICAM-1-bound cells quantified. Activating but not neutral antibodies increased binding to ICAM-1, whereas blocking antibodies were able to block the SDF1-α-induced binding to ICAM-1.
LFA-1 specific antibody binding sites and effects on LFA-1 and α4β1 activity
| . | Chain . | Binding site . | Lfa-1 activity . | α4β1 activity . |
|---|---|---|---|---|
| CBR LFA-1/2 | β2 | I-EGF-3 | Activating | Inhibiting |
| MEM48 | β2 | I-EGF-3 | Activating | Inhibiting |
| MEM148 | β2 | Hybrid | Activating | Inhibiting |
| 7E4 | β2 | Hybrid | Inhibiting | Inhibiting |
| TS1/18 | β2 | β I-LIKE | Inhibiting | Inhibiting |
| MHM23 | β2 | β I-LIKE | Inhibiting | No effect |
| CBR LFA-1/7 | β2 | I-EGF-1 | No effect | No effect |
| MEM83 | αL | α I-domain | Activating | Inhibiting |
| TS1/22 | αL | α I-domain | Inhibiting | No effect |
| MHM24 | αL | α I-domain | Inhibiting | Inhibiting |
| TS2/4 | αL | β propeller | No effect | No effect |
| . | Chain . | Binding site . | Lfa-1 activity . | α4β1 activity . |
|---|---|---|---|---|
| CBR LFA-1/2 | β2 | I-EGF-3 | Activating | Inhibiting |
| MEM48 | β2 | I-EGF-3 | Activating | Inhibiting |
| MEM148 | β2 | Hybrid | Activating | Inhibiting |
| 7E4 | β2 | Hybrid | Inhibiting | Inhibiting |
| TS1/18 | β2 | β I-LIKE | Inhibiting | Inhibiting |
| MHM23 | β2 | β I-LIKE | Inhibiting | No effect |
| CBR LFA-1/7 | β2 | I-EGF-1 | No effect | No effect |
| MEM83 | αL | α I-domain | Activating | Inhibiting |
| TS1/22 | αL | α I-domain | Inhibiting | No effect |
| MHM24 | αL | α I-domain | Inhibiting | Inhibiting |
| TS2/4 | αL | β propeller | No effect | No effect |
Activating, blocking, and neutral antibodies toward LFA-1 affect binding of Jβ2/LFA-1 cells or T blasts to ICAM-1. (A,B) Static adhesion test to ICAM-1. Jβ2.7/LFA-1 cells (A) or T blasts (B) were activated with the chemokine SDF-1α or activating antibodies or with SDF1-α alone or together with blocking or neutral antibodies. Cells were allowed to bind to an ICAM-1-coated surface, unbound cells washed off, and bound cells counted from 6 screens in triplicate. Amounts of bound cells and standard deviations are shown. **P < .01.
Activating, blocking, and neutral antibodies toward LFA-1 affect binding of Jβ2/LFA-1 cells or T blasts to ICAM-1. (A,B) Static adhesion test to ICAM-1. Jβ2.7/LFA-1 cells (A) or T blasts (B) were activated with the chemokine SDF-1α or activating antibodies or with SDF1-α alone or together with blocking or neutral antibodies. Cells were allowed to bind to an ICAM-1-coated surface, unbound cells washed off, and bound cells counted from 6 screens in triplicate. Amounts of bound cells and standard deviations are shown. **P < .01.
