Key Points
CD19-targeted T-cell immunotherapy reveals that a population of PCs lacking CD19 expression survives long-term, independent of B cells.
Preexisting humoral immunity to vaccine-related antigens can persist in patients despite marked B-cell aplasia after CTL019 immunotherapy.
Abstract
The mechanisms underlying the maintenance of long-lasting humoral immunity are not well understood. Studies in mice indicate that plasma cells (PCs) can survive up to a lifetime, even in the absence of regeneration by B cells, implying the presence of long-lived PCs as a mechanism for long-lasting immunity. Evidence from humans treated with anti-CD20, which depletes circulating B cells, also suggests B-cell–independent long-term survival of some PCs. On the other hand, antibody responses may be sustained solely by short-lived PCs with repopulation from clonally related memory B cells. To explore PC longevity and humoral immunity in humans, we investigated the fate of PCs and their antibodies in adult and pediatric patients who received chimeric antigen receptor–based adoptive T-cell immunotherapy targeting CD19 to treat B-cell lineage malignancies (CTL019). Treatment with CTL019 is frequently associated with B-cell aplasia that can persist for years. Serum antibody titers to vaccine-related antigens were measured, and quantitative assessment of B cells and PCs in blood and bone marrow was performed at various time points before and after CTL019 therapy. While total serum immunoglobulin concentrations decline following CTL019-induced B-cell aplasia, several vaccine/pathogen-specific serum immunoglobulin G and A (IgG and IgA) titers remain relatively stable for at least 6 and 12 months posttreatment, respectively. Analysis of bone marrow biopsies after CTL019 revealed 8 patients with persistence of antibody-secreting PCs at least 25 months post-CTL019 infusion despite absence of CD19+CD20+ B cells. These results provide strong evidence for the existence of memory B-cell–independent, long-lived PCs in humans that contribute to long-lasting humoral immunity.
Introduction
Antibodies are relatively short-lived proteins with serum half-lives ranging from ∼1 week to 1 month. However, antigen-specific antibody responses can last as long as a lifetime.1 Thus, the plasma cells (PCs) that produce them must be maintained long-term. Upon antigen encounter, a B cell proliferates and gives rise to clonally related PCs and memory B cells, the latter giving rise to additional PCs upon antigen reencounter. Long-lived humoral immunity may theoretically be maintained by PCs that are long-lived or replenished from long-lived memory B cells, or both. Cell-labeling studies in rodents show that a fraction of newly generated PCs survive for at least 6 months in mice, supporting the existence of long-lived PCs.2,3 Additionally, B-cell depletion studies in mice suggest that at least some PCs are maintained independent of regeneration from B cells.4-7 Whether these observations also apply to PC longevity in humans is not well known. In patients with rheumatoid arthritis or immune thrombocytopenic purpura, PCs have been demonstrated for 3 to 6 months posttreatment with anti-CD20.8,9 We addressed this question by studying PCs in patients experiencing B-cell aplasia induced by CD19-targeted adoptive T-cell immunotherapy.
CTL019, a CD19-specific chimeric antigen receptor (CAR)-based T-cell therapy, has resulted in long-term disease remissions in some patients with chemotherapy-resistant B-lineage malignancies including chronic lymphocytic leukemia (CLL) and B-cell acute lymphoblastic leukemia (ALL).10-13 CD19 is a pan-B-cell surface protein with expression that spans the development of B cells from early pre-B cells to mature, fully differentiated B cells. Thus, successful therapy with CTL019 is often accompanied by profound and persistent B-cell aplasia.12 Following differentiation of B cells to PCs, CD19 expression is thought to decline. Immunophenotypic analyses of bone marrow–derived PCs demonstrate both CD19+ and CD19− populations.14-17 Little is known about the ontogeny, functions, and fate of these 2 immunophenotypically distinct PC populations. Recent data on human PCs suggest that CD19− PCs are enriched in bone marrow and may include long-lived cells that give rise to long-lasting humoral immunity.8,18
We hypothesized that CTL019 would spare the population of CD19− PCs, leaving previously established humoral immunity relatively intact. Furthermore, we anticipated that tracking the fate of the PCs in the context of CTL019-induced B-cell aplasia would shed light on the question of CD19− PC lifespan and maintenance. In the present study (Figure 1), we use multiple methods to examine the fate of PCs and humoral immunity in the context of CTL019 therapy. We show that CD19− bone marrow PCs are indeed resistant to direct elimination by CTL019 and persist independent of B-cell repopulation for at least 25 months. We further show that a variety of humoral responses established prior to CTL019 infusion are retained even as total immunoglobulin levels decline. These results support the hypothesis that human CD19− PCs can be long-lived to maintain long lasting humoral immunity.
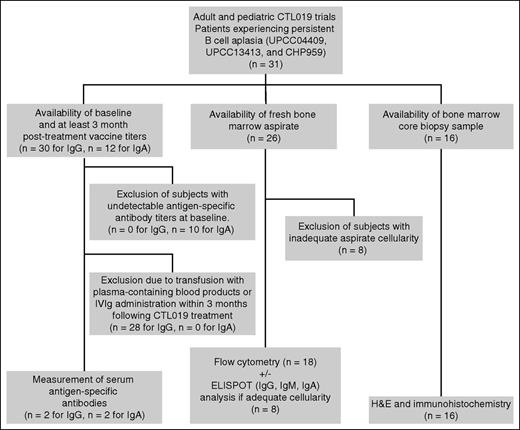
Scheme of subject selection and testing. Samples from patients enrolled in CTL019 clinical trials UPCC04409, UPCC13413, and CHP959 were tested for the presence of B cells, PCs, and serum antibodies to evaluate the state of humoral immunity posttreatment. H&E, hematoxylin and eosin.
Scheme of subject selection and testing. Samples from patients enrolled in CTL019 clinical trials UPCC04409, UPCC13413, and CHP959 were tested for the presence of B cells, PCs, and serum antibodies to evaluate the state of humoral immunity posttreatment. H&E, hematoxylin and eosin.
