Key Points
A classical TGF-β receptor pair counteracts extravasation of myeloid cells by rapidly interfering with integrin activation.
GDF-15 and TGF-β1 inhibit leukocyte integrin activation by targeting the Rap-1 GTPase exchange factor CalDAG-GEF1.
Abstract
Growth differentiation factor 15 (GDF-15) is the first cytokine known to counteract chemokine-induced activation of leukocyte integrins. We showed recently that this activity dampens neutrophil recruitment into inflamed tissue and is required for survival of myocardial infarction in mice. The receptor responsible for this GDF-15–triggered anti-inflammatory mechanism on myeloid cells is not known. Here, we identify this receptor as transforming growth factor β receptor I (TGF-βRI) (activin receptor-like kinase 5 [ALK-5]) and TGF-β receptor II (TGF-βRII). We show that interference with these receptors by small-molecule inhibitors, antibodies, or small interfering RNA, blocked the GDF-15 effect on leukocyte integrin activation. Likewise, gene inactivation of each of the 2 receptors in neutrophils isolated from conditional gene-deficient mice abolished the inhibitory effect of GDF-15 on CXCL1-induced β2-integrin activation and neutrophil diapedesis. Rapid neutrophil arrest induced by CXCL1 in vivo was inhibited by GDF-15 in an ALK-5 and TGF-βRII dependent way. As for GDF-15 gene-deficient mice, we found that extravasation of neutrophils deficient for ALK-5 or TGF-βRII was strongly increased in the interleukin-1β inflamed cremaster. The inhibitory effects of GDF-15 on neutrophil integrin activation and in vivo neutrophil arrest were also found for TGF-β1. Mechanistically, GDF-15 and TGF-β1 interfered with integrin activation by inhibiting the activation of Ras-related protein 1 (Rap-1), an effect that depended on CalDAG- guanine nucleotide exchange factor 1 (GEF1) and cell division control protein 42 homolog. We conclude that both GDF-15 and TGF-β1 counteract chemokine-induced integrin activation on neutrophils via the ALK-5/TGF-βRII heterodimer. This represents a novel, rapid anti-inflammatory activity of the 2 TGF-β receptors and of TGF-β1.
Introduction
Leukocyte extravasation is a central part of the inflammatory process and requires capturing of leukocytes to the endothelium, followed by the actual transmigration through the blood vessel wall.1,2 The selectins mediate leukocyte capturing and rolling, and the signaling by selectin ligands leads to the slowing down of the rolling movement finally resulting in chemokine-induced leukocyte arrest. Both these processes are strictly dependent on the activation of leukocyte integrins, which are αβ-heterodimers that can adapt inactive and active conformations. Such conformational activation events are essential for efficient leukocyte extravasation and are stimulated by complex, rapid signaling pathways stimulated by selectin-ligands and chemokine receptors.3,4
Growth differentiation factor 15 (GDF-15) is a distantly related member of the transforming growth factor β (TGF-β) cytokine superfamily, sharing a structural characteristic such as the 7 highly conserved cysteines that form a distinctive cysteine knot.5 GDF-15 is one of the most divergent members of this family with only 15% to 29% amino acid similarity to other members. Under quiescent, non-activated conditions, GDF-15 is weakly expressed in most tissues, and becomes induced under pathological conditions6 and stimuli such as ischemia, proinflammatory cytokines, or oxidative stress,7 suggesting a role in inflammation.
We have previously shown that GDF-15 acts as a cardioprotective cytokine. We found that GDF-15 gene-inactivated mice suffered from dramatically increased post-infarct mortality, with some animals even showing signs of cardiac rupture.8 This severe increase in damage and mortality was caused by an enhanced recruitment of neutrophils and monocytes into the infarcted tissue. Mechanistically, this effect was due to the capacity of GDF-15 to interfere with the chemokine-induced activation of β2-integrins and integrin α4β1.8 The receptor for GDF-15 on neutrophils and monocytes responsible for these effects is not known.
The TGF-β superfamily comprises more than 35 ligands including TGF-βs, activins, bone morphogenetic proteins, and GDFs.9 Their receptors are heterodimers consisting of type I and type II receptors. The receptor complex is formed upon ligand binding, which leads to transphosphorylation of the type I receptor by the type II receptor, which in turn induces intracellular signaling pathways.10,11 Most of the ligand-induced signaling pathways depend on the activation of Smads, which leads to the regulation of transcription-based responses.12 A minority of signaling mechanisms are mediated by Smad-independent events, which include the activation of, for example, p38, extracellular signal-regulated kinase, and Akt pathways, and can be transcription independent.13,14 TGF-β signaling is important during embryonic development, and in adult organisms it controls tissue homeostasis and a large variety of pathological and immunologic processes.15,16
Here, we have searched for the leukocyte receptor of GDF-15, which is responsible for the rapid inhibitory effect on integrin activation. We found that TGF-β receptor I (activin receptor-like kinase 5 [ALK-5]) and TGF-β receptor II (TGF-βRII) are responsible for these effects and mediate them by blocking the activation of the guanosine triphosphate (GTP)ase Ras-related protein 1 (Rap-1) via steps that involve cell division control protein 42 homolog (Cdc42) and the guanine nucleotide exchange factor (GEF) CalDAG-GEF1. In addition, we found that TGF-β1, a very well characterized ligand for the ALK-5/TGF-βRII receptor complex could also inhibit chemokine-induced integrin activation in vitro and in vivo.
Materials and methods
Mice
ALK-5lox/lox,17 TGF-βRIIlox/lox,18 and Cdc42lox/lox19 mice were crossed with LysMCre+/T mice20 to inactivate the gene for the corresponding receptor in myeloid cells. Deletion of the antigens was controlled by immunoblotting (see supplemental Figure 2, available on the Blood Web site) and peripheral leukocyte counts of the mouse lines were determined (supplemental Table 2). CalDAG-GEFI−/− mice were obtained from Jill Crittenden.21 For controls, gender and age-matched littermates or C57Bl6 mice were used. All experiments were carried out under the German legislation for the protection of animals and were approved by the Landesamt fuer Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen.
Cells, antibodies, and reagents
Cells were cultured as described: THP-1 cells22 and brain endothelial 5 (bEnd.5) cells.23 Antibodies against the following antigens were used: human TGF-βRI (hTGF-βRI) (V-22; Santa Cruz), hTGF-βRII (AF-241-NA; R&D), Rap-1 (07-916; Millipore), α-tubulin (B-5-1-2; Sigma-Aldrich), pSmad-2 (Ser465/467; clone 138D4; Cell Signaling), Smad2 (D43B4; Cell Signaling), Cdc42 (C44; BD Biosciences), α-Actin Ab-5 (C4; BD Biosciences), and mouse α-human immunoglobulin G (hIgG) conjugated with allophycocyanin (APC) (H2; Southern Biotech). Secondary antibodies were purchased from Jackson Immuno Research. The following reagents were used: recombinant human GDF-15 (hGDF-15), mouse chemokine C-X-C motif ligand 1, hCCL2, hVCAM-1-Fc, and the inhibitors SB-431542, GW-788388, and dorsomorphin-1 (DMH-1) (R&D), human TNF-α and TGF-β1 (PeproTech), and hIgG1 (Sigma-Aldrich). Recombinant ICAM-1-Fc and mouse endomucin-Fc (EM-Fc) were produced by our laboratory.
