To the editor:
Acute myeloid leukemia (AML) is a fast-growing and lethal malignancy of the blood and bone marrow, with a 5-year mortality rate of 75%.1,2 Between disease onset and chemotherapy treatment, patients often experience adverse symptoms, such as bleeding and infection, which can lead to worse outcomes.3,4 The prognosis of AML patients aged 65 years and older is especially poor, as many are physically unable to tolerate conventional treatment and have comorbidities that increase the risk of adverse side effects during treatment.5-7 As is the case in complex and invasive procedures,8-12 effective management of AML in older patients may require health professionals with specialized skills and resources, which are more likely to be present in hospitals treating these patients more frequently.13,14 Therefore, the purpose of this study was to better understand the volume-outcome relationship in AML.
We used 2013 Medicare inpatient fee-for-service (FFS) claims for beneficiaries with a primary or secondary diagnosis of nonrelapsed AML (International Classification of Diseases, 9th Revision [ICD-9] codes 205.00 and 205.20). We excluded beneficiaries if they were under the age of 65 years, transferred from another hospital, or not enrolled in Medicare part A/B for the entirety of 2013. After exclusions, 7568 beneficiaries from 1895 hospitals remained for analysis. This study was deemed human subjects exempt by the University of Tennessee Health Science Center Institutional Review Board.
Hospitals were rank-ordered by increasing annual case volume and then placed in 1 of 5 volume categories of equal size: very low, low, moderate, high, and very high. Very-low, low-, and moderate-volume categories had similar case volumes and were pooled to represent low volume more generally. Low-volume (90.0%), high-volume (7.3%), and very-high-volume (2.7%) hospitals treated a median of 2, 10, and 25 cases annually, respectively (Table 1). As hospital volume increased, patients were more likely to be younger, nonwhite, and to receive chemotherapy during their first hospitalization, but they were less comorbid and less likely to be emergent admissions (Table 1).
Characteristics and mortality rates for AML patients aged 65 years and older by hospital volume category (n = 7568)
| Variable . | Total . | Hospital volume category . | P* . | ||
|---|---|---|---|---|---|
| Low (<8 cases) . | High (8-15 cases) . | Very high (16-129 cases) . | |||
| No. of hospitals, % | 1895 (100) | 1706 (90.0) | 138 (7.3) | 51 (2.7) | |
| Median annual cases (IQR) | 2 (1-5) | 2 (1-4) | 10 (9-12) | 25 (19-34) | |
| No. of patients, % | 7568 (100) | 4450 (60.0) | 1499 (19.8) | 1529 (20.2) | |
| Patient characteristics, % | |||||
| Male | 56.7 | 55.9 | 57.0 | 58.7 | .16 |
| Race | .04 | ||||
| White | 88.8 | 88.6 | 90.6 | 87.7 | |
| Black | 6.6 | 6.5 | 6.2 | 7.4 | |
| Other | 4.6 | 4.9 | 3.1 | 5.0 | |
| Age category, y | <.001 | ||||
| 65-69 | 21.5 | 16.5 | 25.4 | 31.5 | |
| 70-79 | 42.7 | 40.3 | 43.6 | 47.3 | |
| 80+ | 36.6 | 43.1 | 31.0 | 21.3 | |
| Index admission type | <.001 | ||||
| Emergent | 58.7 | 65.5 | 52.8 | 44.2 | |
| Nonemergent | 41.3 | 34.5 | 47.2 | 55.8 | |
| Received chemotherapy at index admission | 26.1 | 14.5 | 36.4 | 50.3 | <.001 |
| Mean Charlson Comorbidity Index (SD) | 3.3 (1.5) | 3.4 (1.5) | 3.2 (1.4) | 3.0 (1.4) | <.001 |
| ≤2 | 35.5 | 34.7 | 41.8 | 49.3 | <.001 |
| 3-4 | 43.5 | 45.5 | 42.6 | 38.1 | |
| 5+ | 17.5 | 19.8 | 15.6 | 12.6 | |
| 30-day mortality | |||||
| Unadjusted | 36.0 | 42.1 | 33.8 | 19.8 | <.001 |
| Risk-adjusted | 31.4 | 32.0 | 32.1 | 28.1 | <.001 |
| 1-year mortality | |||||
| Unadjusted | 72.7 | 78.2 | 69.4 | 59.6 | <.001 |
| Risk-adjusted | 71.0 | 71.4 | 71.2 | 69.3 | <.001 |
| Variable . | Total . | Hospital volume category . | P* . | ||
|---|---|---|---|---|---|
| Low (<8 cases) . | High (8-15 cases) . | Very high (16-129 cases) . | |||
| No. of hospitals, % | 1895 (100) | 1706 (90.0) | 138 (7.3) | 51 (2.7) | |
| Median annual cases (IQR) | 2 (1-5) | 2 (1-4) | 10 (9-12) | 25 (19-34) | |
| No. of patients, % | 7568 (100) | 4450 (60.0) | 1499 (19.8) | 1529 (20.2) | |
| Patient characteristics, % | |||||
| Male | 56.7 | 55.9 | 57.0 | 58.7 | .16 |
| Race | .04 | ||||
| White | 88.8 | 88.6 | 90.6 | 87.7 | |
| Black | 6.6 | 6.5 | 6.2 | 7.4 | |
| Other | 4.6 | 4.9 | 3.1 | 5.0 | |
| Age category, y | <.001 | ||||
| 65-69 | 21.5 | 16.5 | 25.4 | 31.5 | |
| 70-79 | 42.7 | 40.3 | 43.6 | 47.3 | |
| 80+ | 36.6 | 43.1 | 31.0 | 21.3 | |
| Index admission type | <.001 | ||||
| Emergent | 58.7 | 65.5 | 52.8 | 44.2 | |
| Nonemergent | 41.3 | 34.5 | 47.2 | 55.8 | |
| Received chemotherapy at index admission | 26.1 | 14.5 | 36.4 | 50.3 | <.001 |
| Mean Charlson Comorbidity Index (SD) | 3.3 (1.5) | 3.4 (1.5) | 3.2 (1.4) | 3.0 (1.4) | <.001 |
| ≤2 | 35.5 | 34.7 | 41.8 | 49.3 | <.001 |
| 3-4 | 43.5 | 45.5 | 42.6 | 38.1 | |
| 5+ | 17.5 | 19.8 | 15.6 | 12.6 | |
| 30-day mortality | |||||
| Unadjusted | 36.0 | 42.1 | 33.8 | 19.8 | <.001 |
| Risk-adjusted | 31.4 | 32.0 | 32.1 | 28.1 | <.001 |
| 1-year mortality | |||||
| Unadjusted | 72.7 | 78.2 | 69.4 | 59.6 | <.001 |
| Risk-adjusted | 71.0 | 71.4 | 71.2 | 69.3 | <.001 |
IQR, interquartile range; SD, standard deviation.
P values based on χ2 tests and Student t tests for categorical and continuous variables, respectively.
The primary study outcomes were 30-day or 1-year mortality based on admission date of the first hospitalization where AML appeared as a primary or secondary diagnosis. We used hierarchical logistic regression models to estimate risk-adjusted 30-day and 1-year mortality rates. Models adjusted for sex, race (white, black, or other), age (65-69, 70-79, or 80+ years), emergent vs nonemergent hospital admission, receipt of chemotherapy at first hospitalization, and Charlson Comorbidity Index category (≤2, 3-4, or 5+). Odds ratios (ORs) and 95% confidence intervals (CIs) estimated the effect of high and very-high hospital volume on mortality, compared with low-volume hospitals.
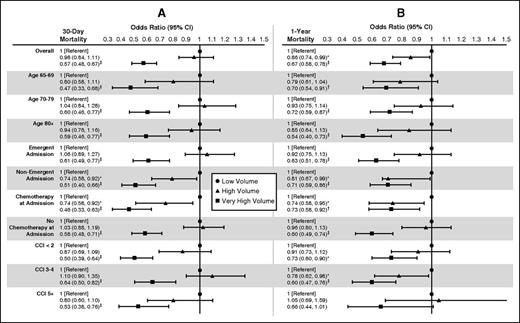
We found evidence of a volume-outcome relationship in Medicare beneficiaries aged 65 years and older with AML. Risk-adjusted 30-day mortality rates for low-, high-, and very-high-volume hospitals were 32.0%, 32.1%, and 28.1%, respectively, and rose to 71.3%, 71.3%, and 68.6% at 1 year (Table 1). Compared with low-volume hospitals, patients treated in very-high-volume hospitals had significantly reduced likelihood of mortality at 30 days (OR = 0.57; 95% CI, 0.48, 0.67; P < .001) and 1 year (OR = 0.67; 95% CI, 0.58, 0.78; P < .001) (Figure 1). This relationship was consistent across many clinically relevant subgroups, including age category, emergent admission status, receipt of chemotherapy at admission, and comorbidity status (Figure 1). Patients treated in high-volume hospitals had significantly lower mortality compared with low-volume hospitals only at 1 year (OR = 0.86; 95% CI, 0.74, 0.99; P < .05). When treated at high-volume hospitals, only nonemergent admissions and patients who received chemotherapy at admission had lower odds of mortality at 30 days and 1 year, compared with low-volume hospitals (Figure 1). We also ran our 1-year mortality analysis on patients who survived to 30 days; we found that the likelihood of mortality was still reduced at 1 year when treated at very-high-volume hospitals, although the effect had attenuated (OR = 0.80; 95% CI, 0.69-0.93; P = .004). A previous study using the Nationwide Inpatient Sample found that in-hospital mortality for AML was significantly lower in high-volume hospitals.14 Our study extends these findings by capturing patient outcomes following hospital discharge in all beneficiaries over 65 years of age, as opposed to a national sample.
Adjusted ORs. Adjusted ORs comparing (A) 30-day and (B) 1-year mortality in very-high- and high-volume hospitals to low-volume hospitals for the overall sample and stratified by selected clinical factors (*P < .05, †P < .01, ‡P < .001). CCI, Charlson Comorbidity Index.
Adjusted ORs. Adjusted ORs comparing (A) 30-day and (B) 1-year mortality in very-high- and high-volume hospitals to low-volume hospitals for the overall sample and stratified by selected clinical factors (*P < .05, †P < .01, ‡P < .001). CCI, Charlson Comorbidity Index.
There are several possible explanations for the association between volume and mortality in AML. With the rapid changes in agents and guidelines used in the treatment of AML,15 higher-volume hospitals may be better positioned to classify AML and administer tailored chemotherapy treatments more quickly and accurately. Higher-volume hospitals may also be better prepared for the life-threatening side effects associated with chemotherapy in older, frailer AML patients, such as bleeding, infection, and treatment resistance.16,17 Our data provide support for this hypothesis, as the effect of very-high-volume hospitals on mortality was greater within the 30-day mortality window, when induction therapy is typically administered. Higher-volume hospitals may also have access to important infrastructure and resources unavailable in low-volume hospitals. Previous studies have shown that hospital characteristics, such as National Cancer Institute designation, are predictive of improved surgical oncology outcomes.18,19 We did not adjust for hospital characteristics in this study because this would identify the independent effect of volume on mortality, over and above hospital characteristics.
The primary limitations of this study come from using Medicare FFS claims data. First, we used ICD-9 coding to identify patients with AML, as we did not have detailed diagnosis and treatment information in Medicare FFS data to augment case identification. Second, as we limited our study to beneficiaries aged 65 years and older, our results may not be generalizable to those under 65 years of age. However, our results apply to a majority of AML patients, whose average age at diagnosis is 67 years.20 Third, claims data do not contain important clinical information found in cancer registries such as chemotherapy type or intensity, functional status, or outpatient treatment. Fourth, because we only have 2013 data, we may have overestimated 30-day and 1-year mortality rates because patients identified early in 2013 as an index admission may have been diagnosed prior to 2013. Last, we were unable to identify patients with acute promyelocytic leukemia, a more treatable subtype of AML that makes up 10% to 12% of AML cases.21 However, the primary strength of this study is that we likely included the vast majority of all AML cases in this population, which is not obtainable through cancer registries such as the Surveillance, Epidemiology, and End Results registry.
Our findings provide many opportunities for future research. Research is needed to distinguish factors associated with disparities in care between high- and low-volume hospitals, such as the specificity and timing of treatments and staff responsiveness to adverse events. Research should explore the relationship between hospital infrastructure, resources, and certifications and patient outcomes, particularly those factors characteristic of very-high-volume hospitals. Research is also needed to understand how socioeconomic and community factors determine where patients seek treatment, and how those factors may influence potential referral policies. Our findings also motivate the need to explore the volume-outcome relationship in other rare malignancies. Answering these questions will help explain the observed volume-outcome relationship, and inform effective and equitable policies regarding AML patient referral.
In conclusion, we found that Medicare beneficiaries with AML have significantly reduced likelihood of mortality within 1 year of diagnosis when treated at very-high-volume hospitals, but more research is needed to explain our findings, and to understand the potential impact of referral policies.
Authorship
Contribution: M.P.T., T.M.W., E.K.K., C.N.M., and M.G.M. were all involved in the conceptual design and analysis planning of the project, interpretation of analysis findings, and critical revision of the manuscript; T.M.W., E.K.K., and M.G.M. were involved in the acquisition of the data; M.P.T. and C.N.M. performed the statistical analysis of the data; M.P.T. drafted the manuscript; and T.M.W. and M.G.M. supervised all aspects of the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael P. Thompson, Department of Preventive Medicine, University of Tennessee Health Science Center, 66 N. Pauline, Suite 633, Memphis, TN 38163; e-mail: mthompson@uthsc.edu.