Materials and methods
Human subjects
The present study included 4 subjects from adult CTL019 trials at the University of Pennsylvania (Penn; ClinicalTrials.gov #NCT01029366 and #NCT02030834) and 12 from a pediatric CTL019 trial at the Children’s Hospital of Philadelphia (CHOP; ClinicalTrials.gov #NCT01626495) (Table 1). Written informed consent for participation was obtained from patients or their guardians according to the Declaration of Helsinki and protocols were approved by the institutional review boards of Penn and CHOP.
Patient demographics
| Subject ID . | Age at infusion, y . | Diagnosis . | Disease response . | Onset of CD19+ B-cell aplasia* . | Duration of CD19+ B-cell aplasia . | Pre-CART19 chemotherapy† . | Prophylactic immunoglobulin start, schedule . |
|---|---|---|---|---|---|---|---|
| UPN-1 | 64 | CLL | CR | Day +24‡ | To day +1827, last time point tested | Pentostatin/ Cyclophosphamide | IVIg day +259, then Q3-4 mo |
| UPN-2 | 60 | CLL | CR→ deceased (PR)§ | Day +11 | To day +428 | Pentostatin/ Cyclophosphamide | IVIg day +288, then monthly |
| UPN-3 | 55 | ALL | CR | Day +29 | To day +741, last time point tested | Cyclophosphamide/ Vincristine | IVIg on day +223, once |
| UPN-4 | 44 | FL | CR | Day −1 | To day +234 (day of death) | Fludarabine/ Cyclophosphamide | IVIg monthly, started >5 y prior to CTL019 |
| CHP-1 | 21 | ALL | CR→ deceased with sCD19− relapse | Day −1 | To day +261 (time of relapse) | Fludarabine/ Cyclophosphamide | IVIg day +126, then monthly |
| CHP-2 | 9 | ALL | CR | Day 0 | To day +735, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +2, then monthly, then switched to weekly subQ from day +255 |
| CHP-3 | 15 | ALL | CR | Day −1 | To day +745, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +35, then monthly, then switched to Q2 wk subQ from day +400 |
| CHP-4 | 9 | ALL | CR | Day −1 | To day +629, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +30, then monthly, then switched to weekly subQ from month +16 |
| CHP-5 | 22 | ALL | CR→ relapse (CD19− on day +687) | Day −2 | To day +250, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +2, then monthly |
| CHP-6 | 16 | ALL | CR | Day −1 | To day +642, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +7, then monthly, then switched to weekly subQ from day +548 |
| CHP-7 | 21 | ALL | CR | Day −1 | To day +640, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +18, then monthly |
| CHP-8 | 5 | ALL | CR | Day +1 | To day +647, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +17, then monthly, then switched to weekly subQ from day +630 |
| CHP-9 | 13 | ALL | CR→ relapse (CD19−) | Day −1 | To day +268, (time of relapse) | Fludarabine/ Cyclophosphamide | IVIg day +8, then monthly |
| CHP-10 | 19 | ALL | CR | Day −2 | To day +545, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +146, then monthly |
| CHP-11 | 9 | ALL | CR→ Allo Txp in CR | Day −1 | To day +93 → Allo Txp in CR | Fludarabine/ Cyclophosphamide | IVIg day +24, then monthly |
| CHP-12 | 17 | ALL | CR | Day −1 | To day +500, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +7, then monthly |
| Subject ID . | Age at infusion, y . | Diagnosis . | Disease response . | Onset of CD19+ B-cell aplasia* . | Duration of CD19+ B-cell aplasia . | Pre-CART19 chemotherapy† . | Prophylactic immunoglobulin start, schedule . |
|---|---|---|---|---|---|---|---|
| UPN-1 | 64 | CLL | CR | Day +24‡ | To day +1827, last time point tested | Pentostatin/ Cyclophosphamide | IVIg day +259, then Q3-4 mo |
| UPN-2 | 60 | CLL | CR→ deceased (PR)§ | Day +11 | To day +428 | Pentostatin/ Cyclophosphamide | IVIg day +288, then monthly |
| UPN-3 | 55 | ALL | CR | Day +29 | To day +741, last time point tested | Cyclophosphamide/ Vincristine | IVIg on day +223, once |
| UPN-4 | 44 | FL | CR | Day −1 | To day +234 (day of death) | Fludarabine/ Cyclophosphamide | IVIg monthly, started >5 y prior to CTL019 |
| CHP-1 | 21 | ALL | CR→ deceased with sCD19− relapse | Day −1 | To day +261 (time of relapse) | Fludarabine/ Cyclophosphamide | IVIg day +126, then monthly |
| CHP-2 | 9 | ALL | CR | Day 0 | To day +735, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +2, then monthly, then switched to weekly subQ from day +255 |
| CHP-3 | 15 | ALL | CR | Day −1 | To day +745, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +35, then monthly, then switched to Q2 wk subQ from day +400 |
| CHP-4 | 9 | ALL | CR | Day −1 | To day +629, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +30, then monthly, then switched to weekly subQ from month +16 |
| CHP-5 | 22 | ALL | CR→ relapse (CD19− on day +687) | Day −2 | To day +250, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +2, then monthly |
| CHP-6 | 16 | ALL | CR | Day −1 | To day +642, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +7, then monthly, then switched to weekly subQ from day +548 |
| CHP-7 | 21 | ALL | CR | Day −1 | To day +640, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +18, then monthly |
| CHP-8 | 5 | ALL | CR | Day +1 | To day +647, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +17, then monthly, then switched to weekly subQ from day +630 |
| CHP-9 | 13 | ALL | CR→ relapse (CD19−) | Day −1 | To day +268, (time of relapse) | Fludarabine/ Cyclophosphamide | IVIg day +8, then monthly |
| CHP-10 | 19 | ALL | CR | Day −2 | To day +545, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +146, then monthly |
| CHP-11 | 9 | ALL | CR→ Allo Txp in CR | Day −1 | To day +93 → Allo Txp in CR | Fludarabine/ Cyclophosphamide | IVIg day +24, then monthly |
| CHP-12 | 17 | ALL | CR | Day −1 | To day +500, last time point tested | Fludarabine/ Cyclophosphamide | IVIg day +7, then monthly |
Allo Txp, allogeneic bone marrow transplant; PR, partial response; Q, every; sCD19, surface-CD19; subQ, subcutaneous.
B-cell aplasia was defined as CD19+ B cells <1% of peripheral blood mononuclear cells. In the majority of samples, the percentage was 0% to 0.1%.
Chemotherapy aimed at lymphodepletion was given within 1 wk prior to CTL019 infusion, timed so that the last dose was given 2 to 6 d prior to infusion.
First postinfusion flow cytometry assessment, true onset of B-cell aplasia may be earlier.
UPN-2 was considered CR at month 6, then PR at month 14 based on radiology findings (at month 14, neither B cells nor CLL was detected in blood and bone marrow).
B-cell aplasia was defined as <1% CD19+CD20+ B cells of peripheral blood mononuclear cells as determined by flow cytometry. In the vast majority of time points, the frequency of circulating B cells was <0.1% (supplemental Figure 1, available on the Blood Web site). For samples from UPN-1 and UPN-2 in particular, in the posttreatment samples with CD19+CD20+ frequencies above 0.1%, κ and λ light-chain staining was performed and showed that events in the CD19+C20+ gate were light-chain negative, thus, likely representing technical artifact.
In the adult and pediatric trials, administration of immunoglobulin as replacement therapy was left to the discretion of the treating physician. As such, there was variation in treatment but, in most cases, it was given when serum IgG was below the normal range.
For UPN-1, UPN-3, and CHP-7 for whom antigen-specific titers are shown, vaccination history could not be obtained due to retrospective nature of serum sample testing.
Flow cytometry and cell sorting
Flow cytometry for assessment of peripheral blood B cells was done by the Translational Correlative Studies Laboratory of the Translational Research Program at the University of Pennsylvania as previously described.12
Flow cytometry of bone marrow aspirate cells was performed using ∼1 to 5 × 106 total cells per condition. Surface staining was performed using anti-CD3 allophycocyanin (APC)-Cy7 (SP34-2), anti-CD14-APC-H7 (M5E2), anti-CD16-APC-H7 (3G8), anti-CD20 peridinin chlorophyll (L27), anti-CD38BV605 (HB7), anti-CD45 fluorescein isothiocyanate (HI30), anti-CD138BV421 (MI15) all from BD Biosciences, anti-CD19 phycoerythrin-Cy7 (J3-119; Beckman Coulter) and anti-CD27VB711 (O323; BioLegend). Samples were stained for viability using eFluor780 (eBioscience). Following permeabilization using Permeabilization Medium-B (Invitrogen), intracellular staining was performed using anti-λ-phycoerythrin (MHL-38) and anti-κ-APC (MHK-49) from BioLegend. Samples were analyzed using an LSR II (BD Biosciences). For cell sorting, mononuclear cells isolated by Ficoll density centrifugation were stained with surface antibodies and eFluor780 and sorted using a FACSAria (BD Biosciences).
ELISPOT
To enumerate total immunoglobulin G, A, and M (IgG, IgA, and IgM)-secreting cells, bone marrow aspirate mononuclear cells or sorted cells were plated at 100 to 2.5 × 105 cells per well of enzyme-linked immunospot (ELISPOT) plates. ELISPOTs for total IgG-, IgM-, and IgA-secreting cells were performed using ELISPOT kits (Mabtech) according to the manufacturer’s instructions.
ELISPOTs for antigen-specific antibody-secreting cells were performed by modification of the Mabtech ELISPOT protocol. Tetanus toxoid (2 μg/mL; Santa Cruz Biotechnology), measles (10 μg/mL; AbD Serotec), or mumps (10 μg/mL; AbD Serotec), each diluted in phosphate-buffered saline, were used to coat wells overnight at 4°C. Wells were then washed and blocked with culture medium followed by addition of cells for overnight culture. Spots were then developed using detection reagents in the Mabtech IgG ELISPOT kit according to the manufacturer’s instructions.
ELISA
For measurement of antigen-specific IgG levels, only patients with available serum, who also had protective antibody levels prior to CTL019 therapy, were tested. Additionally, for specific IgG levels, only patients who had not been treated with intravenous immunoglobulin (IVIg) for at least 3 months post-CTL019 infusion were included and only serum samples from time points prior to IVIg initiation were analyzed.
Antibodies were measured using the following kits according to the manufacturer’s instructions: anti-measles IgG (Serion Immunodiagnostica GmbH), anti-mumps IgG (Calbiotech), anti-rubella IgG (Phoenix Pharmaceuticals Inc), anti-Streptococcus pneumoniae capsular polysaccharide IgG (The Binding Site), anti-Haemophilus influenzae type b capsular polysaccharide IgG (The Binding Site), anti-tetanus toxoid IgG (The Binding Site), and anti-herpes simplex virus (HSV) type 1/2 IgA (Serion Immunodiagnostica GmbH). Protective levels were defined as >0.15 IU/mL for anti-tetanus toxoid IgG,19 ≥120 mIU/mL for anti-measles IgG,20 >15 IU/mL for anti-rubella IgG,21 and >0.15 mg/mL for anti-H influenzae type b capsular polysaccharide IgG.22 For anti-mumps IgG and anti-S pneumoniae capsular polysaccharide IgG, the limits of antibody positivity were an index of 1.1 and 5 mg/L, respectively.
Statistical analysis
Due to the exploratory nature of the study, statistical analyses on subjects were primarily descriptive. Overall and subject-specific patterns over time were described qualitatively. Wilcoxon signed-rank test was used to analyze data in Figure 6. Statistical analysis was performed using GraphPad Prism v.5.00.
Results
Patient demographics
In the present study, we investigated the fate of humoral immunity in adult and pediatric patients with either CLL, ALL, or follicular lymphoma (FL) who were treated with CD19-specific CAR T cells on 1 of 3 clinical trials (NCT01029366, NCT02030834, and NCT01626495) (Table 1). Our goal was to understand the fate of preexisting humoral immunity after successful CTL019 treatment and to explore a fundamental question about the maintenance of long-lived PCs.
Study subjects were selected based on availability of samples from a cohort of 16 patients, all of whom achieved a complete response (CR) at the 1-month time point following CTL019 therapy. Among these patients, the median time of B-cell aplasia onset was day −1 (range, −2 to +29; mean = 3). The median duration of B-cell aplasia was 589 days (range, 94-1803; mean = 571). Four of these 16 subjects experienced disease relapses. In 3 of these cases, CHP-1, CHP-5, and CHP-9, relapse was surface CD19− and B-cell aplasia remained; the fourth subject, UPN-2, was subsequently considered a partial responder based on radiology findings although CLL and B cells were undetectable in the blood and bone marrow. Finally, autopsy findings are presented from UPN-4, a patient treated for FL who achieved and remained in CR until death.
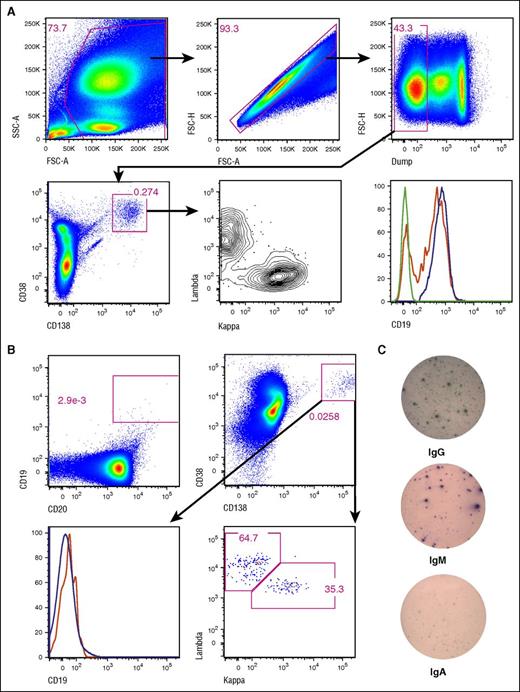
CTL019 therapy, which specifically targets CD19, would be expected to eliminate the CD19+ PC subset, but spare the CD19− fraction. Using bone marrow aspirates from healthy donors, we confirmed by flow cytometry that normal CD138+CD38+ PCs are composed of CD19+ and CD19− fractions (Figure 2A). The CD19+ fraction varied from ∼54% to 92% (mean, 71%; median, 72.5%; n = 7). We evaluated pre- and postinfusion bone marrow aspirates on patients selected from our cohort (Table 1) based upon the availability of fresh bone marrow aspirate material, which was only available from the pediatric CTL019 trial (NCT01626495). PCs were not detectable in all patients or at all time points. This may be attributable to sampling biases inherent with the low frequency of PCs and their nonhomogeneous distribution within the bone marrow (supplemental Table 1). We were able to identify 3 CTL019-complete responders (CHP-3, CHP-7, CHP-11) with the absence of CD19+ cells, including B cells, in their bone marrow, but with a distinct population of CD138+CD38+ PCs with a normal ratio of κ:λ light-chain expression and lacking CD19 (Figure 2B; supplemental Table 1). ELISPOT analysis of cells from these samples also confirmed the presence of antibody-secreting PCs (Figure 2C). These results show that CTL019 effectively eliminates both B cells and CD19+ PCs, but spares a CD19− population of PCs.
CD19− bone marrow PCs resist elimination by CTL019. (A) Bone marrow aspirate from healthy donors were stained and analyzed by flow cytometry. The bottom right panel shows CD19 expression on CD138+CD38+ PCs (red) and CD20+CD19+ cells (blue); the green histogram represents CD138+CD38+ cells with fluorescence-minus-one (CD19) staining. Representative data from 7 donors are shown. The “dump” channel contains stains for viability, CD3, CD14, and CD16. (B) Bone marrow aspirate cells from CHP-11 on day 28 post-CTL019 infusion were analyzed as in panel A. Gating for B cells (top left), PCs (top right) is shown. The bottom left panel shows CD19 expression on CD38+CD138+ cells (red histogram) and on all singlet events in the forward (FSC) and side scatter (SSC) gate (blue histogram). In both panels A and B, expression of light chains was assessed by intracellular staining. (C) Two hundred thousand cells from the aspirate material shown in panel B were plated in ELISPOT wells, which were stained for total IgG, IgM, and IgA after overnight incubation.
CD19− bone marrow PCs resist elimination by CTL019. (A) Bone marrow aspirate from healthy donors were stained and analyzed by flow cytometry. The bottom right panel shows CD19 expression on CD138+CD38+ PCs (red) and CD20+CD19+ cells (blue); the green histogram represents CD138+CD38+ cells with fluorescence-minus-one (CD19) staining. Representative data from 7 donors are shown. The “dump” channel contains stains for viability, CD3, CD14, and CD16. (B) Bone marrow aspirate cells from CHP-11 on day 28 post-CTL019 infusion were analyzed as in panel A. Gating for B cells (top left), PCs (top right) is shown. The bottom left panel shows CD19 expression on CD38+CD138+ cells (red histogram) and on all singlet events in the forward (FSC) and side scatter (SSC) gate (blue histogram). In both panels A and B, expression of light chains was assessed by intracellular staining. (C) Two hundred thousand cells from the aspirate material shown in panel B were plated in ELISPOT wells, which were stained for total IgG, IgM, and IgA after overnight incubation.
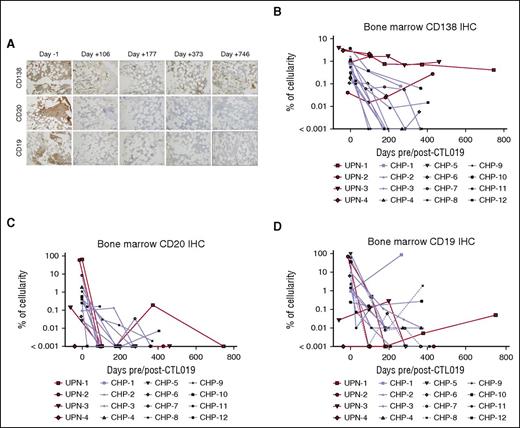
Given the inaccuracy of enumerating PCs in bone marrow aspirates by flow cytometry and because hemodilution and cell viability may additionally affect the analysis of bone marrow aspirate specimens, we also performed immunohistochemistry (IHC) analysis of bone marrow core biopsies that were available from both the adult and pediatric CTL019 trials. Stains for CD20, CD138, and CD19 were performed to identify B cells and PCs. Overall, consistent with previous observations, bone marrow PCs generally declined in frequency over time after CTL019 treatment. However, similar to results by flow cytometry, we noted several cases in which CD138+ cells remained despite peripheral B-cell aplasia as well as absence of CD19+CD20+ cells by IHC in the biopsy (Figure 3; supplemental Table 2). In UPN-2, the subject with the longest post-CTL019 follow-up, we found that PCs persisted for at least 746 days in the absence of B cells (Figure 3A). Interestingly, although the number of patients studied is limited, we noted that, in the adult cohort (UPN), CD138+ cells remained above the approximate limit of detection (0.001%) in all 4 subjects whereas, in contrast, PCs remained above this level in only 4 of the 12 pediatric subjects (Figure 3B). With 1 exception (CHP-1), CD20 and CD19 IHC (Figures 3C-D) confirmed postinfusion mature B-cell aplasia with the occasional presence of rare CD20+ or CD19+ cells likely indicative of early B-cell development not proceeding to full B-cell maturation. CHP-1 experienced surface CD19− leukemia relapse with continued normal B-cell aplasia; the subject continued to demonstrate CD138+ PCs at day 261. Overall, the presence of CD138+ cells in biopsies with the absence of or extremely low-level CD19 staining was consistent with findings by flow cytometry indicating preservation of CD19− PCs.
Immunohistochemical analysis demonstrates long-term presence of bone marrow PCs and B-cell aplasia after CTL019 treatment. (A) Bone marrow core biopsies from subject UPN-1 at baseline and serial time points post-CTL019 infusion were stained, separately, for CD138, CD20, and CD19 by IHC. Representative regions at ×20 magnification are shown. (B-E) The frequency of CD138+ (B), CD20+ (C), and CD19+ cells (D) among total nucleated cells in bone marrow biopsies from adult (red curves) and pediatric (blue curves) patients was determined by digital image analysis for the indicated time points pre- and post-CTL019.
Immunohistochemical analysis demonstrates long-term presence of bone marrow PCs and B-cell aplasia after CTL019 treatment. (A) Bone marrow core biopsies from subject UPN-1 at baseline and serial time points post-CTL019 infusion were stained, separately, for CD138, CD20, and CD19 by IHC. Representative regions at ×20 magnification are shown. (B-E) The frequency of CD138+ (B), CD20+ (C), and CD19+ cells (D) among total nucleated cells in bone marrow biopsies from adult (red curves) and pediatric (blue curves) patients was determined by digital image analysis for the indicated time points pre- and post-CTL019.
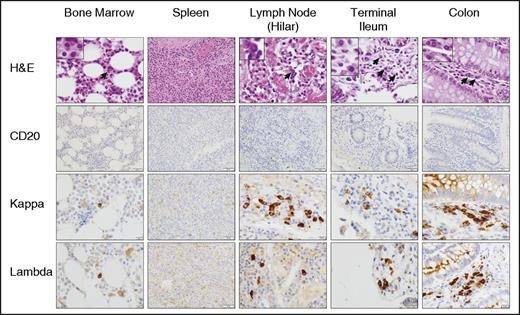
While conducting these studies, which were limited to blood and bone marrow, autopsy material from a patient with follicular lymphoma (UPN-4) who was treated with CTL019 (#NCT02030834, ClinicalTrials.gov) became available. At the time of death on day 234 (clinical details of this case have been reported23 ), the patient had persistent B-cell aplasia. Extensive clinical testing failed to show any signs of infection around the time of death. IHC analysis of bone marrow, spleen, lymph nodes, small and large intestine revealed the absence of CD20+ (Figure 4), Pax5+, or CD19+ (data not shown) cells. CD138 staining was weak, likely related to its well-described instability ex vivo.24 However, morphologic assessment in addition to κ and λ light-chain staining clearly demonstrated PCs in bone marrow, lymph nodes, and small and large intestine (Figure 4). In this patient, PCs were rare but evident in the bone marrow and most abundant in the lamina propria of the colon. In contrast, sections of the spleen showed no evidence of PCs. IgG, IgA, and IgM staining revealed that the majority of the PCs were IgG+ or IgA+ although all 3 subsets were detected in the gastrointestinal tract (supplemental Figure 2). The weak staining observed in scattered cells by CD138, CD20, and CD19 IHC was confirmed to represent an artifact due to residual endogenous myeloperoxidase activity in maturing myeloid cells (supplemental Figure 3). Mei et al previously described a population of CD19+ plasmablasts circulating in blood in rituximab-treated patients. These cells, suggested to derive from rituximab-resistant mucosal B cells, were mostly positive for IgA and Ki67 and appeared to have a mucosal phenotype.25 In contrast, we did not see any CD19+ cells in circulation following CTL019. Moreover, dual staining for Ki67 and IgA/κ/λ in the gastrointestinal tract showed no dual-positive cells; >200 cells of each type (IgA+, κ+, λ+) were analyzed per section (supplemental Figure 4). This supports the notion that the CD19− PCs observed represent a terminally differentiated, nonproliferating, long-lived population.
CTL019 results in B-cell depletion in primary and secondary lymphoid tissues. Sections of bone marrow, spleen, lymph nodes, terminal ileum, and colon, obtained at autopsy from a CTL019-treated subject (day 234), were analyzed by H&E and IHC (CD20, κ, λ). Representative sections are shown at ×20 (all spleen and CD20 images) and ×40 magnification (all other images). H&E insets are shown at ×100 magnification.
CTL019 results in B-cell depletion in primary and secondary lymphoid tissues. Sections of bone marrow, spleen, lymph nodes, terminal ileum, and colon, obtained at autopsy from a CTL019-treated subject (day 234), were analyzed by H&E and IHC (CD20, κ, λ). Representative sections are shown at ×20 (all spleen and CD20 images) and ×40 magnification (all other images). H&E insets are shown at ×100 magnification.
The ontogeny and role of CD19+ and CD19− PCs in humoral immunity has yet to be fully defined. However, recently, Mei et al suggested that the CD19− PC fraction represents a long-lived population of PCs capable of producing antibodies to vaccine antigens.8 We hypothesized, therefore, that a long-lived CD19− PC might play such a role also in CTL019-treated patients. In order to evaluate this further, we quantitatively measured vaccine/pathogen-specific IgG antibodies in subjects prior to and after receiving CTL019. We restricted antibody testing by several criteria to ensure measurements were made during deep, sustained CD20+CD19+ B-cell aplasia, which would be most informative regarding the question of B-cell dependence of the antibody-specific PCs. Additionally, only subjects with baseline antigen-specific IgG levels at or above commonly accepted protective thresholds (tetanus, measles, rubella, H influenzae) or above the limit of detection (mumps, S pneumoniae), and only time points prior to immunoglobulin-replacement therapy with IVIg were assessed. Due to these stringent criteria and limited serum sample availability, data presented in Figure 5 are limited to UPN-1 and UPN-4, but are nonetheless informative.
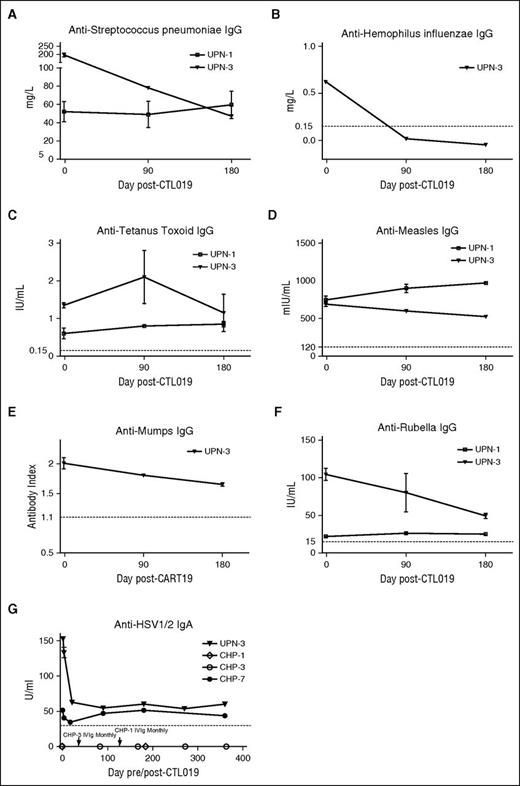
Antigen-specific antibody titers at baseline and after CTL019 infusion. (A) S pneumoniae–, (B) H influenzae type B–, (C) tetanus toxoid–, (D) measles-, (E) mumps-, and (F) rubella-specific IgG, and (G) HSV1/2-specific IgA levels were measured in CTL019-treated patients who experienced persistent B-cell aplasia. For anti-H influenzae, -tetanus toxoid, -rubella, and anti-measles, thresholds of protective IgG levels are indicated by the dashed lines. For anti-S pneumoniae, anti-mumps, and anti-HSV1/2 IgA, the dashed lines indicate the lower limit of detection. Error bars indicate standard deviations.
Antigen-specific antibody titers at baseline and after CTL019 infusion. (A) S pneumoniae–, (B) H influenzae type B–, (C) tetanus toxoid–, (D) measles-, (E) mumps-, and (F) rubella-specific IgG, and (G) HSV1/2-specific IgA levels were measured in CTL019-treated patients who experienced persistent B-cell aplasia. For anti-H influenzae, -tetanus toxoid, -rubella, and anti-measles, thresholds of protective IgG levels are indicated by the dashed lines. For anti-S pneumoniae, anti-mumps, and anti-HSV1/2 IgA, the dashed lines indicate the lower limit of detection. Error bars indicate standard deviations.
UPN-1 experienced B-cell aplasia from day 24 (first postinfusion time point measured) which has been sustained to at least day 1827 and began IVIg replacement from day 259. This subject remains in CR. In UPN-3, B-cell aplasia was documented from day 29 to at least day 741. This subject, who also remains in CR, did not receive IVIg until day 223. For both subjects, antigen-specific titers were measured at baseline, 90 and 180 days post-CTL019 infusion.
Serum concentrations of IgG specific to the T-cell–dependent protein antigens from tetanus toxoid, measles virus, mumps virus, and rubella virus remained relatively stable and within the protective range (Figure 5C-F) with the exception of anti-rubella virus IgG in UPN-3. This titer declined by ∼53% of baseline level over 180 days but remained in the protective range. Evaluation of IgG responses to capsular polysaccharide antigens from S pneumoniae and H influenzae type B (Figure 5A-B) showed differing results between UPN-1 and UPN-3. Whereas anti-S pneumoniae IgG was stable in UPN-1, antibodies to both S pneumoniae and H influenzae type B declined substantially in UPN-3.
Because IgA titers may also be sustained following natural infection,26,27 we evaluated IgA titers to HSV1/2. We identified 2 subjects with detectable anti-HSV1/2 IgA at baseline (Figure 5G). IgA to HSV1/2 remained detectable and largely stable for up to 360 days following CTL019-induced B-cell aplasia. In UPN-3, the initial decline in titer may reflect a loss of CD19+ HSV-specific PCs or may represent normal decline following an active immune response although no evidence of active infection was noted. IgA levels were tested even during periods of Ig replacement therapy as IV and subcutaneous Ig preparations contain a relatively small amount of IgA that is diluted in the recipient and is expected to decline rapidly with a half-life of about 1 week. As controls, 2 subjects without detectable anti-HSV1/2 IgA at baseline, but who began receiving IVIg due to hypogammaglobulinemia, were also tested (Figure 5G; subjects CHP-1 and CHP-3). Both continued to demonstrate undetectable anti-HSV1/2 IgA.
In addition to antigen-specific antibody concentrations measured in UPN-1, UPN-3, and CHP-7, total serum IgG, IgA, and IgM concentrations were measured (supplemental Figure 5). Except for IgG in CHP-7, who received IVIg from day 18 onward, total serum IgG, IgA, and IgM concentrations declined after CTL019.
ABO blood group antibodies were also evaluated at baseline and after CTL019-induced B-cell aplasia. Anti-A and anti-B titers were maintained in the setting of continued B-cell aplasia and, to the best of our knowledge, absence of transfusion (supplemental Table 3).
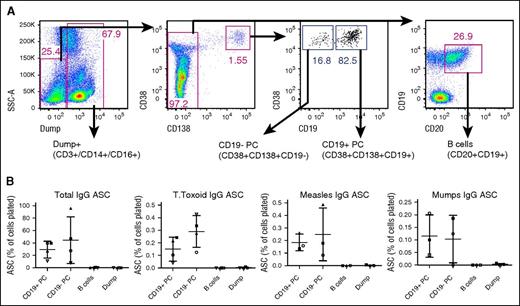
To evaluate the specificity and function of CD19− PCs that remain following CTL019 therapy, we performed flow cytometry–based sorting of bone marrow PCs into CD19+ and CD19− populations (Figure 6A), and assessed their total and antigen-specific IgG secretion by ELISPOT. Due to limitations of sample quantity, these studies were performed using bone marrow from healthy donors. Similar to data reported by others, we observe that the CD19− PC fraction contains PCs capable of producing tetanus toxoid–, mumps virus–, and measles virus–specific IgG (Figure 6B). Overall, these results confirm the presence of vaccine-specific PCs within the CD19− fraction of PCs that remain following CTL019 therapy.
CD19− PCs produce vaccine/pathogen-specific antibodies. (A) Bone marrow aspirate cells from healthy donors were sorted into 4 populations (CD19+ PCs, CD19− PCs, B cells, and Dump+) and were analyzed by ELISPOT. (B) Total IgG, tetanus toxoid–, measles-, or mumps-specific IgG-producing cells were enumerated by ELISPOT. Each symbol represents an individual donor. Means and standard deviations are indicated. For total IgG, all pairwise comparisons demonstrated a P value of .0625 except CD19+ PC vs CD19− PC and B cells vs Dump. For tetanus toxoid, all pairwise comparisons demonstrated a P value of .0625 except B cells vs Dump. For measles, only CD19+ PC vs B cells and CD19+ vs Dump comparisons demonstrated a P value of .0625. P values for all other pairwise comparisons were .1088 or greater (Wilcoxon signed-rank test). ASC, antibody-secreting cells.
CD19− PCs produce vaccine/pathogen-specific antibodies. (A) Bone marrow aspirate cells from healthy donors were sorted into 4 populations (CD19+ PCs, CD19− PCs, B cells, and Dump+) and were analyzed by ELISPOT. (B) Total IgG, tetanus toxoid–, measles-, or mumps-specific IgG-producing cells were enumerated by ELISPOT. Each symbol represents an individual donor. Means and standard deviations are indicated. For total IgG, all pairwise comparisons demonstrated a P value of .0625 except CD19+ PC vs CD19− PC and B cells vs Dump. For tetanus toxoid, all pairwise comparisons demonstrated a P value of .0625 except B cells vs Dump. For measles, only CD19+ PC vs B cells and CD19+ vs Dump comparisons demonstrated a P value of .0625. P values for all other pairwise comparisons were .1088 or greater (Wilcoxon signed-rank test). ASC, antibody-secreting cells.
Discussion
Recent evidence in humans suggests that long-lived humoral immunity resides within the CD19− PC fraction.8,18 Phenotypic analysis in mice suggests that loss of CD19 expression represents progressive differentiation, marking long-lived PCs.28 Similar to depletion studies in mice,5-7 significant reductions in the number of CD19+ PCs in patients’ bone marrow have been observed following anti-CD20 therapy without a significant change in the CD19− subset, suggesting that the CD19+ PC subset represents a short-lived population requiring replenishment from CD20+ B cells.8 Although total IgG may decline, stability of preexisting antigen-specific IgG following rituximab-induced B-cell depletion has been demonstrated.29-33 However, several studies have shown that rituximab therapy effectively eliminates B cells from the peripheral blood but fails to effectively deplete all B cells within secondary lymphoid tissues.34-39 Thus, whether PCs remaining in this setting are long-lived or dependent on replenishment by the residual B cells is unknown.
Overall, our results indicate that a population of PCs lacking CD19 can persist in the bone marrow following an effective CTL019–mediated B-cell depletion. IHC studies performed on autopsy material from 1 subject with a complete tumor response suggest highly effective B-cell depletion in secondary lymphoid organs by CTL019. Serology results from 3 subjects indicate that the remaining CD19− PCs are capable of secreting antibodies to vaccine and pathogen-related antigens. Our results further suggest that CD19− PCs are likely long-lived given the absence of B cells to replenish this pool, and these cells most likely contribute to stable serum antibody titers observed in patients with CTL019-induced B-cell aplasia. Our analysis of PCs following CTL019 treatment allowed assessment of CD19− PCs only. It is possible that some CD19+ PCs may also have the capacity to survive long-term; in fact, we and Mei et al found that ex vivo–sorted CD19+ PCs could also contribute to antibody responses that are relatively long-lived such as responses to tetanus toxoid, and measles and mumps viruses.8 In contrast, for unknown reasons, Halliley et al did not detect these antibody responses in the CD19+ PC fraction.18
Several CD19-directed antibody-based immunotherapeutic approaches are currently in clinical trials for the treatment of B-cell lineage malignancies.40-42 Neither preclinical nor clinical studies using such approaches have addressed the fate of PC or preexisting antigen-specific antibodies.7,40,43 The recent introduction of CD19-directed CAR T-cell–based therapy has provided a unique opportunity to study the impact of prolonged B-cell aplasia on the CD19− PC population. The duration of continuous B-cell aplasia in the cohort presented here, which was observed for at least 1827 days in 1 subject, depends on the duration of CAR T-cell persistence.13 Thus, B-cell aplasia is likely maintained by the continuous action of CAR T cells. The pharmacokinetic profile of CAR T cells is vastly different from that of passively transferred B-cell–depleting antibodies and may translate to more robust B-cell depletion. Consistent with this notion, autopsy material from UPN-4 demonstrated total B-cell elimination from all tissues analyzed (Figures 3 and 4). In this context of marked and sustained B-cell aplasia, we found that some previously generated antigen-specific antibody responses are preserved for at least 6 months (IgG) and up to ∼1 year (IgA) despite absence of normal B cells. Given the 3- to 4-week half-life of IgG and much shorter half-life of IgA, antigen-specific IgG and IgA concentrations would be expected to be reduced by >10-fold without their continued synthesis. This stability strongly argues for the existence of a long-lived pool of antibody-producing CD19− PCs that do not require replenishment by CD19+ precursors. Declines in some antibody levels (eg, anti-rubella virus IgG and anti-HSV1/2 IgA in UPN-3) were noted but these did not occur at the rate expected if the antibody-producing cells were directly targeted by CTL019 cells. Thus, their decline may represent a combination of initial CTL019-mediated loss of antigen-specific CD19+ PCs as well as the turnover of CD19− PCs. Longer follow-up in CTL019-treated patients in the absence of IVIg therapy would be interesting to determine the long-term kinetics of these responses.
Results of our IHC studies raise interesting questions regarding long-lived PCs. Although the lack of identified PCs in some patients may simply reflect their rarity and limitations of tissue sampling, it may be that not all CTL019-treated patients maintain bone marrow PCs. This may be due to differences in PC-intrinsic properties as well as PC-extrinsic factors that constitute the PC niche. Although not yet precisely understood, the PC niche is thought to include factors such as IL-6, APRIL, BAFF, and CXCL12, and cell-surface molecules such as VCAM1 provided by stromal cells, eosinophils, and possibly, others.44 In our cohort, serum BAFF, but not APRIL or IL-6, was increased post-CTL019 compared with pretreatment but we did not see a difference in individual changes between those in whom we did and did not detect PCs posttreatment (data not shown). However, we cannot rule out the fact that loss of PCs in the context of CTL019, and differences between subjects, may be the result of indirect effects. Differences noted between subjects could also be related to age, disease, or prior therapy. The demonstration of CD19− PCs in compartments outside of the bone marrow (lymph node and gastrointestinal tract; Figure 4; supplemental Figure 4) in a patient ∼8 months after CTL019 infusion and in the context of deep B-cell aplasia suggests that PCs at these sites may also be relatively long-lived.
This is the first report, to our knowledge, of the effect of CD19+ cell depletion on PCs and specific humoral immunity in humans. Our findings contribute to a growing body of evidence that suggests B-cell–independent longevity of some human PCs. The maintenance of preexisting humoral immunity observed in our study challenges assumptions regarding the degree of immunosuppression induced by CTL019. Assessment of mucosal antibodies, which constitute a substantial portion of total body immunoglobulin and an important part of host defense, is also needed to understand the breadth of humoral immune deficiency induced by CTL019 therapy.
Our findings focus on normal PCs that remain following CTL019 treatment. Garfall et al recently reported application of CTL019 for the treatment of multiple myeloma where the vast majority of malignant PCs do not express CD19.45 Although the mechanism of response to CTL019 in this context is unclear, the findings suggest that maintenance of malignant PCs may be different from that of normal PCs analyzed in this study.
Finally, our results have also raised important questions regarding the application of B-cell–directed therapies for the eradication of pathogenic autoimmune or allograft antibodies. The durability of preexisting humoral immunity in CTL019-treated subjects suggests that therapies directed at the CD19− long-lived PCs may be required to fully eradicate these types of pathogenic humoral immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Paul Hallberg, Charles (Hank) Pletcher, and Yolanda Mahnke for flow cytometry technical assistance. The authors thank Dan Martinez, Li-Ping Wang, Amy Ziober, and Andrea Edwards for assistance with IHC studies, and Maya Mudambi and Vanessa Gonzalez for assistance with collection of patient data. The authors thank David Allman and Michael Cancro for helpful discussions.
This work was supported by a National Institutes of Health, National Cancer Institute training grant (T32 CA009140), National Heart, Lung, and Blood Institute K12 Career Development award (K12 HL087064) (V.G.B.), National Cancer Institute research grants (1R01CA165206 [C.H.J.]; and R01CA102646 and R01CA116660 [S.A.G.]), a grant from the Leukemia & Lymphoma Society, and a research agreement with Novartis Pharmaceuticals.
Authorship
Contribution: V.G.B. and M.C.M. designed the research; V.G.B., D.A., C.T.E., and F.N. performed the experiments; J.C., C.A.C., and S.F.L. gathered trial data; V.G.B., D.A., G.W., W.-T.H., J.J.M., M.D.F., A.B., and M.C.M. analyzed the data; A.E.O. and M.A.W. assisted with interpretation of the autopsy material; V.G.B. and M.C.M. wrote the manuscript; and S.L.M., S.S., D.L.P., S.A.G., and C.H.J. designed the clinical trials and read and made comments on the manuscript.
Conflict-of-interest disclosure: M.C.M., C.H.J., and D.L.P. are inventors on patents related to CTL019 that have been licensed to Novartis Pharmaceuticals. These financial conflicts of interest are managed in accordance with policies established by the University of Pennsylvania. The remaining authors declare no competing financial interests.
Correspondence: Michael C. Milone, Hospital of the University of Pennsylvania, 7.103 Founders Pavilion, 3400 Spruce St, Philadelphia, PA 19104; e-mail: milone@mail.med.upenn.edu.