Polymorphonuclear neutrophil (PMN) isolation
Mouse femurs and tibias were rinsed with PMN wash buffer (Hanks balanced salt solution [Gibco] containing 10% fetal calf serum (FCS) and 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) to isolate bone marrow cells. PMNs were separated from other blood cells on a Histopaque 1077/1119 gradient (Sigma-Aldrich) and washed 3 times with PMN wash buffer before overnight cultivation in PMN culture medium (Dulbecco’s modified Eagle medium [DMEM] + 20% FCS, 10% WEHI-3B supernatant, 1% l-glutamin, and 1% penicillin/streptomycin).
Leukocyte adhesion assay
For this, 96-well MaxiSorp flat bottom plates (Nunc) were coated with 5 μg/mL ICAM-1-Fc, hVCAM-1-Fc, mouse EM-Fc, or hIgG1 overnight at 4°C and blocked for 1 hour with DMEM + 10% FCS (low IgG) at 37°C. Before PMNs or THP-1 cells were allowed to adhere for 20 minutes at 37°C, they were suspended at 1.2 × 106 or 0.8 × 106, respectively, in phosphate-buffered saline (PBS) containing 1 mM Mg2+ and 1 mM Ca2+, and pretreated for 20 minutes on ice or for 5 minutes at room temperature (RT) with 10 μg/mL of the Fc-receptor blocking monoclonal antibody 2.4G2 (obtained from Alf Hamann, Berlin). This was followed by preincubation with 100 ng/mL hGDF-15 or TGF-β1 in 100 μL for 20 minutes at 37°C. Subsequently, chemokine (CXCL1 or CCL2) was added at 100 ng/mL for 1 minute followed by washing the cells with 20 mL PBS before addition to the wells. Inhibitors or blocking antibodies were applied for 10 minutes at RT before hGDF-15/TGF-β1 treatment.
Transendothelial migration assay
bEnd.5 cells were grown on laminin (Sigma-Aldrich) (50 μg/mL)-coated 12-well transwell filter plates (Corning) with 5 μm pore size for 2 days. bEnd.5 cells were stimulated with 5 nM TNF-α 14 to 16 hours before PMNs were added in migration assay medium (DMEM + 5% FCS, 2% l-glutamin, and 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) to the monolayer. And 20 ng/mL CXCL1 were used as chemoattractant in the lower chamber and PMNs were let to migrate for 20 minutes at 37°C (after preincubation with 100 ng/mL GDF-15 or TGF-β1 for 20 minutes at 37°C). The number of transmigrated cells was counted with a CASY cell counter.
Soluble ICAM-1 binding assay
One × 106 PMNs were suspended in 30 μL Hanks balanced salt solution containing 1 mM MgCl2 and CaCl2, and incubated with 100 ng/mL hGDF-15 or hTGF-β1 for 20 minutes at 37°C. After adding 1.5 μg ICAM-1-Fc and 0.75 μg APC-conjugated anti-human IgG1, PMNs were stimulated with 100 ng/mL CXCL1 for 3 minutes at 37°C in a ThermoMixer (300 rpm). Cells were fixed with 7.4% formaldehyde for 10 minutes on ice and stained with anti-Ly6G (1A8)-fluorescein isothiocyanate (BioLegend) (1:200) for 20 minutes on ice. ICAM-1 binding was determined by measuring APC mean fluorescent intensity in a flow cytometer (FACSCanto, BD).
Intravital microscopy (IVM)
Mice were anesthetized with an intraperitoneal injection of 125 mg/kg ketamine hydrochloride (Sanofi) and 12.5 mg/kg xylazine (TranquiVed; Phoenix Scientific). Cremaster muscle and carotid artery were prepared and IVM carried out as previously described.24 Postcapillary venules with a diameter of 20 to 40 μm were chosen for recording using an intravital upright microscope (Olympus BX61 W1) with a ×20 XlumPlan F1 (Olympus) 0.95 numerical aperture saline immersion objective. Inflammation was induced 4 hours before the experiment by intrascrotal injection of interleukin-1β (IL-1β). For chemokine-induced arrest studies in cremaster venules, 4 μg GDF-15 or TGF-β1 were injected via the carotid artery 15 minutes before stimulation with 600 ng CXCL1 through the same vessel.
Immunoblotting
Cells were lysed in lysis buffer (20 mM imidazol [pH 6.8], 100 mM NaCl, 2 mM CaCl2, 1% Triton X-100, 0.04% NaN3, 5× complete EDTA-free protease inhibitor cocktail [Roche]), and lysates were centrifuged for 10 minutes at 20,000 g. Afterward, lysates were separated by electrophoresis on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Schleicher & Schuell) by wet blotting. Blots were analyzed as previously described.25
Results
Pharmacologic inhibitors, small interfering RNA (siRNA) treatment, or antibodies determine ALK-5 and TGF-βRII as potential receptors for GDF-15
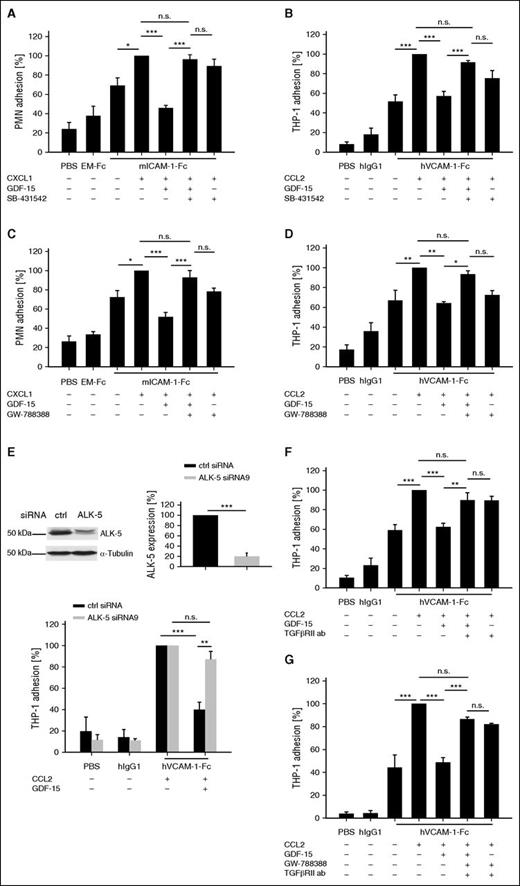
Despite the distant sequence similarity of GDF-15 with other members of the TGF-β family, we started our search for the GDF-15 receptor on neutrophils and monocytes by testing whether pharmacologic inhibitors against different TGF-β superfamily receptors would block the inhibitory effect of GDF-15 on chemokine-induced integrin activation. We first applied the inhibitor SB-431542 against TGF-β type I receptors, ALK-4, -5, and -7, and tested adhesion of mouse bone marrow-derived neutrophils and the human monocytic cell line THP-1 to immobilized β2-integrin ligand ICAM-1-Fc or α4β1-integrin ligand VCAM-1-Fc, respectively. Twenty minutes pre-incubation with GDF-15 inhibited CXCL1-induced adhesion of neutrophils to ICAM-1-Fc as previously seen,8 whereas the inhibitor SB-431542 completely reversed this effect (Figure 1A). Similar results were obtained for THP-1 cells adhering on VCAM-1-Fc, where SB-431542 compensated most of the inhibitory effect of GDF-15 resulting in 89.2% (± 3.5%) of the original binding efficiency of THP-1 cells treated with CCL2 only (Figure 1B). To determine which of the 3 type I TGF-β receptors is responsible for the observed GDF-15 effects, we tested the ALK-5 specific inhibitor GW-788388 in similar experiments. This compound reversed the inhibitory effect of GDF-15 on chemokine-induced neutrophil adhesion to ICAM-1-Fc and THP-1 adhesion to VCAM-1-Fc almost completely (Figure 1C-D). Another inhibitor for a type I TGF-β receptor, the reagent dorsomorphin-1, which blocks ALK-2, did not interfere with the GDF-15 effect (supplemental Figure 1A). Our results suggest that ALK-5 mediates the inhibitory effects of GDF-15 on leukocyte binding to ICAM-1 and VCAM-1.