The binding of Jβ2.7 cells and T blasts to VCAM-1 is mediated through α4β1, as blocking antibodies to α4β1 abolish VCAM-1-binding.13 To study the effect of the LFA-1-specific antibodies on α4β1 activity, cells were treated with different antibodies, and the amount of cells adhering to VCAM-1 under flow quantified. Incubation with the activating antibodies to β2 and αL leads to a decrease of Jβ2.7/LFA-1 cells binding to VCAM-1. This was LFA-1-dependent, as Jβ2.7 cells lacking LFA-1 showed no changes in VCAM-1 binding (Figure 2A). Next, we tested how blocking antibodies to β2 or αL affected α4β1 binding to VCAM-1, expecting to see no effect on α4β1 activity. The blocking antibodies MHM23 and TS1/22 did not affect adhesion to VCAM-1, but incubation of Jβ2.7/LFA-1 cells with the β2 blocking antibody 7E4 resulted in a strong decrease of α4β1 binding to VCAM-1. A smaller effect was seen with the β2 blocking TS1/18 and the αL blocking antibody MHM24. Incubation with neutral antibodies did not affect VCAM-1 binding. To test that the lack of effect of neutral antibodies was not caused by insufficient amounts of antibody, we tested increasing amounts of neutral antibodies CBR LFA-1/7 or TS2/4, with no change in VCAM-1 binding. Higher concentrations of blocking antibodies than the ones used did not change their effect on cells significantly, as shown for 7E4 (Figure 2B). The inhibitory effect of 7E4 on α4β1 activity was not caused by crosslinking of LFA-1 molecules, as purified monovalent Fab fragments of the 7E4 antibody caused inhibition of VCAM-1-binding equally well as the full antibody (Figure 2C). We also tested a set of antibodies on human T cells and T blasts (Figure 2D). Naive T cells bound poorly to VCAM-1 under flow. A similar pattern of α4β1 inhibition as in Jβ2.7/LFA-1 cells was seen for T blasts. The results show that specific antibodies to LFA-1 can affect the activity of α4β1 in the same cell. Blocking ICAM-1 or ICAM-2 with specific antibodies did not affect the cross-talk between LFA-1 and α4β1 initiated by LFA-1 antibodies (supplemental Figure 2A), and the presence of Mg2+ was not a prerequisite for cross-talk, as cells in PBS containing Ca2+ but no Mg2+ bound less to VCAM-1 when LFA-1 was activated. The most efficient transdominant inhibition of α4β1 was seen with PBS containing Mg2+ but lacking Ca2+, which may be a result of the inhibitory effect of Ca2+ on LFA-120 (supplemental Figure 2B).
Treatment of Jβ2/LFA-1 cells or T blasts with activating or blocking antibodies leads to transdominant inhibition of α4β1. Jβ2.7 or Jβ2.7/LFA-1 cells (A) or naive T cells or T blasts (D) were treated with SDF1-α, indicated LFA-1 antibodies, or left untreated. (B) Jβ2.7/LFA-1 cells were treated with different concentrations of the LFA-1 blocking antibody 7E4 or the neutral antibodies CBR LFA-1/7 or TS2/4. (C) Jβ2.7/LFA-1 cells were treated with the 7E4 antibody or an equal molar amount of the Fab fragment of the antibody. Adhesion to VCAM-1 under flow was quantified from 6 screens in triplicate. Amounts of bound cells and standard deviations shown. *P < .05; **P < .01, compared with untreated control of the same cell type.
Treatment of Jβ2/LFA-1 cells or T blasts with activating or blocking antibodies leads to transdominant inhibition of α4β1. Jβ2.7 or Jβ2.7/LFA-1 cells (A) or naive T cells or T blasts (D) were treated with SDF1-α, indicated LFA-1 antibodies, or left untreated. (B) Jβ2.7/LFA-1 cells were treated with different concentrations of the LFA-1 blocking antibody 7E4 or the neutral antibodies CBR LFA-1/7 or TS2/4. (C) Jβ2.7/LFA-1 cells were treated with the 7E4 antibody or an equal molar amount of the Fab fragment of the antibody. Adhesion to VCAM-1 under flow was quantified from 6 screens in triplicate. Amounts of bound cells and standard deviations shown. *P < .05; **P < .01, compared with untreated control of the same cell type.
Integrins α4β1 and α5β1 on T cells bind fibronectin. Cells were incubated with blocking antibodies to α4 or α5 before LFA-1 antibodies and allowed to adhere to fibronectin. LFA-1-mediated inhibition of α4β1 is specific for VCAM-1 binding, as cells with activated LFA-1 bound equally well to fibronectin after blocking α5β1. Cells with active LFA-1, however, increased binding to fibronectin mediated by α5β1 (Figure 3A-B). The same could be seen in a migration assay over fibronectin-coated transwell filters toward SDF1-α (Figure 3C). Activation of α5β1 after blocking α4β1 has previously been shown.21 Whether the increased binding of α5β1 to fibronectin is mediated by LFA-1 or a consequence of α4β1 inhibition by LFA-1 is not known.
Treatment of Jβ2/LFA-1 cells or T blasts with activating or blocking antibodies enhances α5β1 and does not reduce α4β1 adhesion and migration on fibronectin. Jβ2.7/LFA-1 cells (A) or T blasts (B) were treated with α4 or α5 blocking antibodies and the LFA-1 activating antibody CBR LFA-1/2 or neutral antibody TS2/4 or left untreated. Cells were allowed to adhere to fibronectin, unbound cells washed off, and bound cells quantified from 6 screens in triplicate. Amounts of bound cells and standard deviations are shown. (C) T blasts were treated as earlier and allowed to migrate over a fibronectin-coated filter for 1 hour. Migrated cells were quantified from 3 independent experiments. *P < .05.
Treatment of Jβ2/LFA-1 cells or T blasts with activating or blocking antibodies enhances α5β1 and does not reduce α4β1 adhesion and migration on fibronectin. Jβ2.7/LFA-1 cells (A) or T blasts (B) were treated with α4 or α5 blocking antibodies and the LFA-1 activating antibody CBR LFA-1/2 or neutral antibody TS2/4 or left untreated. Cells were allowed to adhere to fibronectin, unbound cells washed off, and bound cells quantified from 6 screens in triplicate. Amounts of bound cells and standard deviations are shown. (C) T blasts were treated as earlier and allowed to migrate over a fibronectin-coated filter for 1 hour. Migrated cells were quantified from 3 independent experiments. *P < .05.
Antibodies to LFA-1 cause changes in β2 and PLC phosphorylation
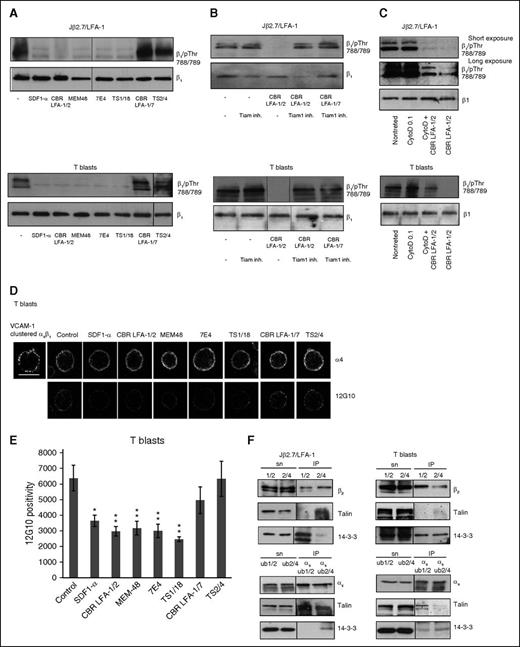
LFA-1-mediated inhibition of α4β1 activity in anti-CD3 or SDF1-α-activated T cells includes β2-chain phosphorylation on Thr-758.13 After SDF1-α activation, phospho-Thr-758 could be detected in cell lysates (100 kDa) and by immunofluorescence with a phospho-specific antiserum (supplemental Figure 3A-B). The additional band in β2 pThr-758 (Figure 4A) may be intracellular β2. Jβ2.7 cells showed no reactivity with the phospho-Thr-758-antiserum, indicating that it is LFA-1-specific. Cells incubated with activating antibodies, but also with the blocking antibody 7E4, were phosphorylated on Thr-758. This could not be seen with the blocking antibody TS1/18 or the neutral antibodies (Figure 4A-B). Phosphorylation of β2 Thr-758 was also seen by immunofluorescence of cells allowed to settle on poly-l-lysine and stained for Thr-758 and phalloidin after incubation with β2-activating antibodies or 7E4 (Figure 4C).
Treatment of Jβ2.7/LFA-1 cells or T blasts with activating or blocking antibodies results in phosphorylation of β2, PLCβ3, and PLCγ1. Jβ2.7/LFA-1 cells (A) or T blasts (B) were treated with SDF1-α, antibodies or left untreated. Cells were lysed and analyzed by western blotting, with the specific antibodies indicated to the right. (C) T blasts were allowed to settle on poly-l-lysine, were treated with SDF-1α, the indicated antibodies, or left untreated. Cells were fixed and stained with phalloidin or the β2/pThr-758 antiserum. Scale bar represents 10 μM.
Treatment of Jβ2.7/LFA-1 cells or T blasts with activating or blocking antibodies results in phosphorylation of β2, PLCβ3, and PLCγ1. Jβ2.7/LFA-1 cells (A) or T blasts (B) were treated with SDF1-α, antibodies or left untreated. Cells were lysed and analyzed by western blotting, with the specific antibodies indicated to the right. (C) T blasts were allowed to settle on poly-l-lysine, were treated with SDF-1α, the indicated antibodies, or left untreated. Cells were fixed and stained with phalloidin or the β2/pThr-758 antiserum. Scale bar represents 10 μM.
SDF1-α can activate PLCβ3 and PLCγ1.22 PLC catalyzes the cleavage of PIP2 into diacylglycerol, which results in the activation of PKC, which in turn phosphorylates Thr-758 on β2.19 We therefore tested whether PLCs are activated after LFA-1 antibody treatments. Incubating cells with the β2-activating antibodies or the blocking antibody 7E4 resulted in increased phosphorylation of PLCβ3 on Ser-1105 and PLCγ1 on Tyr-783, indicating that they are activated. Lower amounts of phosphorylated PLC was also seen after other antibody incubations, especially in Jβ2.7/LFA-1 cells. These results indicate that incubation of cells with activating antibodies to LFA-1 or the blocking antibody 7E4 leads to PLC activation and β2 Thr-758 phosphorylation (Figure 4A-B). A difference in the total amount of PLCβ3 was present both in the soluble and pellet fractions. The amount of PLCβ3 remained low in control cells after MG132 or leupeptin treatment. Cells treated with 7E4 showed more PLCβ3, and the amount remained unchanged after MG132 treatment, but leupeptin reduced the amount to similar levels as in untreated cells. This could indicate that calpain regulates a pathway that leads to increased PLCβ3 levels after LFA-1 activation and cross-talk signaling to α4β1 (supplemental Figure 4A).
Inhibition of α4β1 by LFA-1 antibodies requires signaling through Tiam1
Phosphorylation of Thr-758 on β2 leads to the binding of 14-3-3 and signaling through Tiam1 and Rac1.14 Cells were incubated with LFA-1 antibodies with or without pretreatment with the Tiam1 inhibitor. The reduction of binding seen with CBR LFA-1/2 or 7E4 was reversed by the inhibition of Tiam1. (Figure 5A-B). Because the Tiam1 inhibitor also can inhibit TrioN, we transfected Jβ2.7/LFA-1 cells with siRNA for Tiam1 or a control. Tiam1 siRNA reduced Tiam1 to 35% of untreated cells and caused a reduction of cross-talk to α4β1 (Figure 5C).
Inhibitory signaling from LFA-1 to α4β1 in Jβ2.7/LFA-1 cells or T blasts requires Tiam1 and an intact cytoskeleton. (A) Jβ2.7 or Jβ2.7/LFA-1 cells or (B) T blasts were treated with SDF1-α, the LFA-1 activating (CBR LFA-1/2), blocking (7E4), or neutral (CBR LFA-1/7) antibodies with or without a Tiam1 inhibitor. (C) Jβ2.7/LFA-1 cells transfected with Tiam1 siRNA or control siRNA and treated with activating (CBR LFA-1/2) or blocking (7E4) antibodies. (D) Jβ2.7/LFA-1 cells were treated with cytochalasin D (0.1 or 10 μg/mL) and LFA-1 activating (MEM48), blocking (7E4), or neutral (CBR LFA-1/7) antibodies. Adhesion to VCAM-1 under flow was quantified from 6 screens in triplicate. Amounts of bound cells and standard deviations are shown. Phalloidin staining of F-actin of Jβ2.7/LFA cells treated with 0.1 or 10 μg/mL cytochalasin D. Scale bar represents 10 μM. (E) Jβ2.7 or Jβ2.7/LFA-1 cells treated with LFA-1 activating antibody (CBR LFA-1/2) or blocking antibody (7E4) were lysed and Rac-GTP pulled down from lysates. Immunoblot shows Rac-GTP from pulldown and total Rac or actin from cell lysates. (F) Jβ2.7 or Jβ2.7/LFA-1 cells were incubated with the Rac inhibitor or left untreated and with the LFA-1 activating antibody (CBR LFA-1/2), and cells adhering to VCAM-1 under flow quantified as earlier. *P < .05; **P < .01.
Inhibitory signaling from LFA-1 to α4β1 in Jβ2.7/LFA-1 cells or T blasts requires Tiam1 and an intact cytoskeleton. (A) Jβ2.7 or Jβ2.7/LFA-1 cells or (B) T blasts were treated with SDF1-α, the LFA-1 activating (CBR LFA-1/2), blocking (7E4), or neutral (CBR LFA-1/7) antibodies with or without a Tiam1 inhibitor. (C) Jβ2.7/LFA-1 cells transfected with Tiam1 siRNA or control siRNA and treated with activating (CBR LFA-1/2) or blocking (7E4) antibodies. (D) Jβ2.7/LFA-1 cells were treated with cytochalasin D (0.1 or 10 μg/mL) and LFA-1 activating (MEM48), blocking (7E4), or neutral (CBR LFA-1/7) antibodies. Adhesion to VCAM-1 under flow was quantified from 6 screens in triplicate. Amounts of bound cells and standard deviations are shown. Phalloidin staining of F-actin of Jβ2.7/LFA cells treated with 0.1 or 10 μg/mL cytochalasin D. Scale bar represents 10 μM. (E) Jβ2.7 or Jβ2.7/LFA-1 cells treated with LFA-1 activating antibody (CBR LFA-1/2) or blocking antibody (7E4) were lysed and Rac-GTP pulled down from lysates. Immunoblot shows Rac-GTP from pulldown and total Rac or actin from cell lysates. (F) Jβ2.7 or Jβ2.7/LFA-1 cells were incubated with the Rac inhibitor or left untreated and with the LFA-1 activating antibody (CBR LFA-1/2), and cells adhering to VCAM-1 under flow quantified as earlier. *P < .05; **P < .01.
Treatment of cells with low concentration of cytochalasin D partly reversed the inhibition of VCAM-1 binding seen with MEM48 and 7E4 (Figure 5D). This indicates that an intact cytoskeleton is required for the cross-talk between LFA-1 and α4β1 or is a result of increased mobility of integrins.23 The amount of active Rac1 in antibody-treated cells was not changed (Figure 5E). Rac1 has been implicated in the regulation of α4β1 activity.24 We saw that Rac1 inhibition decreased binding to VCAM-1 also in Jβ2.7 cells lacking LFA-1. Therefore, we were unable to study the role of LFA-1 antibodies on α4β1 in cells with inactive Rac1 (Figure 5F).
Antibodies to LFA-1 affect β1 phosphorylation and activation
We next studied the phosphorylation and activation state of α4β1. Cells were treated with LFA-1 antibodies, and the phosphorylation state of β1 studied using a phospho-specific Thr-788/789 antiserum. The phosphorylation was reduced by activating LFA-1 antibodies or the blocking antibodies 7E4 and TS1/18, but not by neutral antibodies (Figure 6A). In cells treated with the Tiam1 inhibitor before CBR LFA-1/2, the amount of β1 phosphorylation was not reduced (Figure 6B), whereas cells treated with 0.1 μg/mL cytochalasin D before LFA-1/2 showed an intermediate amount of phosphorylated β1 (Figure 6C). Activating and blocking antibodies toward LFA-1 caused a reduction in the intensity of 12G10 staining, indicating that there is less active β1 in the cells. There were no changes in α4 clustering on the cell surface of antibody-treated cells (Figure 6D-E).
Antibody treatment of LFA-1 on Jβ2.7 cells or T blasts leads to changes in integrin β1-phosphorylation, activation epitope, and protein complexes. (A) Jβ2.7/LFA-1 cells or T blasts were treated with SDF1-α or antibodies to LFA-1. Cells were lysed and analyzed by western blotting for β1/pThr-788/789 or β1. (B) Cells were preincubated with Tiam1 inhibitor or (C) cytochalasin D (0.1 μg/mL) (C) and LFA-1 antibodies CBR LFA-1/2 or CBR LFA-1/7, and lysates analyzed as earlier. (D) T blasts were treated as earlier, allowed to adhere on poly-l-lysine, and fixed before immunofluorescence staining with an α4 antibody or the antibody for activated integrin β1 (12G10-488). α4β1 clustered by soluble VCAM-1 and VCAM-1 antibody. (E) Positive pixels from 50 cells stained with 12G10-488 were quantified using ImageJ. Standard deviations shown and statistical significance compared with control. *P < .05; **P < .01. Scale bar represents 10 μM. (F) Jβ2.7/LFA-1 cells or T blasts were lysed, immunoprecipitated with the activating CBR LFA-1/2 (1/2) or neutral TS2/4 (2/4) antibody, and immunoblotted for β2, talin or 14-3-3. The unbound fraction of the CBR LFA-1/2 (ub1/2) and TS2/4 (ub2/4) precipitates were further immunoprecipitated with α4 antibody and immunoblotted for α4, talin, and 14-3-3.
Antibody treatment of LFA-1 on Jβ2.7 cells or T blasts leads to changes in integrin β1-phosphorylation, activation epitope, and protein complexes. (A) Jβ2.7/LFA-1 cells or T blasts were treated with SDF1-α or antibodies to LFA-1. Cells were lysed and analyzed by western blotting for β1/pThr-788/789 or β1. (B) Cells were preincubated with Tiam1 inhibitor or (C) cytochalasin D (0.1 μg/mL) (C) and LFA-1 antibodies CBR LFA-1/2 or CBR LFA-1/7, and lysates analyzed as earlier. (D) T blasts were treated as earlier, allowed to adhere on poly-l-lysine, and fixed before immunofluorescence staining with an α4 antibody or the antibody for activated integrin β1 (12G10-488). α4β1 clustered by soluble VCAM-1 and VCAM-1 antibody. (E) Positive pixels from 50 cells stained with 12G10-488 were quantified using ImageJ. Standard deviations shown and statistical significance compared with control. *P < .05; **P < .01. Scale bar represents 10 μM. (F) Jβ2.7/LFA-1 cells or T blasts were lysed, immunoprecipitated with the activating CBR LFA-1/2 (1/2) or neutral TS2/4 (2/4) antibody, and immunoblotted for β2, talin or 14-3-3. The unbound fraction of the CBR LFA-1/2 (ub1/2) and TS2/4 (ub2/4) precipitates were further immunoprecipitated with α4 antibody and immunoblotted for α4, talin, and 14-3-3.
In co-immunoprecipitation experiments, more talin was bound to α4-complexes in cells treated with the LFA-1 activating antibody CBR LFA-1/2 than in cells treated with the neutral antibody TS2/4. Both the 225- and the 190-kDa talin fragments were coprecipitated. Weak talin binding was seen in β2 complexes of TS2/4-treated cells. More 14-3-3 bound to β2 from CBR LFA-1/2 activated cells and less to the TS2/4-treated cells, whereas the opposite was seen in α4-complexes (Figure 6F).13
The blocking antibodies 7E4 and TS1/18 regulate α4β1 activity through the same pathway, but with different intensity
7E4 was most potent in inhibiting α4β1 activity, whereas the TS1/18 antibody showed an intermediate effect. Incubation of cells with 7E4 lead to the phosphorylation of PLC and Thr-758 on β2, and a Tiam1-dependent decrease in α4β1 phosphorylation of Thr-788/789 on β1, 12G10 reactivity, and VCAM-1 binding. We also saw a reduction in β1 phosphorylation, 12G10 reactivity, and VCAM-1 binding with TS1/18 treatment, but it was not as strong as with activating antibodies or 7E4. Although no phosphorylation of Thr-758 was detected with shorter exposures (Figure 4B-C), with long exposures a weaker phospho-Thr-758 signal could be detected after TS1/18 treatment, which may be enough to initiate inhibitory signaling (supplemental Figure 4B). Blocking Tiam1 partly restored VCAM-1 binding, supporting signaling through Tiam1 for α4β1 inhibition (supplemental Figure 4C).
Discussion
Interactions between integrins and their ligands are involved in many pathological conditions, including autoimmune diseases, allograft rejection, and cancer. Blocking LFA-1 and α4β1 ligand interactions is a potential target for disease interventions. Antibodies, peptides, and small molecules toward these integrins have been used to control inflammation and autoimmune diseases as well as metastasis.25,26 We now show that antibodies that bind specifically to LFA-1 can regulate the activity not only of LFA-1 but also another integrin, α4β1. This is important to know when studying integrin antibody effects on cells, but also when designing treatment strategies aiming at a specific integrin.
Leukocytes circulating in the blood are nonadhesive and express inactive forms of the adhesion receptors LFA-1 or α4β1. As they reach the inflammatory site, these integrins are activated in a highly regulated manner to allow for the sequential steps of cell rolling, adhesion, firm adhesion, and transmigration over endothelial cells. Cross-talk between LFA-1 and α4β1 allows the controlled progression of leukocyte adhesion and migration in this stepwise manner.12,13,21 At an early stage, α4β1 mediates rolling and simultaneously may provide activation signals required for firm adhesion enhancing LFA-1 binding to ICAM-1.27,28 Next, the transition from firm adhesion to a migratory phenotype is initiated by LFA-1-mediated transdominant inhibition of α4β1.13,21 Adhesion of T cells via β2 integrins not only decreases α4β1 integrin-mediated adhesion but also enhances α5β1 integrin-mediated transmigration (Figure 3).21 We have previously shown that the trans-dominant inhibition of α4β1 by LFA-1 mediated by intracellular signaling requires an active form of LFA-1 and changes in β1- and β2-chain phosphorylations and protein interactions.13 We now show that this cross-talk can be initiated not only by inside-out activation or ligand binding of LFA-1 but also by specific antibodies.
Antibodies shown to activate LFA-1 ligand binding and an active conformation initiate an inhibitory signal to α4β1, which could not be seen with neutral antibodies. Antibodies that have been shown to inactivate LFA-1 ligand binding show different outcomes, evidently depending on the binding site on the extracellular domains. The LFA-1 antibodies that we have used have been well-characterized and their binding sites carefully mapped (Figure 7).
Schematic figure of LFA-1-specific antibody binding sites and the cross-talk from LFA-1 to α4β1. (A) A schematic picture of LFA-1 αL and β2-chains, with the binding sites of the antibodies used. Antibodies in red induce the inhibitory signal to α4β1; antibodies in blue do not affect α4β1 activity. Binding site for talin and 14-3-3 indicated on the β2 chain. (B) Activation from the T-cell receptor, through chemokines or by LFA-1 antibodies, results in PLC and PKC activation and phosphorylation of Thr-758 in β2, followed by binding of 14-3-3 and Tiam1 and activation of Rac1. The signal is further transferred to α4β1, resulting in dephosphorylation of Thr-788/789 and loss of activity and ligand binding.
Schematic figure of LFA-1-specific antibody binding sites and the cross-talk from LFA-1 to α4β1. (A) A schematic picture of LFA-1 αL and β2-chains, with the binding sites of the antibodies used. Antibodies in red induce the inhibitory signal to α4β1; antibodies in blue do not affect α4β1 activity. Binding site for talin and 14-3-3 indicated on the β2 chain. (B) Activation from the T-cell receptor, through chemokines or by LFA-1 antibodies, results in PLC and PKC activation and phosphorylation of Thr-758 in β2, followed by binding of 14-3-3 and Tiam1 and activation of Rac1. The signal is further transferred to α4β1, resulting in dephosphorylation of Thr-788/789 and loss of activity and ligand binding.
Antibodies that map to the αL I-domain or the β I-like domain are normally inhibitory. Antibodies that bind more C-terminally are often neutral or activating.29 The neutral antibody CBR LFA-1/7 binds to the first I-EGF-domain on β230 and TS2/4 to the β-propeller on αL.31,32 The LFA-1 stalk region provides a crucial link between changes in the transmembrane and cytoplasmic domains and conformational movements in the ligand binding site. Several antibodies that activate ligand binding map to this region. In our experiments, the β2 activating antibodies CBR LFA-1/2, MEM48, and MEM148, as well as the αL activating antibody MEM83, reduced α4β1 binding to VCAM-1. CBR LFA-1/233 and MEM4834 bind to overlapping epitopes including residues 534, 536, 541, 543, and 546 in the I-EGF-3 repeat.29,30 CBR LFA-1/2 and MEM48 have been suggested to act as a wedge to keep the α- and β-subunits apart, and thus induce an extended form of β2 altering the ligand binding part.30,35 This change in conformation also affects protein interactions with the β2 cytoplasmic domain and may induce Thr-758 phosphorylation. MEM148 binds to the hybrid domain in β2 and recognizes an activation epitope, residue Pro-374, on the β2 hybrid domain facing the α-subunit β-propeller (ie, the inner face of the hybrid domain), and it stabilizes hybrid domain swing out and increases affinity toward ligand.36-38
7E4 binds to the hybrid domain, but in contrast to MEM148, it is inhibitory.17 It binds to residue Val-385 on a loop on the outer face of the hybrid domain at the interphase with the β I-like domain, which is remodeled on hybrid domain swingout to the open conformation.39,40 The antibody TS1/1831 binds to the β I-like domain, blocks ligand binding,29,30 and induces a closed head piece.36,41 The epitope maps to the residues that are adjacent in the α1 and α7 helices of the β I-like domain (Arg-133, Gln-332). The β2 blocking antibody MHM2342 binds to Glu-175 in the loop between the β2 and β3 strands in the β I-like domain, but it does not inhibit α4β1. Both 7E4 and TS1/18 localize to regions that are significantly altered when shifting to the open head piece. Binding of these antibodies stabilizes the closed head piece36 and may simultaneously cause structural changes that result in intracellular signaling, similar to that seen with activating antibodies. Therefore, an antibody can be both inhibitory when it comes to ligand binding and activating when it comes to intracellular signaling.
Two of the αL blocking antibodies, TS1/2231,43 and MHM24,42 bind to the α-chain I domain. TS1/22 does not affect α4β1 activity, whereas MHM24 shows a small reduction in α4β1 activity. The αL activating antibody MEM83 also binds to the α-chain I-domain and inhibits α4β1 activity, but less than other activating antibodies. The epitopes of MEM83 and MHM24 are in close proximity, MEM83 was mapped to the β3–α2 loop and the α4 helix residues 153 to 183 and 217 to 248 and MHM24 to the α–-α4 loops containing residues 197 to 201. TS1/22 maps more C-terminal to the β5–α6 loop and the short α6 helix residues 266 to 270.43
The murine antibody MHM24 has been humanized (efalizumab) (Raptiva, Genentech)44 for treating psoriasis45 and can inhibit the extravasation and of T lymphocytes.46 It is of interest to note that according to our studies, MHM24 does not only inhibit ICAM-1 binding but also significantly reduces α4β1 binding to VCAM-1. Thus, the 2 important integrins in transmigration are inhibited.
Antibodies that initiate cross-talk to α4β1 cause phosphorylation of Thr-758 on β2. The mechanism by which antibody binding leads to the phosphorylation of the β2 cytoplasmic tail is only partially understood, but we show that PLC enzymes are activated. The resulting diacylglycerol is known to activate PKCs, the kinases phosphorylating Thr-758.19 Antibodies to LFA-1 may cause structural changes in the integrin, which transfer signals into the cell, activating PLC, and thus PKC.
The reduced binding of α4β1 to VCAM-1 indicates a change in affinity. Cross-talk between integrins have previously been shown to require an intact cytoskeleton,47 and we confirm this, using cytochalasin D. Future studies of different subsets of recently activated effector lymphocytes will shed light on the environmental settings in which this cross-talk takes place.
Here we show that modifying LFA-1 activity by LFA-1-specific antibodies will not only affect LFA-1 ligand binding but concomitantly may change the activity of α4β1. We now have antibodies to LFA-1, which enable us to activate LFA-1 and block α4β1, inhibit both integrins, or only block LFA-1. The findings may make it possible to develop specific pharmaceuticals of high specificity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funding from the Academy of Finland, the Sigrid Jusélius Foundation, the Medicinska Understödsföreningen Liv och Hälsa, the Finska Läkaresällskapet, the Wilhelm and Else Stockmann Foundation, the Ruth och Nils-Erik Stenbäck Foundation and the Magnus Ehrnrooth Foundation (C.G.G., M.G., and F.J.).
Authorship
Contribution: F.J., E.A.B., M.G., S.M., F.A, L.S.H., and L.M.U. performed the experiments; F.J. supervised the students; and M.G. and C.G.G. planned the experiments, analyzed the data, and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carl G. Gahmberg, Division of Biochemistry and Biotechnology, Faculty of Biological and Environmental Sciences, Viikinkaari 9 C (P.O. Box 56), 00014 University of Helsinki, Helsinki, Finland; e-mail: carl.gahmberg@helsinki.fi.
References
Author notes
M.G. and F.J. contributed equally to this study.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal