Key Points
Autologous hematopoietic cell transplantation is safe and effective in patients with HIV-related lymphoma who meet standard transplant criteria.
Patients with HIV-related lymphomas should not be precluded from participating in AHCT clinical trials.
Abstract
Autologous hematopoietic cell transplant (AHCT) for HIV-infected patients is largely limited to centers with HIV-specific expertise. The Blood and Marrow Transplant Clinical Trials Network 0803/AIDS Malignancy Consortium 071 trial is a multicenter phase 2 study of AHCT for patients with HIV-related lymphoma (HRL). Eligible patients had chemotherapy-sensitive relapsed/persistent HRL, were >15 years of age, and had treatable HIV infection. Patients were prepared using carmustine, etoposide, cytarabine, and melphalan and received consistent management of peritransplant antiretroviral treatment. The primary endpoint was 1-year overall survival. Forty-three patients were enrolled; 40 underwent AHCT. Pretransplant HIV viral load was undetectable (<50 copies/mL) in 32 patients (80%); the median CD4 count was 249/μL (range, 39-797). At a median follow-up of 24.8 months, 1-year and 2-year overall survival probabilities were 87.3% (95% confidence interval [CI], 72.1-94.5) and 82% (95% CI, 65.9-91), respectively. The probability of 2-year progression-free survival was 79.8% (95% CI, 63.7-89.4). One-year transplant-related mortality was 5.2%. Median time to neutrophil and platelet recovery was 11 days and 18 days, respectively. Nine patients experienced a total of 13 unexpected grade 3-5 adverse events posttransplant (10 grade 3 and 3 grade 4 events). Twenty-two patients had at least 1 infectious episode posttransplant. At 1 year post-AHCT, median CD4+ T-cell count was 280.3 (range, 28.8-1148.0); 82.6% had an undetectable HIV viral load. Trial patients were compared with 151 matched Center for International Bone Marrow Transplant Research controls. Outcomes between HIV-infected patients and controls were not statistically significantly different. HRL patients should be considered candidates for AHCT if they meet standard transplant criteria. The trial was registered at www.clinicaltrials.gov as #NCT01141712.
Introduction
Infection with HIV is associated with a significantly increased risk of cancer, including Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) even when patients are treated successfully with modern antiretroviral therapy.1-4 Before the advent of effective anti-HIV therapy, HIV-infected patients were not candidates for standard, combination chemotherapeutic regimens because of the significant risk of opportunistic infection and poor malignancy response rates.5 Since 1996, however, the availability of combination anti-retroviral therapy (cART) has altered our previous understanding of how best to manage patients with HIV-related malignancies.6,7 Treatment with cART results in profound suppression of HIV viremia, marked numerical recovery of CD4+ T cells, and decreased risk of opportunistic infections.8-13 Despite this dramatic immunological reconstitution, patients in the post-cART era still have a 25-fold increased incidence of NHL and their risk of HL has not decreased from that in pre-cART era.2,14-16
Patients with HIV-related lymphomas (HRLs) currently receive standard therapeutic regimens and achieve outcomes comparable to those of non–HIV-infected individuals.7,14,17-24 The role of autologous hematopoietic cell transplantation (AHCT) in the care of patients with HRL, however, remains unclear. AHCT is the standard of care for patients with chemotherapy-sensitive relapsed and persistent lymphoma.24,25 HIV infection, however, has long been considered an a priori contraindication to transplantation. Commencing in the late 1990s, groups in both Europe and the United States piloted the use of AHCT in the care of patients with high-risk, relapsed, and persistent HRL.26,27 These initial experiences were encouraging and provoked other investigators to study the use of AHCT for patients with relapsed and persistent HRL.28-33 These data, however, are limited in that many of these studies were retrospective in nature, AHCT was largely limited to centers with significant HIV-related expertise, they included a wide variety of transplant preparative regimens, and management of cART in the peri-HCT period was variable. This has prevented full acceptance of AHCT as the standard of care for patients with relapsed and persistent HRL. AHCT for patients with HRL remains largely limited to centers with significant HIV-specific expertise, and HIV infection remains an exclusion criterion for most transplant-related clinical trials.
Here we report the results of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0803/AIDS Malignancy Consortium (AMC) 071 study. This was a phase 2 clinical trial designed to prospectively evaluate the safety and effectiveness of AHCT for patients with HRL in a multicenter setting.
Patients and methods
Study design
Between April 2010 and March 2013, a prospective phase 2 multicenter trial was conducted by the BMT CTN in collaboration with the AMC. Patients who met trial eligibility criteria underwent AHCT following a preparative regimen that consisted of modified carmustine, etoposide, cytarabine, and melphalan (BEAM) treatment: carmustine 300 mg/m2 (day – 6), etoposide 100 mg/m2 twice daily (days −5 to −2), cytarabine 100 mg/m2 twice daily (days −5 to −2), and melphalan 140 mg/m2 (day −1). Patients underwent AHCT on day 0 and received supportive care per the respective institutional standard through the period of post-AHCT recovery. The primary end point of the trial was overall survival (OS) after AHCT for patients with chemotherapy-sensitive, aggressive B-cell lymphoma or HL in HIV-infected patients. Secondary end points included progression-free survival (PFS), time to progression, complete remission (CR), and overall response (CR plus partial response [PR]) rates at day +100, lymphoma disease-free survival, time to hematopoietic recovery, hematological function at day +100, toxicities, incidence of infections, treatment-related mortality, and immunological reconstitution.
The protocol was approved by the protocol review committee of the National Heart, Lung, and Blood Institute and local institutional review boards. All patients signed the approved informed consent in accordance with the Declaration of Helsinki.
Eligibility criteria.
Patients enrolled onto the trial had documented evidence of HIV infection, were a minimum of 15 years of age with a Karnofsky performance status >70%, and persistent or recurrent diffuse large B-cell, immunoblastic, plasmablastic, Burkitt or Burkitt-like NHL or classical Hodgkin lymphoma. Patients must have received no more than 3 prior treatment regimens and 2 or fewer salvage regimens. Monoclonal antibody therapy and local radiation were considered prior therapies. Patients had to have chemosensitive disease as demonstrated by response to induction or salvage chemotherapy. Adequate organ function for AHCT was required, as was <10% marrow involvement after patients’ most recent salvage therapy.
Patients could not have had prior AHCT or allogeneic hematopoietic cell transplantation. Patients had to initiate their conditioning regimen within 3 months of mobilization or bone marrow harvest. Hematopoietic progenitor cell (HPC) mobilization was per institutional standards. Patients were required to achieve adequate HPC mobilization (>1.5 × 106 CD34+ cells/kg) to be eligible for the protocol. Patients could not have HIV infection resistant to pharmacological therapy or opportunistic infection unresponsive to treatment.
Treatment.
Patients meeting study eligibility criteria were treated with the BEAM transplant preparative regimen followed by AHCT. The day of AHCT was designated day 0. cART was held from the time of initiation of the BEAM regimen and resumed at least 7 days after completion of the preparative regimen or following recovery from transplant-related gastrointestinal toxicities. Because of the long half-life of efavirenz and risk of resistance during even single therapeutic interruptions, efavirenz was held at least 2 weeks before initiation of the preparative regimen and an alternative agent substituted during this period. Use of zidovudine was prohibited following AHCT because of its myelosuppressive effects. Patients received growth factor, transfusion, and antimicrobial supportive care as per the respective institutional standards.
End points.
The primary end point for the trial was 1-year OS, including death from any cause, with the time interval defined from day 0 AHCT to death. Time to progression was defined as the interval from day 0 AHCT to the time of relapse or progression, or to receipt of antilymphoma therapy other than consolidative localized radiotherapy to sites of prior bulky disease. PFS was defined as the interval from day 0 AHCT to death, disease relapse, or progression. Transplant-related mortality (TRM) was defined as death occurring in a patient from causes other than relapse/progression. Patients underwent disease status assessments before AHCT, at day +100, and at 1 year post-AHCT. Lymphoma responses were assessed using the Cheson criteria for determination of CR and CR plus PR rates at day +100.34
Patients underwent post-AHCT laboratory testing twice a week from day 0 through neutrophil recovery and then at 8 weeks, day +100, +6 months, and at 1 and 2 years. Time to hematopoietic recovery was defined as the first of 2 consecutive days of >500 neutrophils/μL following nadir and time to platelet count >20 000/μL for the first of 2 consecutive laboratory tests with no platelet transfusions during the prior 7 days. Post-AHCT hematopoietic recovery was defined as the patient achieving an absolute neutrophil count >1500/μL, untransfused hemoglobin >10 g/dL, and untransfused platelets >100 000/μL. Post-AHCT toxicities were graded using the Common Terminology Criteria for Adverse Events, version 3. Grade 3 and higher toxicities were collected. Microbiologically documented infections were collected by site of disease, date of onset, severity, and resolution from day 0 through 1 year post-AHCT. Recovery of immunoglobulin (Ig) levels was assessed on peripheral blood by measuring quantitative immunoglobulin levels (IgM, IgG, and IgA) on days +60, +180, and +365 post-AHCT. Reconstitution of CD4+ T cells was assessed on peripheral blood by flow cytometry on days +60, +180, and +365 post-AHCT. The HIV viral load was also assessed at each of these time points.
Statistical analysis
The date for cutoff for data analysis was October 26, 2015. End point analysis was limited to the 40 patients undergoing AHCT. Patient, disease, and treatment characteristics were summarized by median and range for continuous variables and by frequency and percentage for categorical variables. Probabilities of OS and PFS were calculated using the Kaplan-Meier estimator.35 Cumulative incidences of relapse or progression, TRM, and neutrophil and platelet recovery were calculated using the cumulative incidence function to accommodate competing risks.35 For TRM, relapse or progression was the competing event; for relapse or progression, TRM was the competing event. For neutrophil and platelet recovery, death without the event was considered the competing risk. In all analyses, patients without an event were censored at last follow-up.
Outcomes of transplant recipients from this trial (cases) were compared with outcomes of a cohort of non-HIV transplant recipients selected from the Center for International Bone Marrow Transplant Research (CIBMTR) database (controls). Up to 4 matched controls were selected for each case based on the following criteria: age (within 10 years), Karnofsky performance score, disease, and disease stage. The marginal Cox proportional hazards model of Lee, Wei, and Amato was used to compare the risk of overall mortality, treatment failure, relapse, or progression, TRM, and neutrophil and platelet recovery between the cases and the controls.36 The marginal Cox model accounts for potential correlation of outcomes between cases and matched controls.36
The immunological reconstitution data were summarized by median and range. Undetectable HIV infection was defined as a viral load of <50 copies/mL. All analyses were done using SAS software (version 9.3, Cary, NC).
Results
Patients
Forty-three HIV-infected patients with relapsed/persistent NHL or classical HL were enrolled onto the clinical trial. Median follow-up for the study population was 24.8 months (range, 2.8 -27.7 months). Three patients did not undergo transplantation because of disease progression before conditioning; they are not included in the study analysis. There were 6 protocol violations, including 1 patient who received a cytarabine dose of 200 mg/m2 rather than 100 mg/m2, 1 who refused to stop cART during the conditioning regimen, and 1 who received a lower than calculated dose of etoposide.
Forty patients underwent AHCT at 16 different transplant centers. Thirty-five patients were male. Median patient age was 46.9 years (range, 22.5-62.2). All patients received <3 prior treatment regimens. Patient characteristics are detailed in Table 1.
Demographic and clinical characteristics for patients undergoing autologous hematopoietic cell transplantation
| Characteristics . | N (%) . |
|---|---|
| Total transplanted | N = 40 (100.0%) |
| Sex | |
| Female | 5 (12.5%) |
| Male | 35 (87.5%) |
| Ethnicity | |
| Hispanic or Latino | 9 (22.5%) |
| Not Hispanic or Latino | 29 (72.5%) |
| Unknown | 1 (2.5%) |
| Not answered | 1 (2.5%) |
| Race | |
| Black or African American | 13 (32.5%) |
| White | 24 (60.0%) |
| Unknown | 3 (7.5%) |
| Age, y | |
| Median (range) | 46.9 (22.5-62.2) |
| Performance status | |
| 100 | 11 (27.5%) |
| 90 | 20 (50.0%) |
| 80 | 7 (17.5%) |
| 70 | 2 (5.0%) |
| Patient diagnosis | |
| Hodgkin lymphoma | 15 (37.5%) |
| Non-Hodgkin lymphoma | 25 (62.5%) |
| Diffuse large B-cell lymphoma | 16 |
| Plasmablastic lymphoma | 2 |
| Burkitt or Burkitt-like lymphoma | 7 |
| Disease stage at AHCT | |
| Complete remission | 30 (75.0%) |
| Partial remission | 9 (22.5%) |
| Relapsed or progressive disease | 1 (2.5%) |
| Characteristics . | N (%) . |
|---|---|
| Total transplanted | N = 40 (100.0%) |
| Sex | |
| Female | 5 (12.5%) |
| Male | 35 (87.5%) |
| Ethnicity | |
| Hispanic or Latino | 9 (22.5%) |
| Not Hispanic or Latino | 29 (72.5%) |
| Unknown | 1 (2.5%) |
| Not answered | 1 (2.5%) |
| Race | |
| Black or African American | 13 (32.5%) |
| White | 24 (60.0%) |
| Unknown | 3 (7.5%) |
| Age, y | |
| Median (range) | 46.9 (22.5-62.2) |
| Performance status | |
| 100 | 11 (27.5%) |
| 90 | 20 (50.0%) |
| 80 | 7 (17.5%) |
| 70 | 2 (5.0%) |
| Patient diagnosis | |
| Hodgkin lymphoma | 15 (37.5%) |
| Non-Hodgkin lymphoma | 25 (62.5%) |
| Diffuse large B-cell lymphoma | 16 |
| Plasmablastic lymphoma | 2 |
| Burkitt or Burkitt-like lymphoma | 7 |
| Disease stage at AHCT | |
| Complete remission | 30 (75.0%) |
| Partial remission | 9 (22.5%) |
| Relapsed or progressive disease | 1 (2.5%) |
All patients received peripheral blood stem cell grafts. The median transplanted HPC dose was 3.9 × 106 CD34+ cells/kg (range, 1.6-11.0). No patients failed to mobilize HPCs. The median number of apheresis collections was 2 (range, 1-5).
The pretransplant HIV viral load was undetectable in 32 patients (80%). In patients with a detectable viral load, the median viral load was 80 copies/mL (range, 50-17 455). The median pretransplant CD4+ T-cell count was 249.0 CD4+/μL (range, 39-797). Thirty-seven of 40 patients had planned interruptions in their cART during AHCT; data on cART interruption are available for 32 patients. The median duration of cART interruption was 15.5 days (range, 11-40 days).
OS, PFS, and post-AHCT disease response.
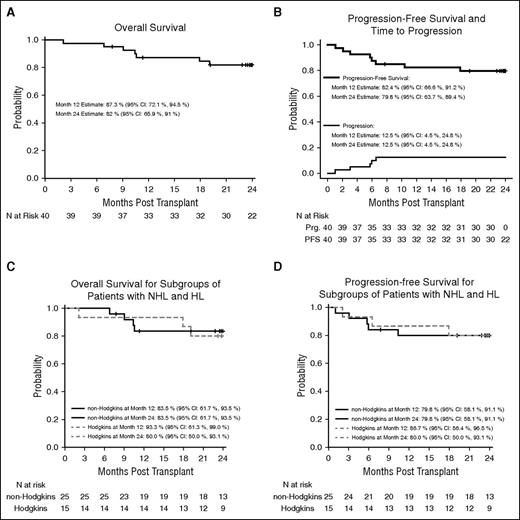
With a median follow-up of 24.8 months (range, 2.8-27.7 months), 7 patients died; 5 of whom died within 1 year of transplant. The 1-year OS probability was 87.3% (95% confidence interval [CI], 72.1-94.5). The OS probability at 2 years was 82% (95% CI, 65.9-91) (Figure 1A).
OS and PFS. OS (A) and PFS (B) for 40 patients undergoing autologous hematopoietic cell transplantation. OS (C) and PFS (D) for the subgroups of patients with non-Hodgkin and Hodgkin lymphoma, respectively. Prg., progression.
OS and PFS. OS (A) and PFS (B) for 40 patients undergoing autologous hematopoietic cell transplantation. OS (C) and PFS (D) for the subgroups of patients with non-Hodgkin and Hodgkin lymphoma, respectively. Prg., progression.
At the time of transplant, 30 patients (75%) were in CR, 9 (22.5%) were in PR, and 1 patient with disseminated HL (2.5%) had progression at a single nodal site immediately before transplant after achieving a CR to salvage therapy. At day +100, 36 patients (92.3%) were in CR, 1 (2.6%) was in PR, 2 (5.1%) had relapsed/progressive disease, and 1 who died on day +64 was unevaluable. Six patients experienced relapse/progression posttransplant; 4 of whom have died. The probability of 2-year PFS for the study group was 79.8% (95% CI, 63.7-89.4). The cumulative incidence of relapse/progression at 2 years was 12.5% (95% CI, 4.5-24.8) (Figure 1B).
The 2-year probabilities for OS and PFS for the subgroups of patient with either NHL or HL were comparable (Figure 1C-D).
Posttransplant mortality
Five patients experienced relapse/progression posttransplant within 1 year; 4 of them died (3 of them within the first year, 1 died after 1 year), 1 died of organ failure, and 1 from fungal infection. The estimated risk of 1-year TRM was 5.2% (95% CI, 0.9-15.2).Two patients died after 1 year: 1 from recurrent/progressive disease and 1 from organ failure (Table 2).
Causes of patient death with 1 year of autologous hematopoietic cell transplantation
| Primary cause of death . | N . |
|---|---|
| Lymphoma recurrence/persistence | 3 |
| Infection (fungal) | 1 |
| Organ failure (cardiac arrest) | 1 |
| Total | 5 |
| Primary cause of death . | N . |
|---|---|
| Lymphoma recurrence/persistence | 3 |
| Infection (fungal) | 1 |
| Organ failure (cardiac arrest) | 1 |
| Total | 5 |
Note: 2 patients who died 1 y after transplant were not included in the table: 1 died from recurrence/persistence and 1 died from cardiac failure.
Time to hematopoietic recovery/hematologic function post-AHCT
The median time to posttransplant neutrophil recovery was 11 days. The cumulative incidence of neutrophil recovery by day +28 was 97.5% (95% CI, 77.7-99.7). The median time to platelet recovery was 18 days. Two patients’ platelet counts did not nadir below 20 000/μL. One patient died before achieving platelet recovery. The cumulative incidence of platelet recovery by day +100 was 92.5% (95% CI, 75.9-97.8). Two patients achieved platelet recovery after day +100.
At day +100 posttransplant, 11 of 38 evaluable patients (28.9%) achieved recovery of hematological function. At 1 year, 23 of 31 evaluable patients (74.2%) achieved recovery of hematological function.
Infections post-AHCT
Twenty-two of 40 patients (55%) experienced at least 1 infectious event within 1 year after transplant, including 11 patients with severe infection. A total of 57 infection events occurred post-AHCT, including 25 from bacteria, 22 from viruses, 6 from fungal organisms, 2 from protozoa, and 2 caused by other organisms. None of the patients developed Pneumocystis jiroveci pneumonia post-AHCT.
Organ toxicities post-AHCT
Nine patients experienced a total of 13 unexpected grade 3-5 adverse events posttransplant. There were 10 grade 3 (severe) events and 3 grade 4 (life-threatening) events. These events included infection/sepsis (including 1 patient with disseminated fungemia) (5), venous thromboembolism (2), esophageal candidiasis (1), enteritis (1), hyperglycemia (1), hypernatremia (1), acute appendicitis (1), and acute coronary syndrome (1).
Sixteen patients required readmission following their initial transplant admission. There were a total of 34 readmissions. The most common causes for readmission were infection18 and fever.6 Two patients were readmitted for management of relapsed/progressive disease. Three patients required 4 or more readmissions. Twelve of the readmissions occurred more than 1 year post transplant.
Immunological reconstitution post-AHCT
Immunological reconstitution studies were performed assessing both recovery of immunoglobulin levels and CD4+ T cells through 1-year post-AHCT. By 1-year post-AHCT, median IgG, IgA, and IgM levels had recovered to 1090 mg/dL (range, 270.0-2331.0), 123 mg/dL (range, 6-534), and 48.5 mg/dL (range, 24-254), respectively.
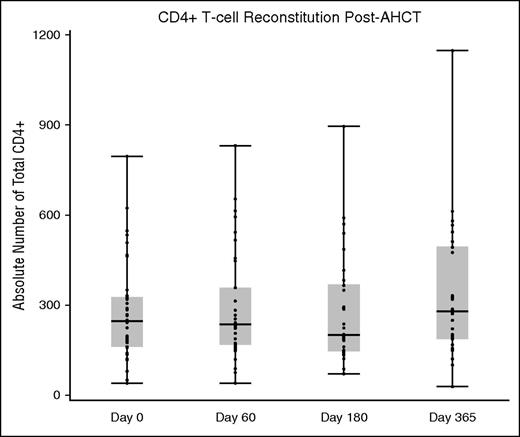
Before AHCT, the median CD4+ T-cell count was 249.0/μL (range, 39-797). At the day +60, +180, and +365 analysis windows, the median CD4+ T-cell counts were 234.9 (range, 39.9-832.5), 200.6 (range, 72.6-897.7), and 280.3 (range, 28.8-1148.0), respectively (Figure 2).
CD4+ T-cell reconstitution before and after AHCT. CD4+ T cells are expressed as an absolute number.
CD4+ T-cell reconstitution before and after AHCT. CD4+ T cells are expressed as an absolute number.
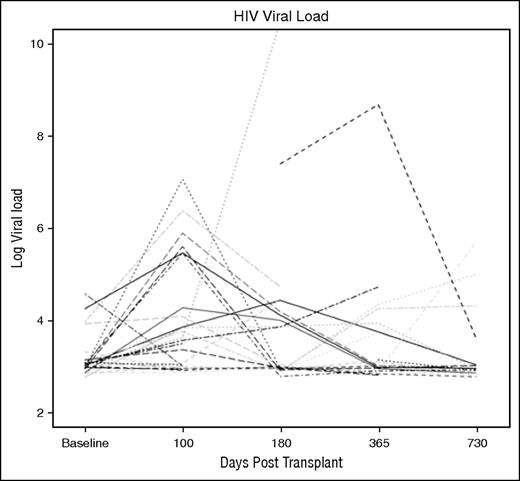
HIV viral load assessments were performed before AHCT and at 100, 180, 365 days, and 730 days posttransplant (Figure 3). The results of these analyses are shown in Table 3 and Figure 3. Although some patients had a transient rise in HIV viral load post-AHCT, by day 365, 82.6% of the patients had an undetectable viral load.
Spaghetti diagram of HIV viral load for undergoing AHCT for HRL. The HIV viral load is expressed as a log viral load. The detection limits of the assay were 50 copies HIV/mL.
Spaghetti diagram of HIV viral load for undergoing AHCT for HRL. The HIV viral load is expressed as a log viral load. The detection limits of the assay were 50 copies HIV/mL.
HIV viral load following AHCT
| Time . | N undetectable . | N detectable . | Median viral load in patients with detectable HIV . | Range . |
|---|---|---|---|---|
| Baseline | 32 | 8 | 80 | 50-17 455 |
| Day 100 | 19 | 8 | 298 | 58-1 100 |
| Day 180 | 20 | 9 | 84 | 55-36 815 |
| Day 365 | 19 | 4 | 97 | 75-5 097 |
| Day 730 | 21 | 5 | 130 | 75-351 |
| Time . | N undetectable . | N detectable . | Median viral load in patients with detectable HIV . | Range . |
|---|---|---|---|---|
| Baseline | 32 | 8 | 80 | 50-17 455 |
| Day 100 | 19 | 8 | 298 | 58-1 100 |
| Day 180 | 20 | 9 | 84 | 55-36 815 |
| Day 365 | 19 | 4 | 97 | 75-5 097 |
| Day 730 | 21 | 5 | 130 | 75-351 |
The table indicates the number of patients assessed at each time point, the number of patients with an undetectable (<50 copies/mL) viral load, and the median and range for the viral load of those patients with detectable HIV.
Comparison with CIBMTR comparison data set
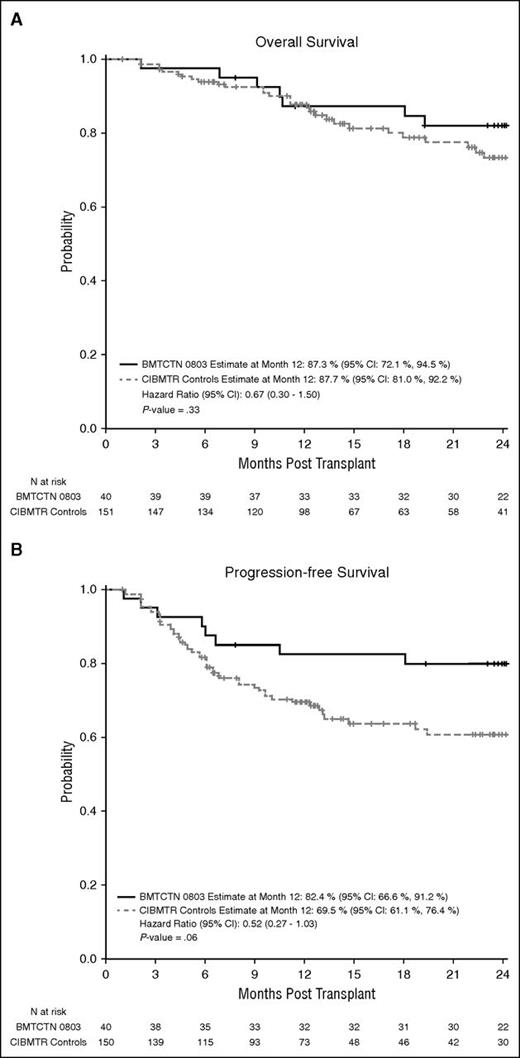
OS and PFS for the study group was compared with a control group of patients identified through the CIBMTR database. Controls were matched for age, performance status, primary disease, and disease status at AHCT. A total of 151 matched controls were identified for the 40 cases: 34 cases (85%) had 4 matched controls, 4 (10%) had 3 matched controls, 1 (2.5%) had 2 matched controls, and 1 (2.5%) had 1 matched control. The age differences between a case and its matched controls were small, with 127 (84%) of matched controls were within 1 year of the case age, 13 (9%) between 1 to 2 years, and the remaining 11 (7%) between 5 and 8 years of the case age. The probability of 1-year OS for the control group was 87.7% (95% CI, 80.9-92.2). The probability of PFS for the control group was 69.5% (95% CI, 61.1-76.4).
Results of the comparison demonstrate that posttransplant outcomes of HIV-infected study patients were not significantly different from outcomes of CIBMTR controls. The hazard ratio for overall mortality in the HIV-infected patient group was 0.67 (95% CI, 0.30-1.50; P = .33) (Figure 4A) compared with the controls. The hazard ratio for treatment failure in the HIV-infected patient group was 0.52 (95% CI, 0.2927-1.03; P = .06) (Figure 4B) compared with the controls. Other comparisons include lymphoma progression (HR, 0.56; 95% CI, 0.29-1.11; P = .0954), treatment-related mortality (HR, 1.05; 95% CI, 0.25-4.47; P = .9428), time to neutrophil recovery (HR, 0.94; 95% CI, 0.74-1.19; P = .61), and time to platelet recovery (HR, 0.88; 95% CI, 0.60-1.28; P = .49).
OS and PFS for HIV-infected and noninfected patients. Comparison of OS (A) and PFS (B) between HIV-infected patients treated under BMT CTN 0803/AMC 071 vs 151 matched controls from the CIBMTR data registry.
OS and PFS for HIV-infected and noninfected patients. Comparison of OS (A) and PFS (B) between HIV-infected patients treated under BMT CTN 0803/AMC 071 vs 151 matched controls from the CIBMTR data registry.
Discussion
The availability of effective anti-HIV therapy has altered our previous understanding of the best management for patients with HRL and has allowed these patients to benefit from standard chemotherapeutic regimens.7,14,17-23 Patients with HRL now have treatment outcomes comparable to those of HIV uninfected patients. For NHL, regimens such as etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab and short-course etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab, including a double dosing of rituximab, produce remarkable PFS rates of 73% to 90% in patients with HIV-related NHL.14,17-19,37 Similarly, for patients with HL, use of regimens such as Adriamycin, bleomycin, vinblastine, and dacarbazine produces OS rates comparable with those of non–HIV-infected individuals.20-23
The Parma group trial and subsequent clinical studies established AHCT as the standard of care for managing patients with chemotherapy-sensitive, relapsed/persistent NHL and HL.24,25 HIV infection has historically been viewed as a contraindication to AHCT. Before the advent of cART, few studies seriously attempted to extend AHCT to HIV-infected patients. Commencing in 2000, however, groups in Europe and the United States began to investigate the role of AHCT in the management of patients with HRL and treatment-responsive HIV infection.26,29 These early efforts demonstrated the feasibility of extending AHCT to patients with chemotherapy-sensitive, high-risk, relapsed or persistent HRL.
Other groups expanded upon the success of these initial trials.33,38,39 In a multicenter prospective trial of 14 patients with high-risk or relapsed HRL, at a median follow-up of 30 months, with a variety of preparative regimens, Serrano and colleagues reported an estimated event-free survival of 65%.33 In the first US cooperative group trial, the AMC used a consistent preparative regimen (busulfan-cyclophosphamide) regimen.38 Their results in 20 transplanted patients demonstrated that lymphoma relapse or progression was the main cause of treatment failure and that HIV-associated opportunistic infections did not contribute to mortality. In a European, prospective, intent-to-treat trial of 50 patients with HRL, at a median follow-up of 44 months, the estimated OS and PSF were 74.6% and 75.9%, respectively, for the subgroup of 27 patients who underwent AHCT.39 In the retrospective, European Group for Blood and Marrow Transplantation study, 68 patients with HRL who had undergone AHCT were retrospectively identified and reviewed. The group included 18 patients with HL. The patients were treated with either BEAM or radiation-containing preparative regimens and treated at 20 different centers. The estimated 3-year OS and PFS for the group of patients were 61% and 56%, respectively, at a median follow-up of 32 months.40
In addition to demonstrating the effectiveness and feasibility of AHCT in HIV-infected patients, this trial also demonstrates the prompt reconstitution of immunoglobulin levels and CD4+ T-cell counts post-AHCT. The median CD4+ T-cell counts returned to pretransplant levels by the day +60 analysis window and were maintained through day +365. These data demonstrate that there does not appear to be any long-term loss of CD4+ T cells following AHCT and that significant, long-term worsening of T-cell immunity is not a complication of transplant.
The BMT CTN 0803/AMC 071 trial represents the largest prospective study for the use of AHCT in patients with HRL. Unlike many of the previously published studies, this trial used a consistent preparative regimen and strategy for management of peritransplant cART. An important finding of this trial is that the comparison of the trial population with a matched population of control patients from the CIBMTR data registry revealed that the outcomes for patients with HRL did not differ statistically from that of non–HIV-infected patients. This is consistent with the finding of prior case-control studies demonstrating similar outcomes for AHCT in HIV-infected vs HIV-uninfected patients.30,41 Together, these trials validate the concept that controlled HIV infection should not preclude consideration of AHCT for patients who otherwise meet standard transplant eligibility criteria. The non–HIV-related inclusion and exclusion criteria for this trial were adapted from those used in the previous BMT CTN 0401 trial, a study that was designed for a non–HIV-infected population.
Some important HIV-related clinical issues are germane to the care of patients with HRL that merit consideration before AHCT. Given the pharmacology of anti-HIV drugs, it is important to review the cART regimen before transplantation for possible drug–drug interactions with either the preparative regimen or post-AHCT supportive care.23 Several anti-HIV medications may have significant impact upon drug metabolism. Ritonavir-boosted protease inhibitors may be particularly problematic because of strong CYP3A4 inducers and should be discontinued before initiating the preparative regimen.23 In addition, given the significant myelosuppressive effects of zidovudine, this agent should not be used in the peri-AHCT period. Also, as noted in this trial, efavirenz has a long half-life and discontinuation of efavirenz-based regimens before AHCT reduces the risk of patients developing antiretroviral resistance; hence, a substitution was made at least 2 weeks before AHCT. Finally, decisions related to continuation or noncontinuation of cART therapy throughout the peritransplant period should be made carefully to avoid repetitive starts and stops of the cART regimen that might increase the risk of HIV resistance. Although there is no optimal cART regimen for use during treatment of HRL, integrase inhibitors appear to be important agents to consider in this context. In a review of 158 HIV-infected patients with cancer, including 58% with hematological malignancies who were treated at MD Anderson Cancer Center between 2001 and 2012, the authors of the study found that protease inhibitors (PI) pose a challenge in the setting of treating HIV-related malignancies because of significant drug–drug interactions. They also found that integrase inhibitors had effectiveness comparable to that of non-nucleoside reverse transcriptase inhibitors while appearing to be better tolerated than either PIs or non-nucleoside reverse transcriptase inhibitors.42 Thus integrase inhibitor-containing cART regimens should be considered in the context of AHCT for patients with HRL, whereas PI, efavirenz, and AZT-containing regimens may require modification before high-dose chemotherapy.
This trial builds on a body of previously published data to demonstrate clearly that HIV infection alone should not be considered a contraindication to AHCT in patients who otherwise meet transplant inclusion criteria. AHCT should be considered the standard of care for patients with HRL, provided that the HIV infection is treatment-responsive. Patients with HRL should also be considered appropriate potential participants for future AHCT clinic trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this trial; the physicians, nurses, and clinical staff who supported these patients throughout their care; the support of the Blood and Marrow Transplant Clinical Trials Network in the development, administration, and completion of the clinical trip; and the AIDS Malignancy Consortium (AMC) for its partnership in this trial. The authors also thank Mateusz Makowski from The Emmes Corporation for his assistance in trial data review and analysis.
This work is supported by grants from the National Institutes of Health National Cancer Institute (NCI; grant HHSN21200622012C-009) and the AMC through NCI (grant U01CA121947). The Blood and Marrow Transplant Clinical Trials Network infrastructure is supported in part by the National Heart, Lung, and Blood Institute and NCI (grant U10HL069294).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.C.A., J.L.R., Y.W., R.F.L., G.A., E.A., S.D., R.B., L.K., A.N., U.P., J.T., M.M.H., A.M., A.L., A.K., S.J.F., W.H.N., and R.A. participated in the trial development; J.C.A., R.A., J.L.R., Y.W., W.H.N., A.L., and S.J.F. participating in the writing and editing of the manuscript; S.D., R.B., and G.L. performed assessments of T-cell immunological posttransplant T-cell reconstitution; G.A., E.A., S.D., L.K., A.N., U.P., J.H., L.E.M., A.K., and R.A. enrolled patients on the trial; J.L.R. and Y.W. performed all statistical analysis; and J.T. and A.M. administered the trial.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph C. Alvarnas, City of Hope National Medical Center, 1500 E Duarte Rd, MOB3001, Duarte, CA 91010; e-mail: jalvarnas@coh.org.