Interfering with ALK-5 and TGF-βRII abolishes the inhibitory effect of GDF-15 on myeloid cell adhesion. PMNs (A,C) or THP-1 cells (B,D-G) were pre-incubated with the inhibitors SB-431542 (1 μM) (A-B) or GW-788388 (200 nM) (C-D), or 50 μg/mL anti–hTGF-βRII blocking antibody or control antibody (F), or GW-788388 (200 nM) in combination with 50 μg/mL anti–hTGF-βRII blocking antibody, or control antibody (G) for 10 minutes followed by 20 minutes preincubation with hGDF-15 (100 ng/mL) at 37°C. Where indicated, CXCL1 or CCL2 was added for 1 minute. Subsequently, cells were allowed to bind to immobilized mouse ICAM-1-Fc or mouse EM-Fc (A,C), or to hVCAM-1-Fc or control hIgG1 (B,D,F-G). (E) THP-1 cells were treated with ctrl siRNA or ALK-5 siRNA (as indicated) for 3 days. Resulting expression levels of ALK-5 were analyzed by immunoblotting, and quantification is shown beside. Adhesion of siRNA-treated THP-1 cells to immobilized VCAM-1-Fc or hIgG1 was analyzed as for (B,D,F-G). Experiments were done 4- to 9-times and for each adhesion assay 6 wells were analyzed per condition. Data are shown as mean ± standard error of the mean (SEM). *P < .05; **P < .01; ***P < .001. ab, antibody; ctrl, control; h, human; m, mouse; n.s., not significant.
Interfering with ALK-5 and TGF-βRII abolishes the inhibitory effect of GDF-15 on myeloid cell adhesion. PMNs (A,C) or THP-1 cells (B,D-G) were pre-incubated with the inhibitors SB-431542 (1 μM) (A-B) or GW-788388 (200 nM) (C-D), or 50 μg/mL anti–hTGF-βRII blocking antibody or control antibody (F), or GW-788388 (200 nM) in combination with 50 μg/mL anti–hTGF-βRII blocking antibody, or control antibody (G) for 10 minutes followed by 20 minutes preincubation with hGDF-15 (100 ng/mL) at 37°C. Where indicated, CXCL1 or CCL2 was added for 1 minute. Subsequently, cells were allowed to bind to immobilized mouse ICAM-1-Fc or mouse EM-Fc (A,C), or to hVCAM-1-Fc or control hIgG1 (B,D,F-G). (E) THP-1 cells were treated with ctrl siRNA or ALK-5 siRNA (as indicated) for 3 days. Resulting expression levels of ALK-5 were analyzed by immunoblotting, and quantification is shown beside. Adhesion of siRNA-treated THP-1 cells to immobilized VCAM-1-Fc or hIgG1 was analyzed as for (B,D,F-G). Experiments were done 4- to 9-times and for each adhesion assay 6 wells were analyzed per condition. Data are shown as mean ± standard error of the mean (SEM). *P < .05; **P < .01; ***P < .001. ab, antibody; ctrl, control; h, human; m, mouse; n.s., not significant.
To obtain evidence that is independent of pharmacologic inhibitors, we performed siRNA experiments to repress ALK-5 levels in THP-1 cells. ALK-5 expression levels could be reduced by 80% (± 6.4%) (siRNA9) (Figure 1E) or 80% (± 3.9%) (siRNA7) (supplemental Figure 1B). Chemokine-induced adhesion of the ALK-5 siRNA-treated cells to VCAM-1-Fc could no longer be significantly inhibited by GDF-15, whereas GDF-15 reduced adhesion of THP-1 cells treated with control siRNA by 59% (± 6.8%) (Figure 1E). Thus, ALK-5 is a likely candidate for a type I receptor of GDF-15.
ALK-5 often interacts with the TGF-βRII as a type II receptor. For lack of specific pharmacologic inhibitors of TGF-βRII, we used neutralizing antibodies to test a possible relevance of this receptor for GDF-15. We found that the antibody almost fully restored the chemokine-stimulated adhesion of GDF-15–treated THP-1 cells to similar levels as detected for cells not treated with GDF-15 (Figure 1F). This suggests TGF-βRII is a potential type II receptor for the inhibitory effect of GDF-15 on leukocyte integrin activation. When we used the blocking antibody against TGF-βRII in combination with the ALK-5 inhibitor GW-788388, we found that this restored the chemokine-stimulated adhesion of GDF-15–treated THP-1 cells to similar levels as each of the inhibitors alone (compare Figure 1G with 1D,F). This suggests that ALK-5 and TGF-βRII act as a heterodimer receptor for GDF-15.
GDF-15 cannot inhibit adhesion and transmigration of neutrophils isolated from ALK-5 and TGF-βRII conditional gene-inactivated mice
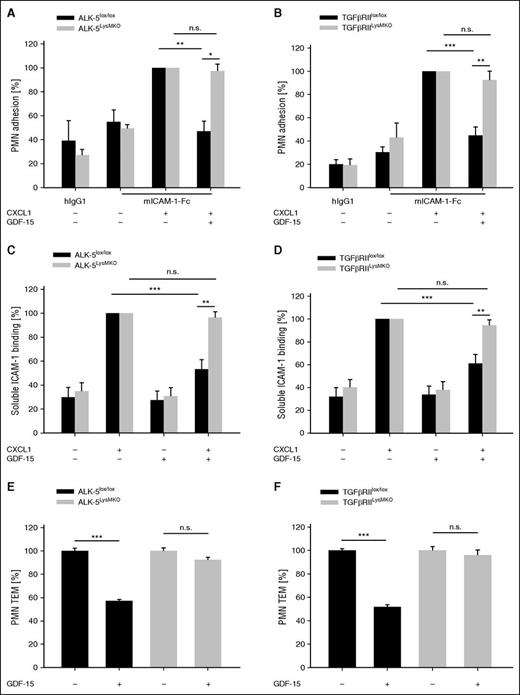
To verify the relevance of ALK-5 and TGF-βRII for the GDF-15 effects on neutrophils independent of inhibitory reagents, we generated mice with a myeloid cell-specific deletion of the respective genes by breeding ALK-5lox/lox 17 or TGF-βRIIlox/lox mice18 with LysM-Cre mice20 expressing Cre in myeloid cells. Both resulting mouse lines (ALK-5LysMKO or TGF-βRIILysMKO) were viable and did not show any developmental defects. Neutrophils isolated from these mice or their corresponding controls (littermates without Cre) were applied in static adhesion assays. As shown in Figure 2A-B, GDF-15 could inhibit chemokine-induced adhesion of control neutrophils to immobilized ICAM-1-Fc by 53% (± 8.5%) or 55% (± 7.4%), respectively, whereas significant inhibitory effects were lost with neutrophils isolated from ALK-5LysMKO or TGF-βRIILysMKO mice.
The inhibitory effect of GDF-15 on PMN adhesion, and transendothelial migration is abolished in ALK-5 and TGF-βRII null PMNs. (A-D) PMNs of ALK-5LysMKO or TGF-βRIILysMKO mice or littermate controls without the Cre transgene (ALK-5lox/lox or TGF-βRIIlox/lox) were preincubated with GDF-15 (100 ng/mL) for 20 minutes followed by stimulation with CXCL1 (100 ng/mL) for 1 minute (where indicated), before adhesion to hIgG1 or ICAM-1-Fc coated support was determined (A-B); or binding to soluble ICAM-1-Fc (1.5 μg/1 × 106 cells) and α-human IgG-APC antibody (0.75 μg/1 × 106 cells) was determined by flow cytometry (C-D). (E-F) Transendothelial migration of PMNs from ALK-5lox/lox, TGF-βRIIlox/lox, ALK-5LysMKO, or TGF-βRIILysMKO mice (as indicated) through a TNF-α–stimulated bEnd.5 cell monolayer in transwell filters along a CXCL1 chemotactic gradient was analyzed. Where indicated, PMNs were incubated with GDF-15 (100 ng/mL) for 20 minutes before transmigration. Data are pooled from 4 (A,C,F), 3 (B,D), and 5 (E) independent experiments with 6 replicates in each adhesion experiment and with at least 3 replicates in each transendothelial migration assay. Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001. n.s., not significant; TEM, transendothelial migration.
The inhibitory effect of GDF-15 on PMN adhesion, and transendothelial migration is abolished in ALK-5 and TGF-βRII null PMNs. (A-D) PMNs of ALK-5LysMKO or TGF-βRIILysMKO mice or littermate controls without the Cre transgene (ALK-5lox/lox or TGF-βRIIlox/lox) were preincubated with GDF-15 (100 ng/mL) for 20 minutes followed by stimulation with CXCL1 (100 ng/mL) for 1 minute (where indicated), before adhesion to hIgG1 or ICAM-1-Fc coated support was determined (A-B); or binding to soluble ICAM-1-Fc (1.5 μg/1 × 106 cells) and α-human IgG-APC antibody (0.75 μg/1 × 106 cells) was determined by flow cytometry (C-D). (E-F) Transendothelial migration of PMNs from ALK-5lox/lox, TGF-βRIIlox/lox, ALK-5LysMKO, or TGF-βRIILysMKO mice (as indicated) through a TNF-α–stimulated bEnd.5 cell monolayer in transwell filters along a CXCL1 chemotactic gradient was analyzed. Where indicated, PMNs were incubated with GDF-15 (100 ng/mL) for 20 minutes before transmigration. Data are pooled from 4 (A,C,F), 3 (B,D), and 5 (E) independent experiments with 6 replicates in each adhesion experiment and with at least 3 replicates in each transendothelial migration assay. Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001. n.s., not significant; TEM, transendothelial migration.
Similar results were found when binding of soluble ICAM-1-Fc to neutrophils was analyzed by flow cytometry (Figure 2C-D). Thus, ALK-5 and TGF-βRII are required for GDF-15–triggered inhibition of chemokine-induced β2-integrin activation.
We have shown before that GDF-15 also inhibits the transmigration of neutrophils through cultured monolayers of endothelial cells grown on transwell filters.8 As shown in Figure 2E-F, this inhibitory effect was also abolished when neutrophils devoid of ALK-5 or TGF-βRII were analyzed in these assays.
The identified GDF-15 receptor-pair is relevant for neutrophil extravasation in vivo
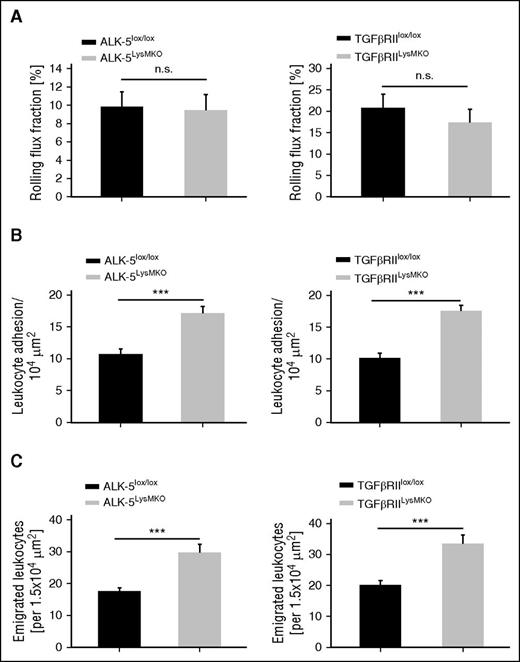
To determine the in vivo relevance of the identified GDF-15 receptor complex, we performed IVM of the cremaster muscle. We have shown before that GDF-15 gene inactivation causes enhanced neutrophil extravasation in the IL-1β–stimulated cremaster.8 This established that beyond the cardiac infarction model, GDF-15 represents an endogenous anti-inflammatory cytokine that counteracts neutrophil recruitment into inflamed tissues. To test whether the identified receptor-pair is indeed relevant for this GDF-15 effect in vivo, we injected ALK-5LysMKO and TGF-βRIILysMKO mice, and for comparison littermates (lacking the Cre transgene) intrascrotally with 50 ng of IL-1β and analyzed neutrophil rolling, adhesion, and extravasation 4 hours later by IVM. As shown in Figure 3, ALK-5 gene inactivation enhanced intraluminal neutrophil adhesion by 59.7% (± 10.2%) and extravasation by 69.5% (± 14.2%). Likewise, TGF-βRII gene inactivation increased intraluminal neutrophil adhesion by 72.6% (± 8.8%) and extravasation by 66% (± 13.9%). The slight reduction of the rolling flux fraction was statistically not significant. Hemodynamic parameters were not affected (supplemental Table 1). Our results reveal that the GDF-15 receptor-pair ALK-5/TGF-βRII acts as an anti-inflammatory receptor in vivo that counteracts leukocyte extravasation.
Gene inactivation of ALK-5 or TGF-βRII in myeloid cells enhances intraluminal neutrophil adhesion and extravasation in cremaster venules. IVM of the inflamed cremaster of ALK-5lox/lox, TGFβRIIlox/lox, ALK-5LysMKO, and TGF-βRIILysMKO mice 4 hours after intrascrotal injection of 50 ng IL-1β. (A) Rolling flux fraction, (B) leukocyte adhesion, and (C) leukocyte extravasation in postcapillary venules. Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001.
Gene inactivation of ALK-5 or TGF-βRII in myeloid cells enhances intraluminal neutrophil adhesion and extravasation in cremaster venules. IVM of the inflamed cremaster of ALK-5lox/lox, TGFβRIIlox/lox, ALK-5LysMKO, and TGF-βRIILysMKO mice 4 hours after intrascrotal injection of 50 ng IL-1β. (A) Rolling flux fraction, (B) leukocyte adhesion, and (C) leukocyte extravasation in postcapillary venules. Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001.
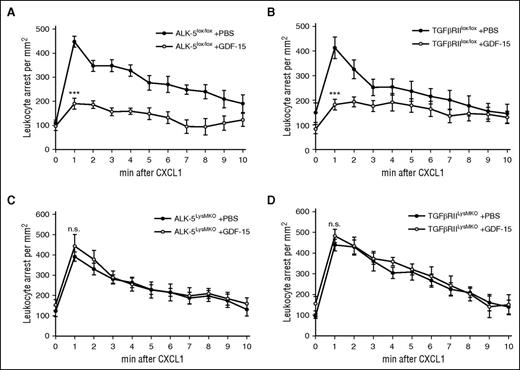
Because the ALK-5/TGF-βRII receptor-pair is known to mediate anti-inflammatory processes by various indirect ways that require the modulation of gene expression, we wanted to test whether this receptor-pair is indeed mediating in vivo the rapid GDF-15 effect on chemokine-induced integrin activation that leads to immediate leukocyte arrest. To this end, GDF-15 was injected into the circulation 15 minutes before recording, followed by intracarotid injection of CXCL1. Leukocyte arrest in the cremaster muscle postcapillary venules was assessed for 15 seconds before and starting again 1 minute after intra-arterial chemokine injection for 10 minutes. In control mice (littermates, no Cre transgene), GDF-15 reduced the number of arrested leukocytes 1 minute after chemokine injection by >50% when compared with PBS injections (Figure 4A-B). In contrast, the GDF-15 effect was completely abolished in ALK-5LysMKO and TGF-βRIILysMKO mice (Figure 4C-D). Thus, these receptors mediate the rapid effect by which GDF-15 counteracts chemokine-induced neutrophil arrest.
ALK-5 and TGF-βRII are required for GDF-15–induced inhibition of chemokine-triggered neutrophil arrest in cremaster venules. IVM of chemokine-induced leukocyte arrest in postcapillary venules of the cremaster muscle of ALK-5lox/lox (A) or TGF-βRIIlox/lox (B) control mice, and of ALK-5LysMKO (C) and TGF-βRIILysMKO (D) mice. Fifteen-second movies were taken before and 1 minute after intra-arterial injection of CXCL1 (600 ng) every minute for 10 minutes. GDF-15 (4 μg) or vehicle was intra-arterially injected 15 minutes before the experiment. Number of analyzed mice were 6 (A-B,D) and 5 (C). Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001.
ALK-5 and TGF-βRII are required for GDF-15–induced inhibition of chemokine-triggered neutrophil arrest in cremaster venules. IVM of chemokine-induced leukocyte arrest in postcapillary venules of the cremaster muscle of ALK-5lox/lox (A) or TGF-βRIIlox/lox (B) control mice, and of ALK-5LysMKO (C) and TGF-βRIILysMKO (D) mice. Fifteen-second movies were taken before and 1 minute after intra-arterial injection of CXCL1 (600 ng) every minute for 10 minutes. GDF-15 (4 μg) or vehicle was intra-arterially injected 15 minutes before the experiment. Number of analyzed mice were 6 (A-B,D) and 5 (C). Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001.
TGF-β1 inhibits neutrophil adhesion and transmigration in vitro, and rapid leukocyte arrest in vivo
The receptor complex we have identified for GDF-15 is also the classical receptor for TGF-β1. Therefore, we tested whether TGF-β1 would cause anti-inflammatory effects by a similar rapid mechanism as GDF-15. We found that TGF-β1 could inhibit CXCL1-induced neutrophil adhesion to immobilized ICAM-1-Fc in a dose-dependent manner, resulting in a 61.8% (± 8.5%) inhibition of adhesion with 100 ng/mL TGF-β1 (Figure 5A). Likewise, binding of soluble ICAM-1-Fc to CXCL1-stimulated neutrophils was reduced by 47.7% (± 4.2%), as was determined by flow cytometry (Figure 5B). In addition, TGF-β1 inhibited transmigration through a bEnd.5 monolayer along a CXCL1 gradient by 52% (± 3%) (Figure 5C).
TGF-β1 inhibits PMN interaction with ICAM-1, transendothelial migration, and in vivo intraluminal arrest. (A) Adhesion of WT PMNs to hIgG1 or ICAM-1-Fc coated on tissue culture dishes. PMNs were incubated with 20, 50, 100, or 200 ng/mL TGFβ-1 for 20 minutes at 37°C before stimulation with 100 ng/mL CXCL1 for 1 minute. (B) PMNs were incubated with ICAM-1-Fc (1.5 μg/1 × 106 cells) and α-human IgG-APC antibody (0.75 μg/1 × 106 cells) after preincubation, where indicated, for 20 minutes with TGFβ-1 (100 ng/mL) and/or with CXCL1 (100 ng/mL) for 3 minutes at 37°C. (C) Transendothelial migration of WT PMNs through a TNF-α–stimulated bEnd.5 cell monolayer in transwell filters along a CXCL1 chemotactic gradient. PMNs were incubated with TGF-β1 (100 ng/mL) or solute for 20 minutes before transmigration. (D) IVM of chemokine-induced leukocyte arrest in postcapillary venules of the cremaster muscle of WT mice. Fifteen-second movies were taken before and 1 minute after intra-arterial injection of CXCL1 (600 ng) every minute for 10 minutes. TGF-β1 (4 μg) or vehicle was intra-arterially injected 15 minutes before the experiment. (E-F) Binding of soluble ICAM-1-Fc (1.5 μg/1 × 106 cells) after a 20 minute preincubation with TGF-β1 (100 ng/mL) and a 3 minute stimulation with CXCL1 (100 ng/mL) (where indicated) to ALK-5lox/lox and ALK-5LysMKO PMNs (E) and TGF-βRIIlox/lox and TGF-βRIILysMKO PMNs (F). (G-I) Adhesion of PMNs (G) or THP-1 cells (H-I) to either ICAM-1-Fc or EM-Fc (G), or hVCAM-1-Fc or hIgG1 (H-I). Cells were preincubated with GW-788388 (200 nM) (G) or with 50 μg/mL anti-hTGF-βRII blocking antibody or control antibody (H) or both (I) for 10 minutes at RT before 20 minutes TGF-β1 incubation (100 ng/mL). Where indicated, CXCL1 or CCL2 (100 ng/mL) was added for 1 minute. Data are pooled from 4 (A,C,E-H), 3 (B,I), and 6 (D) independent experiments. Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001. TEM, transendothelial migration; WT, wild-type.
TGF-β1 inhibits PMN interaction with ICAM-1, transendothelial migration, and in vivo intraluminal arrest. (A) Adhesion of WT PMNs to hIgG1 or ICAM-1-Fc coated on tissue culture dishes. PMNs were incubated with 20, 50, 100, or 200 ng/mL TGFβ-1 for 20 minutes at 37°C before stimulation with 100 ng/mL CXCL1 for 1 minute. (B) PMNs were incubated with ICAM-1-Fc (1.5 μg/1 × 106 cells) and α-human IgG-APC antibody (0.75 μg/1 × 106 cells) after preincubation, where indicated, for 20 minutes with TGFβ-1 (100 ng/mL) and/or with CXCL1 (100 ng/mL) for 3 minutes at 37°C. (C) Transendothelial migration of WT PMNs through a TNF-α–stimulated bEnd.5 cell monolayer in transwell filters along a CXCL1 chemotactic gradient. PMNs were incubated with TGF-β1 (100 ng/mL) or solute for 20 minutes before transmigration. (D) IVM of chemokine-induced leukocyte arrest in postcapillary venules of the cremaster muscle of WT mice. Fifteen-second movies were taken before and 1 minute after intra-arterial injection of CXCL1 (600 ng) every minute for 10 minutes. TGF-β1 (4 μg) or vehicle was intra-arterially injected 15 minutes before the experiment. (E-F) Binding of soluble ICAM-1-Fc (1.5 μg/1 × 106 cells) after a 20 minute preincubation with TGF-β1 (100 ng/mL) and a 3 minute stimulation with CXCL1 (100 ng/mL) (where indicated) to ALK-5lox/lox and ALK-5LysMKO PMNs (E) and TGF-βRIIlox/lox and TGF-βRIILysMKO PMNs (F). (G-I) Adhesion of PMNs (G) or THP-1 cells (H-I) to either ICAM-1-Fc or EM-Fc (G), or hVCAM-1-Fc or hIgG1 (H-I). Cells were preincubated with GW-788388 (200 nM) (G) or with 50 μg/mL anti-hTGF-βRII blocking antibody or control antibody (H) or both (I) for 10 minutes at RT before 20 minutes TGF-β1 incubation (100 ng/mL). Where indicated, CXCL1 or CCL2 (100 ng/mL) was added for 1 minute. Data are pooled from 4 (A,C,E-H), 3 (B,I), and 6 (D) independent experiments. Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001. TEM, transendothelial migration; WT, wild-type.
To test the short-term anti-inflammatory effects of TGF-β1 in vivo, we administered the cytokine in chemokine arrest experiments into the circulation, 15 minutes before intra-arterial injection of CXCL1, followed by instantaneously reporting leukocyte arrest in postcapillary venules of the cremaster. We found that TGF-β1 reduced the number of arrested leukocytes by 59% (± 5.9%) within 1 minute after chemokine injection (Figure 5D). Collectively, these results suggest that TGF-β1 counteracts rapid integrin activation and neutrophil arrest in vivo.
To determine whether TGF-β1 acts via ALK-5 and TGF-βRII, we analyzed whether TGF-β1 could still inhibit chemokine-induced binding of soluble ICAM-1-Fc to ALK-5LysMKO or TGF-βRIILysMKO PMNs. We found that the TGF-β1 effect was abolished in both types of knockout PMNs (Figure 5E-F). Furthermore, the ALK-5 inhibitor GW-788388 and the TGF-βRII–blocking antibody almost completely blocked the TGF-β1 effect in WT PMNs or THP-1 cells, respectively (Figure 5G-H). Simultaneous blocking of both receptor chains had no additive effects (Figure 5I), again suggesting that ALK-5 and TGF-βRII act as heterodimers in TGF-β1–mediated inhibition of integrin activation.
GDF-15 and TGF-β1 inhibit Rap-1 activation, which requires Cdc42 and CalDAG-GEF1
We have shown before that GDF-15 counteracts chemokine-induced activation of Rap-1, a central GTPase for the activation of leukocyte integrins.8 Employing a Rap-1 GTP pull-down assay, we found that preincubation of mouse PMNs with 100 ng/mL TGF-β1 for 20 minutes could also block CXCL1-induced Rap-1 activation (Figure 6A).
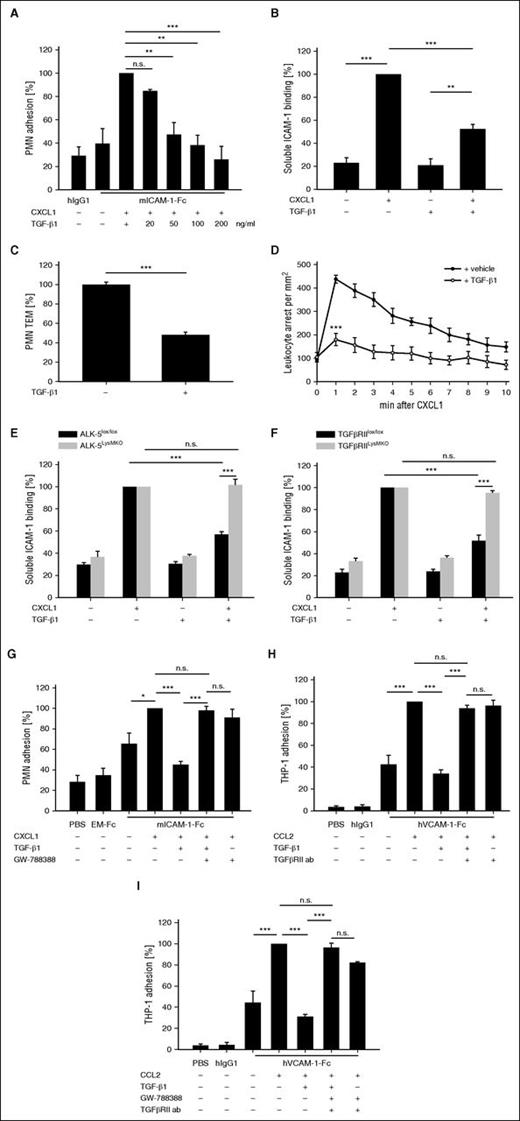
GDF-15 and TGF-β1 inhibit chemokine-induced activation of Rap-1 via Cdc42. (A) PMNs were either untreated (-) or treated with 100 ng/mL TGF-β1 for 20 minutes and/or treated with 100 ng/mL CXCL1 for 1 minute, followed by isolating activated GTP-bound Rap-1, total Rap-1, and immunoblotting. Minimal (GDP) and maximal (GTPγS) activation levels of Rap-1 are shown on the right. (B) PMNs from either WT or Cdc42LysMKO mice were untreated (-) or treated with either 100 ng/mL TGF-β1 or 100 ng/mL hGDF-15 for 20 minutes, and/or treated with 100 ng/mL CXCL1 for 1 minute (as indicated), followed by isolating activated GTP-bound Rap-1, total Rap-1, and immunoblotting. Quantification of band intensities (for 3 experiments) is given below. (C) PMNs from either WT or Cdc42LysMKO mice were treated with TGF-β1, GDF-15, and CXCL1 as above, before binding to soluble ICAM-1-Fc (1.5 μg/1 × 106 cells) and α-human IgG-APC antibody (0.75 μg/1 × 106 cells) was determined by flow cytometry. (D-E) Cdc42 activation after 20 minutes of TGF-β1 or GDF-15 treatment (100 ng/mL) (where indicated) in ALK-5lox/lox and ALK-5LysMKO PMNs (D), and TGF-βRIIlox/lox and TGF-βRIILysMKO PMNs (E) was analyzed with the Cdc42 G-LISA activation assay. Data were read at 490 nm. The depicted experiments in (A-B) represent 1 of 3 independent experiments with similar results. In (C), data are pooled from 5 independent experiments and in (D-E) from 3 independent experiments. Data are shown as mean ± SEM. *P < .05; **P < .01 ***P < .001. GDP, guanosine diphosphate; GTPγS, guanosine 5′-O-[gamma-thio] triphosphate.
GDF-15 and TGF-β1 inhibit chemokine-induced activation of Rap-1 via Cdc42. (A) PMNs were either untreated (-) or treated with 100 ng/mL TGF-β1 for 20 minutes and/or treated with 100 ng/mL CXCL1 for 1 minute, followed by isolating activated GTP-bound Rap-1, total Rap-1, and immunoblotting. Minimal (GDP) and maximal (GTPγS) activation levels of Rap-1 are shown on the right. (B) PMNs from either WT or Cdc42LysMKO mice were untreated (-) or treated with either 100 ng/mL TGF-β1 or 100 ng/mL hGDF-15 for 20 minutes, and/or treated with 100 ng/mL CXCL1 for 1 minute (as indicated), followed by isolating activated GTP-bound Rap-1, total Rap-1, and immunoblotting. Quantification of band intensities (for 3 experiments) is given below. (C) PMNs from either WT or Cdc42LysMKO mice were treated with TGF-β1, GDF-15, and CXCL1 as above, before binding to soluble ICAM-1-Fc (1.5 μg/1 × 106 cells) and α-human IgG-APC antibody (0.75 μg/1 × 106 cells) was determined by flow cytometry. (D-E) Cdc42 activation after 20 minutes of TGF-β1 or GDF-15 treatment (100 ng/mL) (where indicated) in ALK-5lox/lox and ALK-5LysMKO PMNs (D), and TGF-βRIIlox/lox and TGF-βRIILysMKO PMNs (E) was analyzed with the Cdc42 G-LISA activation assay. Data were read at 490 nm. The depicted experiments in (A-B) represent 1 of 3 independent experiments with similar results. In (C), data are pooled from 5 independent experiments and in (D-E) from 3 independent experiments. Data are shown as mean ± SEM. *P < .05; **P < .01 ***P < .001. GDP, guanosine diphosphate; GTPγS, guanosine 5′-O-[gamma-thio] triphosphate.
For lymphocytes, it was shown that Cdc42 negatively regulates lymphocyte function-associated molecule 1 activation.26 These results had been based on the introduction of a dominant negative mutant of Cdc42 with the help of a fused membrane-permeable transactivator of transcription (TAT)-peptide. We had found previously that introducing the same reagent into neutrophils abolished GDF-15–triggered inhibition of chemokine-driven activation of Rap-1. Because the introduction of a mutant form of Cdc42 by a membrane permeable peptide can have side effects, we now analyzed whether Cdc42 gene inactivation would indeed interfere with the inhibitory effects of GDF-15 or TGF-β1 on Rap-1 activation. To this end, we isolated neutrophils from Cdc42 conditional gene-inactivated mice (Cdc42lox/lox)19 expressing LysMCre. Performing Rap-1 GTP pull-down assays, we found that GDF-15 and TGF-β1 could no longer inhibit chemokine-induced Rap-1 activation when Cdc42 was absent (Figure 6B). Likewise, chemokine-induced binding of ICAM-1-Fc to neutrophils from Cdc42LysMKO mice could no longer be inhibited by GDF-15 or TGF-β1 (Figure 6C). Importantly, TGF-β1 and GDF-15 stimulated the activation of Cdc42 in PMNs as determined by a G-LISA activation assay, and this effect was abolished in ALK-5LysMKO and TGF-βRIILysMKO PMNs (Figure 6D-E). We conclude that GDF-15 and TGF-β1 prevent Rap-1 and integrin activation via Cdc42.
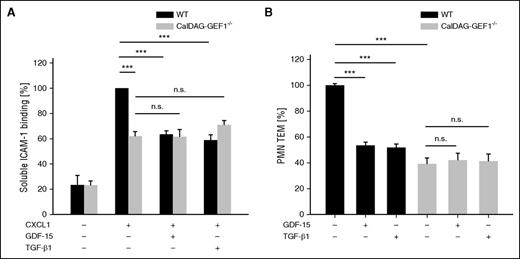
To identify the Rap-1 GEF that is responsible for the GDF-15 and TGF-β1 inhibitory effects on integrin activation, we analyzed neutrophils isolated from CalDAG-GEF1 gene-inactivated mice. CalDAG-GEF1 plays a major role for the activation of β2 integrins on mouse neutrophils.27 We found that CXCL1-induced ICAM-1-Fc binding to neutrophils lacking CalDAG-GEF1 was clearly reduced by 38.1% (± 3.8%) when compared with WT neutrophils, although the chemokine was still able to enhance ICAM-1-Fc binding to the mutant neutrophils several-fold when compared with the PBS control (Figure 7A). Interestingly, neither GDF-15 nor TGF-β1 were able to inhibit chemokine-induced ICAM-1 binding to the mutant neutrophils. This shows that CalDAG-GEF1 is clearly important but not absolutely essential for chemokine-induced integrin activation on neutrophils, whereas this GEF is absolutely essential for the effects of GDF-15 and TGF-β1. Likewise, the inhibitory effects of each of the 2 TGF-β family members on transendothelial migration were completely lost in CalDAG-GEF1 gene-deficient neutrophils (Figure 7B).
GDF-15 and TGF-β1–induced inhibition of integrin activation and leukocyte transmigration depend on CalDAG-GEF1. (A) PMNs from either WT or CalDAG-GEF1−/− mice were incubated for 20 minutes with GDF-15 or TGF-β1 (100 ng/mL), followed by CXCL1 (100 ng/mL) for 3 minutes at 37°C (where indicated) before binding of soluble ICAM-1-Fc (1.5 μg/1 × 106 cells) and α-human IgG-APC antibody (0.75 μg/1 × 106 cells) was determined by flow cytometry. (B) Transendothelial migration of PMNs from WT or CalDAG-GEF1−/− mice (as indicated) through a TNF-α–stimulated bEnd.5 cell monolayer in transwell filters along a CXCL1 chemotactic gradient was analyzed after preincubation of the cells with 100 ng/mL of GDF-15 or 100 ng/mL TGF-β1 (as indicated) for 20 minutes before transmigration. Data of 3 independent experiments were pooled (A-B). Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001.
GDF-15 and TGF-β1–induced inhibition of integrin activation and leukocyte transmigration depend on CalDAG-GEF1. (A) PMNs from either WT or CalDAG-GEF1−/− mice were incubated for 20 minutes with GDF-15 or TGF-β1 (100 ng/mL), followed by CXCL1 (100 ng/mL) for 3 minutes at 37°C (where indicated) before binding of soluble ICAM-1-Fc (1.5 μg/1 × 106 cells) and α-human IgG-APC antibody (0.75 μg/1 × 106 cells) was determined by flow cytometry. (B) Transendothelial migration of PMNs from WT or CalDAG-GEF1−/− mice (as indicated) through a TNF-α–stimulated bEnd.5 cell monolayer in transwell filters along a CXCL1 chemotactic gradient was analyzed after preincubation of the cells with 100 ng/mL of GDF-15 or 100 ng/mL TGF-β1 (as indicated) for 20 minutes before transmigration. Data of 3 independent experiments were pooled (A-B). Data are shown as mean ± SEM. *P < .05; **P < .01; ***P < .001.
Discussion
The activation of leukocyte integrins is a central regulatory step that controls the physical interactions of leukocytes with endothelial cells, which is essential for leukocyte extravasation. GDF-15 is the first endogenous, anti-inflammatory cytokine known to target this process and inhibit the recruitment of neutrophils into inflamed tissue. Here, we have identified the GDF-15 receptor complex on neutrophils, which is responsible for this activity. The type I and type II TGF-β receptors, ALK-5 and TGF-βRII, were each essential for GDF-15–induced inhibition of chemokine-triggered integrin activation and neutrophil arrest in vivo. Likewise, the genetic inactivation of both receptors enhanced neutrophil extravasation in inflamed venules as previously observed for the genetic deletion of GDF-15. TGF-β1, a well-characterized ligand of the ALK-5/TGF-βRII complex had similar activities as GDF-15. Both cytokines inhibited the activation of β2 integrins by the same signaling mechanism, which required the presence of the GTPase Cdc42 and was mediated by the Rap-1 GEF CalDAG-GEF1. In summary, our results establish a novel anti-inflammatory activity for the ALK-5/TGF-βRII receptor complex on neutrophils.
TGF-β signaling is well known for its pleiotropic regulatory role in the inflammatory process.16 TGF-β1 gene inactivation revealed a central role for this factor as an anti-inflammatory cytokine that protects against autoimmune diseases.28 Furthermore, depending on the pathophysiological context, TGF-β signaling can initiate pro- and anti-inflammatory effects, mostly by modulating gene expression. TGF-β1, for example, can induce expression of matrix metalloproteinases29 and β2-integrins,29,30 and modulate the expression of other cytokines.31,32 On the other hand, TGF-β1 can potentially counteract the recruitment of myeloid cells to inflammatory sites by inhibiting the expression of E-selectin, VCAM-1, and IL-8 by cultured endothelial cells.33-35 In contrast to these gene-expression modulating effects, our results reveal a novel regulatory level of TGF-β1–mediated inhibition of leukocyte recruitment by rapidly interfering with the chemokine-signaling that leads to leukocyte integrin activation.
In contrast to the inhibitory effects on leukocyte extravasation, TGF-β1 was also reported to support chemotaxis of monocytes36 (232-235) and neutrophils,37 and to upregulate the expression of integrins and collagenase on monocytes.29 It is possible that such effects are rather relevant for the movement of myeloid cells in tissue.16 In this context, the upregulation of α4-integrin–mediated adhesion of leukocytes to isolated integrin ligands38 might be rather related to extravascular migration than to the extravasation process.
We have shown previously that GDF-15–induced inhibition of leukocyte integrin activation confines the recruitment of myeloid cells into infarcted areas of the mouse heart, which protects from tissue damage and cardiac rupture, and enhances survival.8 The fact that TGF-β1 can also restrict integrin activation and leukocyte recruitment, as shown here, explains why TGF-β1 protects against tissue damage at an early phase after myocardial infarction.39 However, at later stages (ie, several days after myocardial infarction), TGF-β enhances interstitial fibrosis and apoptosis, and it is beneficial to block TGF-β with either antibodies or with a soluble form of TGF-βRII.39-41
The search for GDF-15 receptors in nonhematopoietic cells led to controversial results. In rat cerebellar neurons, GDF-15 was shown to signal via TGF-βRII, but the involvement of TGF-βRI was excluded.42 In mice, signaling of GDF-15 in the hypothalamus was blocked with antibodies against TGF-βRII, but the type I receptor was not identified.43 In contrast, others could not confirm GDF-15 signaling through TGF-β receptors and suggested that GDF-15 promotes in vitro survival of neurons through a cytokine receptor.44 One study with hematopoietic cells suggested that GDF-15 promotes atherosclerosis by enhancing the migration of macrophages. Interestingly, GDF-15 but not TGF-β1 enhanced monocyte chemoattractant protein 1 expression by macrophages and this was dependent on TGF-βRII, whereas signaling via TGF-βRI was excluded.45 In contrast to these studies, we show here that the TGF-βRII and ALK-5 were each essential for GDF-15–induced inhibition of integrin activation and myeloid cell recruitment.
TGF-β signaling mechanisms that rapidly inhibit the activation of integrins have not yet been investigated. For GDF-15, we have previously suggested that it interferes with leukocyte integrin activation by preventing the activation of Rap-1, a central switch in chemokine-induced integrin activation. We had suggested that Cdc42 is involved, because a dominant negative mutant form of Cdc42 transferred into neutrophils by a fused TAT-peptide, abolished the inhibitory effect of GDF-15 on Rap-1 activation. Because the cellular uptake of a TAT-peptide–transferred fusion protein is difficult to control and the use of a dominant negative GTPase mutant can lead to serious side effects,46 it is important that we could demonstrate here that gene inactivation of Cdc42 indeed blocked the GDF-15–mediated (and TGF-β1–mediated) inhibition of Rap-1 activation. Such rapid signaling via Cdc42 is in agreement with a previous study that demonstrated in prostate carcinoma cells the rapid mobilization of actin reorganization and membrane ruffling by TGF-β, which required Cdc42.47
Our results position Cdc42 in the GDF-15 signaling pathway upstream of the inhibitory effect on Rap-1. CalDAG-GEF1 is an important GEF for Rap-1, which is known to trigger chemokine-induced integrin activation in neutrophils.27 Because we found this GEF to be essential for the inhibitory effect of GDF-15 on integrin activation, we propose that GDF-15 (and TGF-β1) activate the GTPase Cdc42, which then impairs by still unknown mechanisms the ability of CalDAG-GEF1 to activate Rap-1 and thereby integrins.
In conclusion, we show that GDF-15 counteracts chemokine-triggered leukocyte integrin activation and neutrophil recruitment into inflamed tissue by interacting with a receptor-pair consisting of ALK-5 and TGF-βRII. The same effects were observed for TGF-β1. This anti-inflammatory activity is based on rapid signaling mechanisms that interfere via Cdc42 and CalDAG-GEF1 with the activation of Rap-1, a central GTPase for leukocyte integrin activation. Because this rapid signaling mechanism dampens inflammation-induced neutrophil recruitment, further studies may provide new opportunities for interfering with inflammatory disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. Crittenden for kindly providing CalDAG-GEF1−/− mice, and Sebastian Bäumer and Wolfgang E. Berdel for providing the scil Vet abc Plus+ hematology counter.
This study was supported by funds from the Deutsche Forschungsgemeinschaft (SFB1009-A1) (D.V.) and the Max Planck Society, and was performed within the Deutsche Forschungsgemeinschaft Excellence Cluster Cells in Motion.
Authorship
Contribution: A.A. performed, analyzed and designed the experiments, and wrote the manuscript; S.B. designed and analyzed the experiments, and wrote the manuscript; and D.V. initiated the study, designed the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dietmar Vestweber, Max Planck Institute for Molecular Biomedicine, Röntgenstr 20, D-48149 Münster, Germany; email: vestweb@mpi-muenster.mpg.de.

![Figure 6. GDF-15 and TGF-β1 inhibit chemokine-induced activation of Rap-1 via Cdc42. (A) PMNs were either untreated (-) or treated with 100 ng/mL TGF-β1 for 20 minutes and/or treated with 100 ng/mL CXCL1 for 1 minute, followed by isolating activated GTP-bound Rap-1, total Rap-1, and immunoblotting. Minimal (GDP) and maximal (GTPγS) activation levels of Rap-1 are shown on the right. (B) PMNs from either WT or Cdc42LysMKO mice were untreated (-) or treated with either 100 ng/mL TGF-β1 or 100 ng/mL hGDF-15 for 20 minutes, and/or treated with 100 ng/mL CXCL1 for 1 minute (as indicated), followed by isolating activated GTP-bound Rap-1, total Rap-1, and immunoblotting. Quantification of band intensities (for 3 experiments) is given below. (C) PMNs from either WT or Cdc42LysMKO mice were treated with TGF-β1, GDF-15, and CXCL1 as above, before binding to soluble ICAM-1-Fc (1.5 μg/1 × 106 cells) and α-human IgG-APC antibody (0.75 μg/1 × 106 cells) was determined by flow cytometry. (D-E) Cdc42 activation after 20 minutes of TGF-β1 or GDF-15 treatment (100 ng/mL) (where indicated) in ALK-5lox/lox and ALK-5LysMKO PMNs (D), and TGF-βRIIlox/lox and TGF-βRIILysMKO PMNs (E) was analyzed with the Cdc42 G-LISA activation assay. Data were read at 490 nm. The depicted experiments in (A-B) represent 1 of 3 independent experiments with similar results. In (C), data are pooled from 5 independent experiments and in (D-E) from 3 independent experiments. Data are shown as mean ± SEM. *P < .05; **P < .01 ***P < .001. GDP, guanosine diphosphate; GTPγS, guanosine 5′-O-[gamma-thio] triphosphate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/4/10.1182_blood-2016-01-696617/4/m_529f6.jpeg?Expires=1765934567&Signature=boP3eBDgWLpOnJJYEJ41cVF25lf1I~JfqivZYJ1WHTBigvxFkav4iVM26HvKQEOLVSVmnPJIQOht4XNgr5JWGv2foHMMtSa-uJqTWx-ceaPMDEuVDCspnCXXGVddjxUQXH9X9qEh-mB2hC1hitlrG3guETRXifyAb7N~0a1fiidteyZKjwbKbzIWjHeaoZosGu46sU5k2MNGcrR2ydHu6nnApHw--0B0XooKLZmQMRJxjHzSZpYBmzuMgq6eX7dvXFF9ZHMhv3Rnazw3CBv8BXHnb0QKpEDa6j31JOKDEXQ97g9MBM1yEE5VUkQGAYWspcddxNzLcyqTqhOOq1~qmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal